Volume 23 Number 3
Scoping review on the treatment of radiodermatitis secondary to radiotherapy treatment of head and neck, and breast cancer
Maryse Beaumier, Sebastian Probst, Dr Mathieu Chamberland, Antony Bertrand-Grenier, Dr Anne Dagnault, Jérémy Laroche, Marie-Ève Daigle, Aude Jalbert-Drouin, Annabelle Prud’homme, Élyse Ménard, Hind Sadiqi, Dania Sakr
Keywords Wound care, wound management, radiodermatitis, radiotherapy, tissue damage
DOI DOI: 10.35279/jowm2022.23.03.07
Abstract
Purpose Almost 95% of patients undergoing radiotherapy treatments will develop a form of radiodermatitis. Despite this prevalence, treatment recommendations lack consensus, and clinical practices differ. The purpose of this scoping review is to examine the literature for radiodermatitis treatment options occurring in persons with head and neck, as well as breast, cancer and to report the pain felt by these populations after receiving radiotherapy.
Methods A scoping review based on the Preferred Reporting Items for Systematic reviews and Meta-analyses extension for Scoping Reviews (PRISMA_Sc-R) checklist was performed. To identify the sources of evidence, the MEDLINE, CINAHL, Cochrane, LiSSA and Google Scholar databases were searched. All available articles published in the French and English languages were included.
Results Two hundred fifty-five studies met the inclusion criteria. The included studies demonstrated heterogeneous results, owing to significant variations in the interventions, the controls and the assessment tools. The quality of the evidence was found to be low and at high risk for biases.
Conclusion This scoping review provides a broad overview of the available data and highlights the paucity of high-quality evidence to guide therapeutic interventions for the optimal management of radiodermatitis. Since radiodermatitis is a common injury of radiotherapy for breast cancer and head and neck cancer, more research is needed to guide the prevention and treatment of radiodermatitis for patients suffering from this complication.
INTRODUCTION
Cancer is among the leading causes of non-accidental deaths worldwide, with more than 19 million people diagnosed each year; nearly 50% of them receive radiation therapy (1). Curative approaches include surgery, radiotherapy and chemotherapy, with an increasing reliance on multimodal therapy to achieve better survival outcomes. Therapy intensification is associated with an increased risk for treatment toxicity.
Head/neck and breast cancers are especially prone to developing radiodermatitis (2). This is due to the intrinsic radiosensitivity of these skin areas, added to increased creasing, risk for maceration, contact with clothing and overall range of motion, in addition to the high doses delivered for curative-intent treatments. Radiotherapy is an integral part of head/neck cancer and breast cancer management (3). Typically, curative patients receive one treatment per day, five days a week for four to eight weeks. Despite advances in technology, cutaneous adverse events in the form of acute radiation-induced skin reactions, or acute radiodermatitis, still arise and reflect the skin toxicity of radiation therapy (Figure 1).
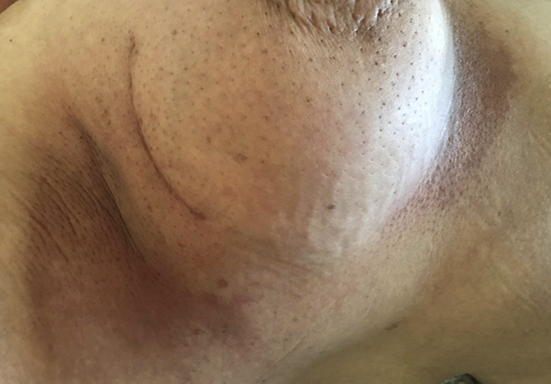
Figure 1: Radiodermatitis of right breast and axilla (shared with patient’s informed consent)
Radiation therapy uses radiation to destroy cancer cells, subjecting healthy tissue to radiobiological risks (4). Evidence demonstrates that almost 95% of patients undergoing radiotherapy treatments will develop some form of radiodermatitis (4, 5). This can manifest in clinical practice mostly as a very mild radiodermatitis that is often treated with the application of a moisturiser to prevent the drying of the skin. Skin irradiation often leads to a complex pattern of tissue injury involving epidermal and endothelial cells within blood vessels’ walls, as well as an inflammatory cell recruitment. Radiodermatitis, also called radiation dermatitis, radiation-induced skin reactions or radiation injury, is a side effect of ionising radiation delivered to the skin, most commonly caused by radiotherapy treatment (4). Conventional two-dimensional radiotherapy (2D-RT) was widely used in recent decades, with satisfactory disease control but a high probability of toxicity. Three-dimensional conformal radiation therapy (3D-CRT) is based on computed tomography or magnetic resonance imaging, which allows for a better delineation of the tumour target and reduced toxicity to normal tissues (6). Compared to conformational radiotherapy, intensity modulated radiotherapy and volumetric modulated arc therapy improve target volume conformity and normal tissue sparing, resulting in reduced acute and late toxicities (7). The radiation beam, technique, fractionation regimen, concomitant chemotherapy or targeted therapy, irradiated site, tumour factors and patient-specific factors each have an impact on the risk and potential severity of radiodermatitis (8).
