Volume 23 Number 3
Dermatoporosis: Clinical features, molecular mechanisms and novel therapeutic targets - A literature review
Dr Aysin Kaya, Dr Hubert B Vuagnat, Prof Gürkan Kaya
Keywords skin tears, wounds, Wound care, dermatology, Deep dissecting haematoma, dermatoporosis
DOI 10.35279/jowm2022.23.03.08
Abstract
‘Dermatoporosis’ is a term proposed 15 years ago to describe an extreme cutaneous insufficiency/fragility syndrome beyond cosmetic aspects. Individuals suffering from this condition are usually elderly, with a history of chronic sun exposure and/or chronic topical or systemic corticosteroid use. The preliminary clinical aspect is skin atrophy, which may be complicated by skin tears and deep dissecting haematoma, a severe complication of dermatoporosis that requires immediate medical attention. We reviewed the literature on dermatoporosis in the PubMed and CINAHL databases from 2006 to 2022 and present here the current knowledge about this emerging skin condition with potential molecular mechanisms and novel therapeutic targets.
Background Dermatoporosis is a particular form of skin atrophy/fragility proposed for the first time in 2007. It has a clear link not only to skin tears, but also to deep dissecting haematomas and pressure ulcers in older adults.
Aim To review the literature published on the subject since its first description.
Method A literature search between 2006 and May 2022 using the keyword ‘dermatoporosis’ was performed on PubMed and CINAHL databases.
Findings A total of 46 papers were identified in the initial search. They describe different aspects of dermatoporosis. From an epidemiological perspective, some papers report its prevalence to be around 30% among elderly hospitalised subjects. Beyond the usual clinical features of dermatoporosis (purpura, pseudoscars, skin tears and deep dissecting haematomas), some papers statistically link dermatoporosis to osteoporosis and pressure ulcers. Other studies describe the histological aspects and molecular mechanisms of dermatoporosis.
Implications for clinical practice These observations should help clinicians to identify patients at risk of developing dermatoporosis and allow them to benefit from global prevention. For those patients already showing the clinical complications of dermatoporosis, an understanding of the underlying process should lead to a better patient care.
INTRODUCTION
Dermatoporosis is a particular form of skin atrophy/fragility that we proposed in 2007 in order to point out its importance and prevalence among elderly people as an emerging entity. We described its molecular aspects and explored the possibility of developing preventive and therapeutic solutions (1-6). With the increasing number of elderly patients, this prevalent skin condition is now also recognised by others as a unique clinical syndrome (7-9). Chronological aging, chronic systemic or topical steroid therapy and chronic exposure to UV irradiation seem to be the main causes (2). A recent study suggested that the use of soaps marketed for skin cleansing containing chemical ingredients that damage human keratinocytes and result in skin barrier subclinical irritation can cause skin atrophy and dermatoporosis (10).
METHODS
A literature search of the PubMed and CINAHL databases using the keyword ‘dermatoporosis’ was performed to identify eligible articles. The publication range was between 2006, the date of the first published article on dermatoporosis, and May 2022. No articles were published on the topic in 2022. A total of 46 papers were identified in the initial search. Abstracts and full texts of all articles were reviewed, and all 46 reports about dermatoporosis in different categories (review, observational study, research study, clinical study, case report, editorial) were included in this review (see Table 1).