Radiodermatitis may affect patients’ quality of life, such as by inducing additional stress or discomfort, restraining certain movements depending on their location or increasing the financial burden associated with treatment. In more severe cases, radiodermatitis might also impact the radiation therapy itself, especially if a modulation of the total dose administered is required, or if the treatment must be temporarily or permanently ceased (9). Pain can also be associated with radiodermatitis. To decrease pain, the area can be covered by wound dressings (10–18); however, their effectiveness remains uncertain (19, 20) and their removal is mandatory before each radiation session. Otherwise, the healthy tissues would receive a higher dose, especially to the skin, due to the phenomenon of dose build-up, also known as the ‘bolus effect’. If radiodermatitis is exposed to the air, patients suffer more, which means their anxiety level is typically higher than if the area were covered with wound dressings. Because of the lack of evidence on the efficacy of products and dressings, there are no therapeutic consensus recommendations for the treatment of radiodermatitis (20–23). The literature notes that interventions are still based on anecdotal data with poor levels of evidence (4, 24). For many types of wounds, advanced dressings are widely used, as they are believed, for example, to lower pain, promote healing and may be kept in place for up to seven days (25–27). Changing a dressing every day may cause skin irritation and result in additional skin rupture, greater pain, a need for closer patient monitoring and higher costs (19, 20).
The prevention and treatment of acute radiodermatitis are of the utmost importance for minimising adverse events, decreasing patient morbidity and increasing patient quality of life (28, 29). To our knowledge, and despite an increase in the number published studies, only a few clinical interventions have emerged as reliable way to prevent or treat radiodermatitis (19). With this in mind, we conducted a scoping review aimed at examining the literature for radiodermatitis treatment options occurring in persons with head/neck and breast cancer and to report on the pain felt by these populations after having radiotherapy.
Scoping review question and objectives:
What treatment is used on radiodermatitis for people with head and neck and/or breast cancer?
How was pain felt by people with a head and neck and/or breast cancer after a radiotherapy treatment?
The objectives were:
1) To examine the extent and nature of the evidence on the skin and wound care treatment of radiodermatitis
2) To report pain felt by people with a head and neck and/or breast cancer after a radiotherapy treatment
3) To identify gaps to improve research and clinical practice for patients’ quality of life during their radiotherapy treatment
METHODS
This scoping review is based on the Preferred Reporting Items for Systematic reviews and Meta-analyses extension for Scoping Reviews (PRISMA-ScR) Checklist (30). No review protocol was registered or published prior to this scoping review.
Eligibility criteria
We included all types of studies, regardless of sample size or year, reporting the prevalence of either female or male head/neck and/or breast cancer patients over the age of 18 and receiving a radiotherapy treatment. We imposed no geographical limitations. No distinctions were made regarding what kind of radiotherapy was received. Only sources of evidence published in English and French were included.
Information sources and search strategy
To identify relevant sources of evidence, the Population-Concept-Context (PCC) method (P=persons with head/neck and/or breast cancer receiving a radiotherapy treatment in a hospital context) recommended by the Joanna Briggs Institute for Scoping Reviews (31) was used. First, MB performed an exploratory search in the databases MEDLINE and CINAHL to identify keywords in the articles’ titles, abstracts and MeSH terms or subheadings. Second, we combined all identified keywords to deliver a complete search strategy for the MEDLINE, CINAHL, Cochrane, LiSSA and Google Scholar databases. We used an exhaustive list of keywords/concepts to define radiodermatitis, radiotherapy, treatment, pain, head and neck cancer and breast cancer. These lists were validated by two researchers (MC, SP) prior to the research and can be found in Table 1. The searches were conducted in May and June 2021.
Selection of sources of evidence
All articles were classified with the bibliographic software Endnote version X9 following the PRISMA-ScR strategy. MB removed duplicates. A second revision was necessary after the retraction of the duplicates, since too many titles and summaries were inappropriate documents. The list can be accessed online as a supplementary file. MB and ABG blindly reviewed all titles and abstracts. We then discussed divergences. A third reviewer was not needed.