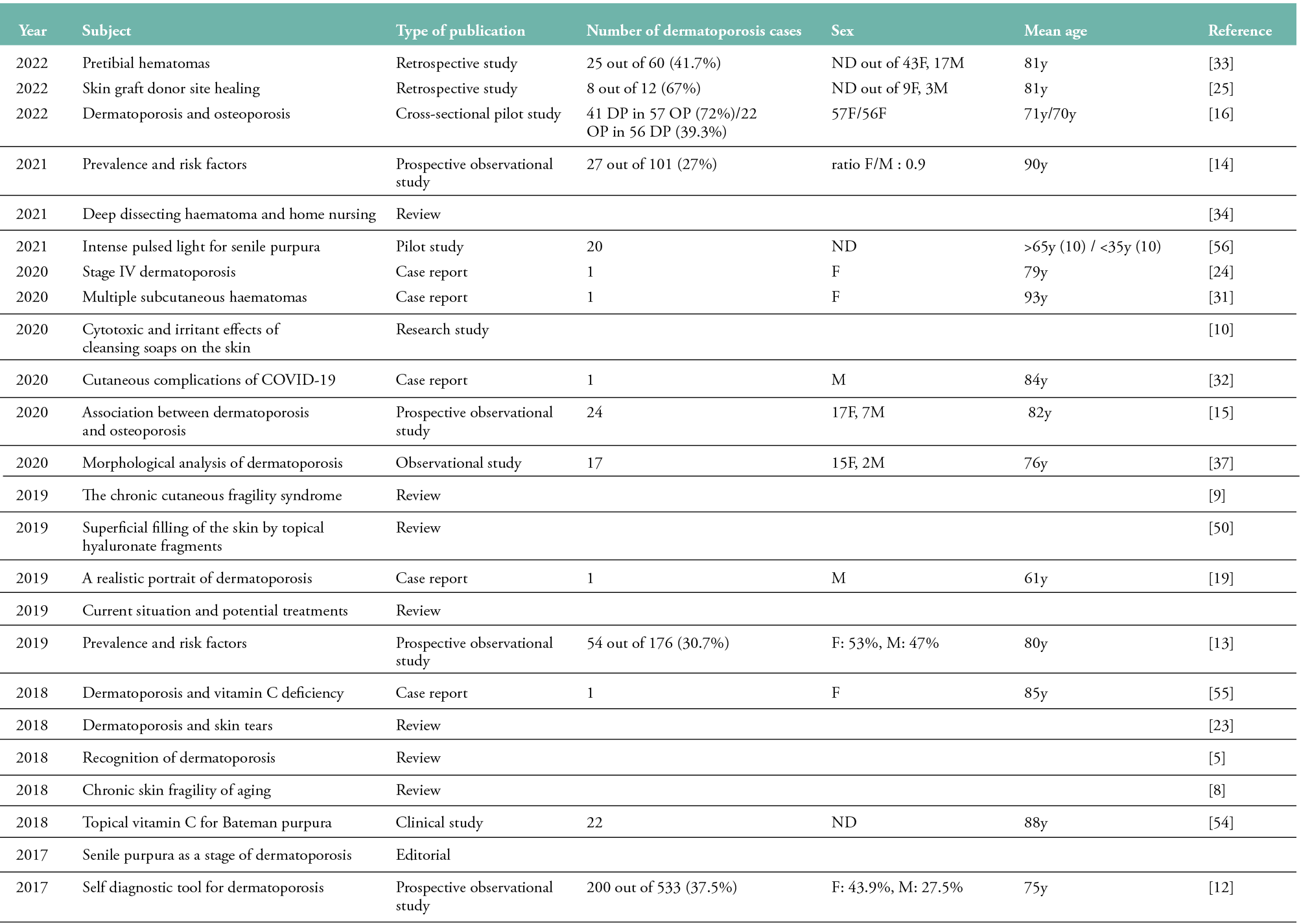
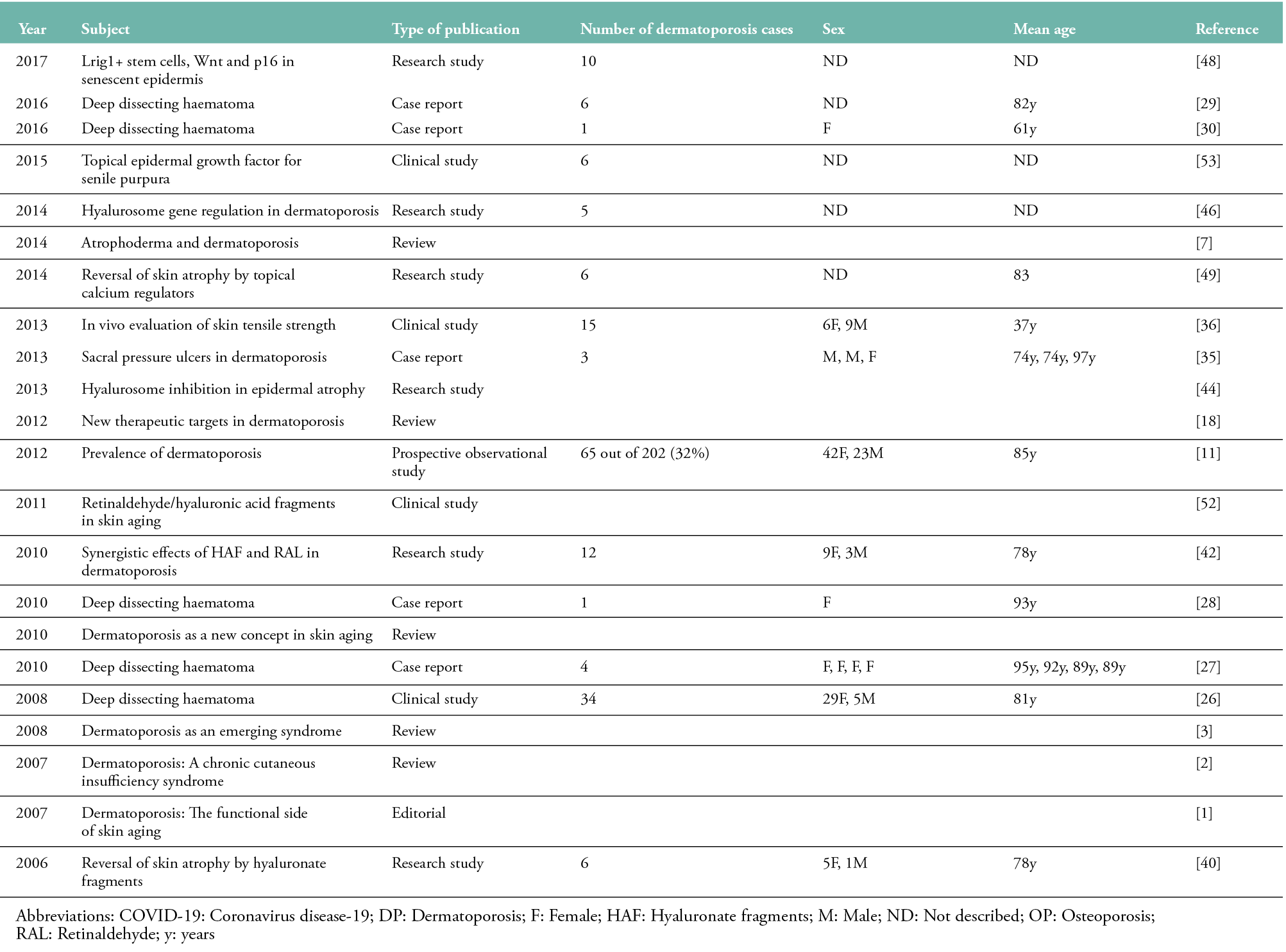
Table 1: Publications concerning dermatoporosis published between 2006 and 2022 (n=46)
Epidemiology of dermatoporosis
A French study reported the prevalence of dermatoporosis to be 32% in geriatric patients (65 of 202 subjects; 42F/23M, mean age: 85 years) (11). Another French study of 533 subjects using a self-diagnosis tool showed an overall prevalence of dermatoporosis of 37.5% (mean age: 75 years), with a F:M ratio of 3:2 (12). A Finnish study found the prevalence of dermatoporosis to be 30.7% in a tertiary care hospital (54 out of 176 subjects; F: 53%, M: 47%; mean age: 80 years) (13). A recent study conducted in France determined the prevalence of dermatoporosis to be 27% in a rehabilitation hospital (27 out of 101 subjects; ratio F/M: 0.9; mean age: 90 years) (14). In another study, where the prevalence of dermatoporosis was 5.6% (24 out of 434 patients; 17F/7M, mean age: 82 years), the presence of dermatoporosis was significantly associated with a history of major osteoporotic fracture (15). In a recent cross-sectional study conducted in Finland, 41 dermatoporosis cases were observed in 57 osteoporosis patients (72%) and 22 osteoporosis cases in 56 dermatoporosis patients (39.3%) (16).
Clinical features of dermatoporosis
The first clinical signs of dermatoporosis are seen after the age of 40 and as wrinkles and appearance modifications; however, classical morphological markers of skin fragility develop after 60 years. Dermatoporosis usually starts with skin atrophy. Other signs, such as senile purpura, pseudoscars and superficial excoriations, may follow as the condition advances (2, 17). The topography of dermatoporosis indicates the role of ultraviolet irradiation in its aetiology: the posterior side of forearms, dorsum of hands, presternal area, scalp and pretibial zones (18). The description of the first clinical signs of dermatoporosis may also be found in old art works, such as The Artist’s Foot, a painting by Adolph von Menzel (1876) (19).
Dermatoporosis has been proposed to have two forms: primary dermatoporosis, the most common type, resulting from chronological aging and long-term unprotected sun exposure, and secondary dermatoporosis, due to the chronic use of topical and/or systemic corticosteroids.
Four stages of dermatoporosis have been proposed (2, 6):
- Stage I: Skin atrophy, senile purpura, pseudoscars and superficial excoriations
- Stage II: IIa, Localised and small superficial lacerations or skin tears up to 3 cm; IIb, Skin tears larger than 3 cm
- Stage III: IIIa, Superficial hematoma; IIIb, Deep dissecting hematoma without skin necrosis
- Stage IV: Large areas of skin necrosis with potential lethal complications
We have recently proposed a scoring system based on clinical signs and ultrasonographic measures of the skin thickness (6). According to this system, dermatoporosis is scored between 0 and 16 (0: no dermatoporosis; 1–7: early stage; 8–9: early intermediate stage; 10–12: late intermediate stage; 13–16: early advanced stage; >16: advanced stage).