Collating, summarising, and reporting the results
Relevant information was extracted from the selected articles using a standardised abstraction form documenting 41 items. To help generate summative statements or recommendations, we grouped key findings into a thematic description. The data extraction was executed by three research teams containing eight medical students each. Descriptive statistics were generated for selected results using Microsoft Excel and expressed as sums, range and arithmetical mean, when relevant.
RESULTS
The initial literature search yielded 2273 sources of evidence and 350 after withdrawal of duplicates, articles in a language other than English or French and unrelated articles to research question. An important characteristic of this literature review was the withdrawal of 49 titles and abstracts for various reasons (see Figure 2), and the incomprehension of finding them in the databases. In the end, the second expert has removed another 51 titles for a total of 249 articles adjusted to 255 with the addition of 6 articles in the June 2021 update. Then, a total of 255 articles were analysed. The PRISMA flowchart (Figure 2) illustrates the process and reasons for exclusion.
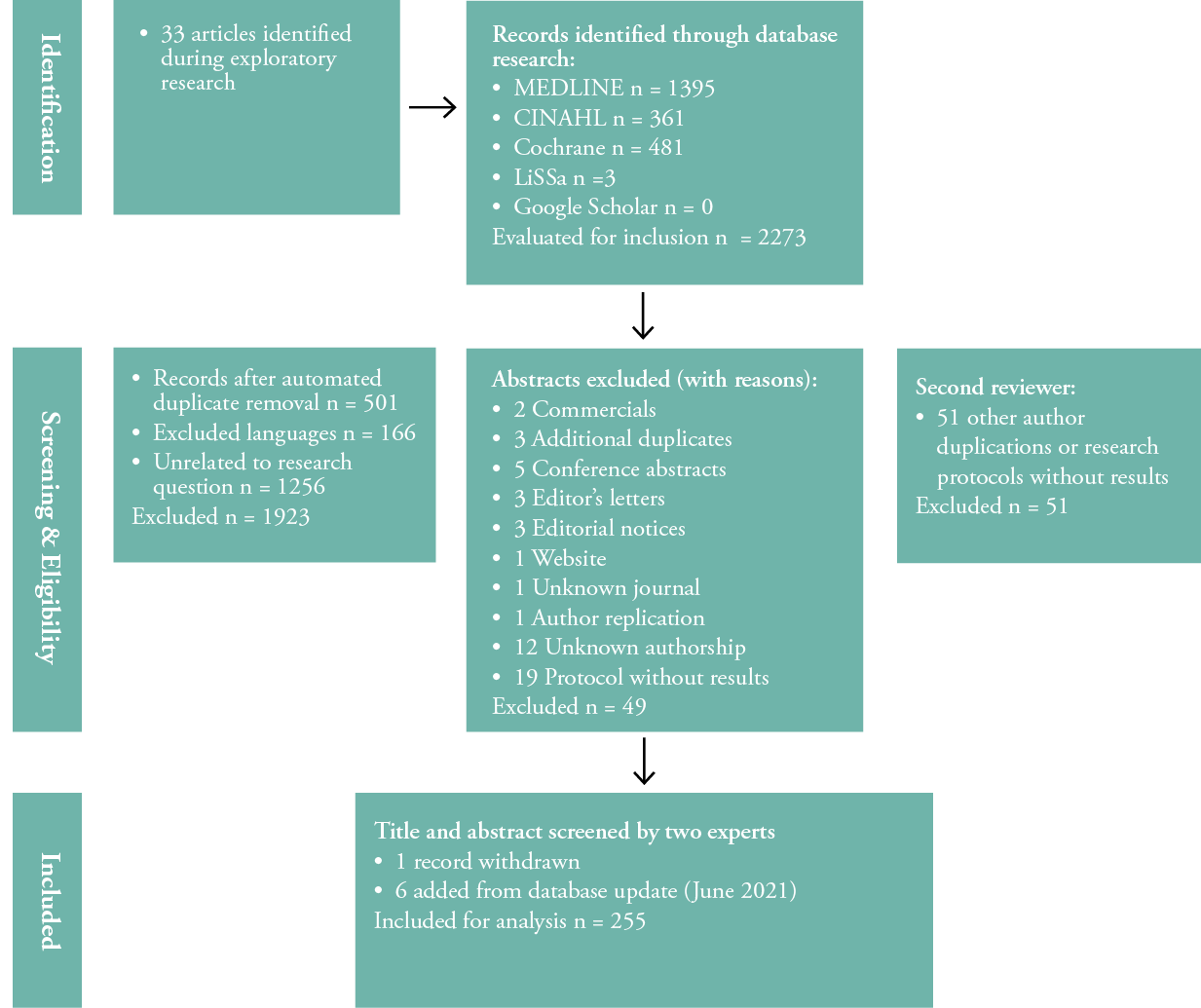
Figure 2: PRISMA-ScR flow diagram
Data extraction
We extracted data into a table including the author name, country, year of publication, study design, patient population with inclusion and exclusion criteria and oncologic treatment modalities. For randomised controlled trials, the interventions and the controls were extracted. Treatment intention was divided into prophylactic or therapeutic intent. Additionally, we extracted investigators’ attitudes towards potential dose build-up effects caused by the intervention. The results of primary studies were listed regarding radiodermatitis and pain, and adverse events were noted. Potential biases within studies were consigned, and internal validity, as well as the methodology itself, was appraised. MC, HS, DS, JL, EM, MED, AP and AJD completed the data extraction. MB and SP reviewed the entire process.