Complications of dermatoporosis Skin tears
Skin tears are considered a significant health problem (20, 21). Their prevalence is estimated to be 3.3–19.8% in acute care, 14.3% in palliative care, 5.5–19.5% in the community and 3.0–26.0% in long-term care (22). The International Skin Tear Advisory Panel defines skin tears as ‘traumatic wounds caused by mechanical forces, including removal of adhesives. Severity may vary by depth (not extending through the subcutaneous layer)’ (20). Patients with dermatoporosis are at increased risk of skin tears (23, 24). Skin tears are seen from clinical stage II. These are superficial wounds with irregular borders most commonly resulting from minor trauma due to extreme skin fragility showing a delayed healing. Split-thickness skin graft harvesting in dermatoporosis patients may result in prolonged donor site healing, which can be managed with regular local wound care (25).
Deep dissecting haematoma
Deep dissecting haematoma (DDH) is the most serious complication of dermatoporosis. Clinically, it presents with a red, hot and swollen limb, resulting from massive bleeding dissecting the subcutaneous fat and muscle fascia, and potentially leading to a medical emergency (26-31). DDH occurs mainly in advanced stages of dermatoporosis; however, according to the degree of the trauma, it can be seen at any stage. In coronavirus disease-19 (COVID-19) patients, haemostasis abnormalities secondary to SARS-CoV-2 infection may cause the progression of minimal dermatoporosis lesions to DDH and lead to lethal consequences. In one published case report, DDH resulted from a combination of various factors, including minimal dermatoporosis, anticoagulation therapy and coagulation abnormalities due to COVID-19 infection (32). Early diagnosis and treatment is of paramount importance for the prognosis of patients with DDH. The rapid evacuation of the haematoma will prevent a large skin injury and, in most cases, save the life of the patient (33). Health care professionals’ awareness of how best to take care of dermatoporosis patients should be enhanced regarding DDH. Early recognition and wound management of DDH is particularly important for home nursing (34).
Pressure ulcers
In three reported cases of sacral ulcers in elderly patients, the presence of clinical signs of dermatoporosis on the forearms suggested an increased risk of pressure ulcer development in dermatoporosis patients (35).
Diagnosis of dermatoporosis
The diagnosis of dermatoporosis is made by clinical examination and cutaneous thickness measurement (2, 6). Clinical examination assesses the features of dermatoporosis, such as skin atrophy, purpuric lesions, pseudoscars and skin tears. The skin thickness measurement (epidermal and dermal thickness until the dermal/subcutaneous fat junction) is conducted using a skin ultrasound system, usually on the dorsal side of the forearms, legs and chest. The normal thickness of the forearm skin is ≥ 1 mm. The skin thickness in dermatoporosis patients is generally between 0.5–1 mm. However, patients with advanced dermatoporosis may show values under 0.5 mm.
The viscoelastic parameters assessed by a Cutometer® using steep and progressive suction procedures are also altered in dermatoporosis (36).
In vivo reflectance confocal microscopy can also be used to diagnose dermatoporosis. Dermal-epidermal atrophy, a reduction of the dermal papillae/area and the thickness of dermal elastosis seem to be the major histometric parameters that characterise dermatoporosis (37).
Histologically, dermatoporotic skin displays an atrophy of the epidermis and dermis. The epidermis is linearised, with the loss of rete ridges, and a significant solar elastosis is found in the dermis. In the dermis, collagen, elastic fibre and mucin content, and the number of blood vessels, are decreased. Senile purpura is characterised by the extravasation of red blood cells into the dermis with no inflammation. The pseudoscar shows a compact subepidermal hypocellular band of collagen in the superficial dermis (2).
Molecular mechanisms of dermatoporosis Decrease of CD44 and hyaluronate
The suppression of epidermal CD44, the principal cell surface receptor of hyaluronate (HA), results in skin atrophy in mice (38). The research in dermatoporosis was based on this observation. HA is found in high amounts in the extracellular matrix of the skin and helps to maintain its normal hydration and viscoelasticity (39). CD44 and HA are decreased in human dermatoporotic skin (40). Exposure to UVA and UVB has been shown to diminish the expression of HA and CD44 in mice (41).
Hyalurosome deficiency
What is new in dermatoporosis research is the hyalurosome model. The term hyalurosome has been proposed to describe an HA factory on a membrane complex including HA synthase 3 (HAS3), the keratinocyte-specific CD44 variant CD44v3 and epidermal growth factor (EGF) receptor (EGFR), which is functionally deficient in cases of dermatoporosis (42). HAS3 is colocalised with CD44v3, HA and EGFR on the keratinocyte membrane (43). HAS3 transfection to cultured epithelial cells induces the formation of filopodia, thin actin-rich plasma-membrane protrusions, and CD44v3, HA and actin are colocalised in the filopodia of cultured keratinocytes (44-45). The expression of hyalurosome molecules is decreased in patients with dermatoporosis, compared to young subjects, as shown by qRT-PCR on skin biopsies (46).
Epidermal stem cells
EGFR inhibitor Lrig1+ stem cells are located in the interfollicular and follicular zones in human skin and conserved in the epidermis of dermatoporotic skin (47,48). Lrig1+ stem cells are located in the isthmus region of the mouse hair follicle and feed all the epidermis in cell lineage tracing experiments performed in CD44 knock-out mice, which show a skin phenotype reminiscent of dermatoporosis (48). These experiments suggest that, in normal epidermis, there are two clusters of basal cells: (i) Lrig1+ clusters as a reservoir of quiescent stem cells, and (ii) EGFR+/CD44v3+ clusters as daily renewing cells. In dermatoporotic skin, there is one cluster, Lrig1+, renewing all the interfollicular epidermis (48).
Wnt/β-catenin pathway
CD44 is a positive regulator of Wnt/β-catenin signalling that is decreased in the epidermis of dermatoporotic skin (48). In normal epidermis, the Wnt pathway is associated with the hyalurosome platform and regulates interfollicular epidermis growth in the EGFR+/CD44v3+ cluster mentioned above. In dermatoporotic skin, the Wnt pathway activity is decreased and the EGFR+/CD44v3+ cluster is lost (48).
Calcium signalling
The expression of the calcium channel Orai-1, the main molecular partner of SOCE (Store-Operated Calcium Entry), located in the epidermal basal layer and involved in keratinocyte proliferation, is decreased in the epidermis of dermatoporosis patients (49).
p16Ink4a pathway
The cyclin-dependent kinase inhibitor CDKN2A, commonly referred to as p16Ink4a or p16, has been established as a general marker of cellular senescence, as p16Ink4a was observed to be expressed in most senescent cells. The number of p16Ink4a-positive cells was shown to be increased in the epidermis of dermatoporotic skin (48).
Treatment strategies
HA fragments
Topical application of HA fragments (HAF) of intermediate size (80–150 kDa; HAFi) induced epidermal hyperplasia and epidermal and dermal HA synthesis by stimulating the molecules of hyalurosome (49). Topical HAFi also prevented corticosteroid-induced skin atrophy in mice (44). Topical treatment with HAFi resulted in a significant clinical improvement in the forearms of dermatoporosis patients after one month by inducing the expression of hyalurosome molecules (40, 46). HAFi also acts as an epicutaneous (or topical) HA filler (50). The combination of HAFi with retinaldehyde (RAL), which increases the expression of CD44 and HA synthases in mouse skin (51), shows a synergistic effect at both the molecular and clinical levels in aged skin (42, 46, 52).
Epidermal growth factor
Topical EGF decreases the appearance of senile purpura and increases the skin thickness, suggesting a preventive effect in late-stage dermatoporosis (53).
Vitamin C
A topical formulation containing 5% ascorbic acid diminishes the number of senile purpura in the forearm of scurvy patients (54). Oral vitamin C improved extensive blistering purpura and spontaneous hematomas in an elderly dermatoporosis patient (55).
Lasers
Recently, it was shown that intense pulsed light lasers are safe and effective for improving the clinical appearance of senile purpura and preventing future lesions by increasing epidermal thickness and restoring collagen and elastic fibre morphology (56).
Other treatments
Other topical treatment modalities for skin atrophy include alpha-hydroxy acids twice daily for at least three months (57), or 1% topical dehydroepiandrosterone cream twice daily for four months (58). Since low protein intake is associated with increased skin fragility, protein-rich nutrition is recommended for elderly patients showing signs of extreme skin aging (8).
Novel molecular targets
Lrig1, β-catenin, Orai-1 and p16Ink4a are interesting potential targets that may lead to the development of efficient preventive and/or therapeutic strategies for dermatoporosis.
IMPLICATIONS FOR CLINICAL PRACTICE
- Patients with dermatoporosis are at increased risk of skin tears. Due to its high prevalence and potential complications, it should be investigated among the elderly population.
- Similar prevention and treatment programmes as those for skin tears and skin health should be proposed.
- Specific treatments should be considered, even if those mentioned are still not used on a wide scale.
Further research
- Epidemiological data should be strengthened.
- Based on current biochemical knowledge, more treatment strategies should be proposed.
- These strategies should be both affordable and easy to use, and a thorough clinical and scientific assessment of their efficacy should be conducted.
Acknowledgments
The authors thank Professor Jean-Hilaire Saurat for his valuable comments and critical reading of the manuscript.
Key messages
- Dermatoporosis is an extreme cutaneous insufficiency/fragility leading to skin tears and deep dissecting haematoma.
- The definition and molecular mechanisms of dermatoporosis will be reviewed.
- Dermatoporosis is frequent among the elderly population. Resulting in skin tears and deep dissecting haematoma, it can lead to significant morbidity and mortality. Better knowledge and treatment of this pathology should be spread among healthcare professionals, to better prevent and treat the resulting lesions.
Author(s)
Dr Aysin Kaya,1 MD, Dr Hubert B Vuagnat,2 MD, Prof Gürkan Kaya,3 MD, PhD
1Department of Clinical Pharmacology and Toxicology, University of Geneva, Geneva, Switzerland
2Wounds and Woundhealing Program, Nursing Directorate, University Hospital of Geneva, Geneva, Switzerland
3Department of Dermatology, University Hospital of Geneva, Geneva, Switzerland
Correspondence: Gürkan Kaya, gkaya@hcuge.ch
Conflict of interest: None
References
- Saurat JH. Dermatoporosis. The functional side of skin aging. Dermatology. 2007; 215:271-2.
- Kaya G, Saurat JH. Dermatoporosis: A chronic cutaneous insufficiency/fragility syndrome. Clinicopathological features, mechanisms, prevention and potential treatments. Dermatology. 2007; 215:284-94.
- Kaya G. Dermatoporosis: An emerging syndrome. Rev Med Suisse. 2008; 4:1078-82.
- Kaya G, Saurat JH. Dermatoporosis: A new concept in skin aging. Eur Geriatr Med. 2010; 1:216-9.
- Kaya G, Kaya A, Sorg O, Saurat JH. Dermatoporosis: A further step to recognition. J Eur Acad Dermatol Venereol. 2018; 32:189-91.
- Kaya G, Kaya A, Sorg O, Saurat JH. Dermatoporosis, a prevalent skin condition affecting the elderly: Current situation and potential treatments. Clin Dermatol. 2019; 37:346-50.
- Piérard-Franchimont C, Hermanns JF, Hermanns-Lê T, Lachapelle JM, Lesuisse M, Piérard GE, et al. Dermatoporosis, a vintage for atrophoderma and transparent skin. Rev Med Liege. 2014; 69:210-3.
- Dyer JM, Miller RA. Chronic skin fragility of aging: Current concepts in the pathogenesis, recognition, and management of dermatoporosis. J Clin Aesthet Dermatol. 2018; 11:13-8.
- Wollina U, Lotti T, Vojvotic A, Nowak A. Dermatoporosis - The chronic cutaneous fragility syndrome. Open Access Maced J Med Sci. 2019; 7:3046-9.
- Castanedo-Cázares JP, Cortés-García JD, Cornejo-Guerrero MF, Torres-Álvarez B, Hernández-Blanco D. Study of the cytotoxic and irritant effects of skin cleansing soaps. Gac Med Mex. 2020; 156:418-23.
- Mengeaud V, Dautezac-Vieu C, Josse G, Vellas B, Schmitt AM. Prevalence of dermatoporosis in elderly French hospital in-patients: A cross-sectional study. Br J Dermatol. 2012; 166:442-3.
- Saurat JH, Mengeaud V, Georgescu V, Coutanceau C, Ezzedine K, Taïeb C. A simple self-diagnosis tool to assess the prevalence of dermatoporosis in France. J Eur Acad Dermatol Venereol. 2017; 31:1380-6.
- Kluger N, Impivaara S. Prevalence of and risk factors for dermatoporosis: A prospective observational study of dermatology outpatients in a Finnish tertiary care hospital. J Eur Acad Dermatol Venereol. 2019; 33:447-50.
- Chanca L, Fontaine J, Kerever S, Feneche Y, Forasassi C, Meaume S, et al. Prevalence and risk factors of dermatoporosis in older adults in a rehabilitation hospital. J Am Geriatr Soc. 2021 Dec 17; 70(4):1252-6. doi: 10.1111/jgs.17618.
- Villeneuve D, Lidove O, Chazerain P, Ziza JM, Sené T. Association between dermatoporosis and history of major osteoporotic fractures: A French prospective observational study in a general practice population. Joint Bone Spine. 2020; 87:511-2.
- Dos Santos Guadanhim LR, Nasser AI, Soares JLM, Sañudo A, Brandão C, Bagatin E. Dermatoporosis and osteoporosis: cross-sectional pilot study. Int J Dermatol. 2022; 61:e331-3.
- Karadag AS, Parish LC, Lambert WC. Senile purpura as a stage of dermatoporosis. Skinmed. 2017; 15:91-2.
- Kaya G. New therapeutic targets in dermatoporosis. J Nutr Health Aging. 2012; 16:285-8.
- Kluger N. The Artist’s Foot by Adolph von Menzel (1876): A realistic portrait of dermatoporosis and venous insufficiency. Ann Dermatol Venereol. 2019; 146:852-3.
- LeBlanc K, Campbell KE, Wood E, Beeckman D. Best practice recommendations for prevention and management of skin tears in aged skin: An overview. J Wound Ostomy Continence Nurs. 2018; 45:540-2.
- Chang YY, Carville K, Tay AC. The prevalence of skin tears in the acute care setting in Singapore. Int Wound J. 2016; 13:977-83.
- Van Tiggelen H, Kottner J, Campbell K, LeBlanc K, Woo K, Verhaeghe S, et al. Measurement properties of classifications for skin tears: A systematic review. Int J Nurs Stud. 2020; 110:103694.
- Vanzi V, Toma E. Recognising and managing age-related dermatoporosis and skin tears. Nurs Older People. 2018; 30:26-31.
- Bandera García C, Aragonés Domínguez AE, Lozano Noriega D, Ginel Mendoza L, Poyato Ramos R. Stage IV dermatoporosis: Report of a case. Gerokomos. 2020; 31(1):32-5.
- Seppälä T, Grünthal V, Koljonen V. Skin graft donor site healing among elderly patients with dermatoporosis - A case series. Int J Low Extrem Wounds. 2022 Mar 15; 15347346221087081.
- Kaya G, Jacobs F, Prins C, Viero D, Kaya A, Saurat JH. Deep dissecting hematoma: An emerging severe complication of dermatoporosis. Arch Dermatol. 2008; 144:1303-8.
- Toutous-Trellu L, Weiss L, Tarteaut MH, Kaya A, Cheretakis A, Kaya G. Deep dissecting hematoma: A plaidoyer for an early and specialized management. Eur Geriatr Med. 2010; 1:228-30.
- Gamo R, Vicente J, Calzado L, Sanz H, López-Estebaranz JL. Deep dissecting hematoma or stage IV dermatoporosis. Actas Dermosifiliogr. 2010; 101:89-90.
- Eto A, Nakamura M, Ito S, Tanaka M, Ichikawa M, Morioka Y, et al. Six cases of deep dissecting hematoma caused by dermatoporosis. Nishinihon J Dermatol. 2016; 78(5):487-90.
- Inokuchi S, Nobeyama Y, Itoh M, Nakagawa H. A case of deep dissecting hematoma: Different managements resulting in similar outcomes. Int J Dermatol. 2016; 55(12):e628–9.
- Vallini V, Rinaldi E, Mangano L, Modesti L, Ghelardini P, Roberts AT, et al. Multiple subcutaneous haematomas of the legs causing skin necrosis in an elderly patient affected by corticosteroid-induced skin atrophy: Case report and review of literature. Int Wound J. 2020; 17(3):540-6.
- Lorenzo-Villalba N, Maouche Y, Syrovatkova A, Pham F, Chahbazian JB, Pertoldi P, et al. Cutaneous complications secondary to haemostasis abnormalities in COVID-19 infection. Eur J Case Rep Intern Med. 2020; 7:001769.
- Seppälä T, Grünthal V, Koljonen V. Pretibial hematomas - A real-world single-center study. JPRAS Open. 2022 Feb 24; 32:79-87.
- Vanzi V, Toma E. Deep dissecting haematoma in patients with dermatoporosis: Implications for home nursing. Br J Community Nurs. 2021; 26(Sup3):S6-13.
- Kurashige Y, Minemuta T, Nagatani T. Three cases of sacral pressure ulcers presenting primary dermatoporosis on the forearms. Case Rep Dermatol. 2013; 5:73-8.
- Piérard GE, Piérard S, Delvenne P, Piérard-Franchimont C. In vivo evaluation of the skin tensile strength by the suction method: Pilot study coping with hysteresis and creep extension. ISRN Dermatol. 2013; 2013:841217.
- Menzinger S, Saurat JH, Kaya G. morphological analysis of dermatoporosis by in vivo reflectance confocal microscopy and ultrasonography. Dermatopathology. 2020; 6:279-87.
- Kaya G, Rodriguez I, Jorcano JL, Vassalli P, Stamenkovic I. Selective suppression of CD44 in keratinocytes of mice bearing an antisense CD44 transgene driven by a tissue-specific promoter disrupts hyaluronate metabolism in the skin and impairs keratinocyte proliferation. Genes Dev. 1997; 11:996-1007.
- Laurent TC, Fraser JR. Hyaluronan. FASEB J. 1992; 6:2397-404.
- Kaya G, Tran C, Sorg O, Hotz R, Grand D, Carraux P, et al. Hyaluronate fragments reverse skin atrophy by a CD44-dependent mechanism. PLoS Med. 2006; 3:e493.
- Calikoglu E, Sorg O, Tran C, Grand D, Carraux P, Saurat JH, et al. UVA and UVB decrease the expression of CD44 and hyaluronate in mouse epidermis which is counteracted by topical retinoids. Photochem Photobiol. 2006; 82:1342-7.
- Barnes L, Tran C, Sorg O, Hotz R, Grand D, Carraux P, et al. Synergistic effect of hyaluronate fragments in retinaldehyde-induced skin hyperplasia which is a CD44-dependent phenomenon. PLoS One. 2010; 5:e14372.
- Barnes L, Carraux P, Saurat JH, Kaya G. Increased expression of CD44 and hyaluronate synthase 3 is associated with accumulation of hyaluronate in spongiotic epidermis. J Invest Dermatol. 2012; 132:736-8.
- Barnes L, Ino F, Jaunin F, Saurat JH, Kaya G. Inhibition of putative hyalurosome platform in keratinocytes as a mechanism for corticosteroid-induced epidermal atrophy. J Invest Dermatol. 2013; 133:1017-26.
- Mattila PK, Lappalainen P. Filopodia: Molecular architecture and cellular functions. Nat Rev Mol Cell Biol. 2008; 9:446-54.
- Nikolic DS, Ziori C, Kostaki M, Fontao L, Saurat JH, Kaya G. Hyalurosome gene regulation and dose-dependent restoration of skin atrophy by retinaldehyde and defined-size hyaluronate fragments in dermatoporosis. Dermatology. 2014; 229:110-5.
- Barnes L, Puenchera J, Saurat JH, Kaya G. Lrig1 and CD44v3 expression in the human folliculosebaceous unit. Dermatology. 2015; 231:116-8.
- Barnes L, Saurat JH, Kaya G. Senescent atrophic epidermis retains Lrig1+ stem cells and loses Wnt signaling, a phenotype shared with CD44KO mice. PLoS One. 2017; 12:e0169452.
- Darbellay B, Barnes L, Boehncke WH, Saurat JH, Kaya G. Reversal of murine epidermal atrophy by topical modulation of calcium signaling. J Invest Dermatol. 2014; 134:1599-608.
- Kaya G, Kaya A, Saurat JH. Induction of hyalurosome by topical hyaluronate fragments results in superficial filling of the skin complementary to hyaluronate filler injections. Dermatopathology. 2019;6:45-9.
- Kaya G, Grand D, Hotz R, Augsburger E, Carraux P, Didierjean L, et al. Upregulation of CD44 and hyaluronate synthase by topical retinoids in mouse skin. J Invest Dermatol. 2005; 124:284-7.
- Cordero A, Leon-Dorantes G, Pons-Guiraud A, Di Pietro A, Asensi SV, Walkiewicz-Cyraska B, et al. Retinaldehyde/hyaluronic acid fragments: A synergistic association for the management of skin aging. J Cosmet Dermatol. 2011; 10:110-7.
- McKnight B, Seidel R, Moy R. Topical human epidermal growth factor in the treatment of senile purpura and the prevention of dermatoporosis. J Drugs Dermatol. 2015; 14:1147-50.
- Humbert P, Fanian F, Lihoreau T, Jeudy A, Pierard GE. Bateman purpura (dermatoporosis): A localized scurvy treated by topical vitamin C - double-blind randomized placebo-controlled clinical trial. J Eur Acad Dermatol Venereol. 2018; 32:323-8.
- Kluger N. Dermatoporosis and vitamin C deficiency. J Eur Acad Dermatol Venereol. 2018;32:e383.
- Siperstein R, Wikramanayake TC. Intense pulsed light as a treatment for senile purpura: A pilot study. Lasers Surg Med. 2021; 53:926-34.
- Van Scott EJ, Ditre CM, Yu RJ. Alpha-hydroxy acids in the treatment of signs of photoaging. Clin Dermatol. 1996; 14(2):217-26.
- Nouveau S, Bastien P, Baldo F, de Lacharriere O. Effects of topical DHEA on aging skin: A pilot study. Maturitas. 2008; 59(2):174-81.