Characteristics of sources of evidence
For this scoping review different kind of evidence have been analysed : 15 systematic reviews (7,89%), 101 randomised controlled trials (53,16%), 11 nonrandomised clinical trials (5,79%), 6 prospective observational studies (3,16%), 6 retrospective studies (3,16%), 12 longitudinal studies (6.32%), 2 cross-sectional studies (1,05%), 13 case reports or case series (6.84%) and 24 clinical guidelines or expert consensus (12%).
Publications ranged from 1979 to 2020. Only 15 sources of evidence were recorded for the years 1979 through 1999. The number of publications per year increased steadily beginning at the turning of the 21st century, though there was a decrease from 2015 to 2020. The geographical origin of the included articles was centred on industrialised countries. There was only a small number of international collaborations among the expert consensus papers, and only a few articles (n = 19) originated in South-East Asia. More than half of these investigated radiodermatitis among head and neck patients.
Study population
All studies included patients with either head and neck and/or breast cancer, or a radiodermatitis treatment not pertaining to any specific tumoral site. Less than half (n=45, 24%) focused on head and neck cancer, while 58% (n = 109) of the articles focused on breast cancer patients. Six percent (n = 12) investigated radiodermatitis in both head and neck and breast cancer patients, and 16% (n = 30) examined radiodermatitis treatment as a whole.
Critical appraisal within sources of evidence
The overall methodological quality of the included sources of evidence was found to be low. Most articles contained a bias jeopardising their results’ reliability. More than 70% (n = 135) contained at least one identifiable bias. Only 13% (n = 25) were found to be of high methodological quality. Of these, 3% (n = 5) were classified as free of biases, and the remaining 11% (n = 20) had minor biases, such as minimising and controlling the potential impact on data. A total of 81 sources of evidence (43%) did not disclose any conflict of interest, and 83 (44%) did not disclose funding. Only 15 (8%) did disclose any conflict of interest among their authors.
The generalisability of the study results to the North American oncology population was found to be good in fewer than 25% (n = 47) of the sources of evidence. There was a wide geographical diversity of study populations, with intrinsic variations in skin phototype, lifestyle habits, cultural tolerance to pain and discomfort, socioeconomic resources, genetic susceptibility and tumour type and site prevalence (e.g., nasopharyngeal carcinoma in South East Asia, versus non- nasopharyngeal carcinoma cancers elsewhere in the world). Head and neck cancer studies assessed both genders, with a strong male predominance, while breast cancer studies exclusively assessed female patients.
Outcomes of individual sources of evidence
The reported outcomes of the included sources varied according to the intervention used to prevent and manage radiation dermatitis. Pain was measured in only 26% (n = 50) of the included studies. It should be highlighted that pain is often included in quality of life outcomes. Characteristics and outcomes included in a selection of the included studies most representative of the higher quality data are available in Table 4.
Synthesis of results
The included studies showed heterogeneous results, as the interventions, controls, assessment tools and outcomes were assessed differently. Figure 3 shows a visual representation of the treatments used. A total of 34 (17%) studies described the use of topical agents, such as different creams or ointments; this was followed by oral applications (n = 4), such as curcumin; wound dressings (n = 3); and cognitive behavioural therapy with hypnosis (n = 2). All treatments were applied either before, during or after a radiation session. Figures 3, 5 and 6 provide a summary of the topical agents and dressings used for the treatment of radiodermatitis and the effects of pain relief.
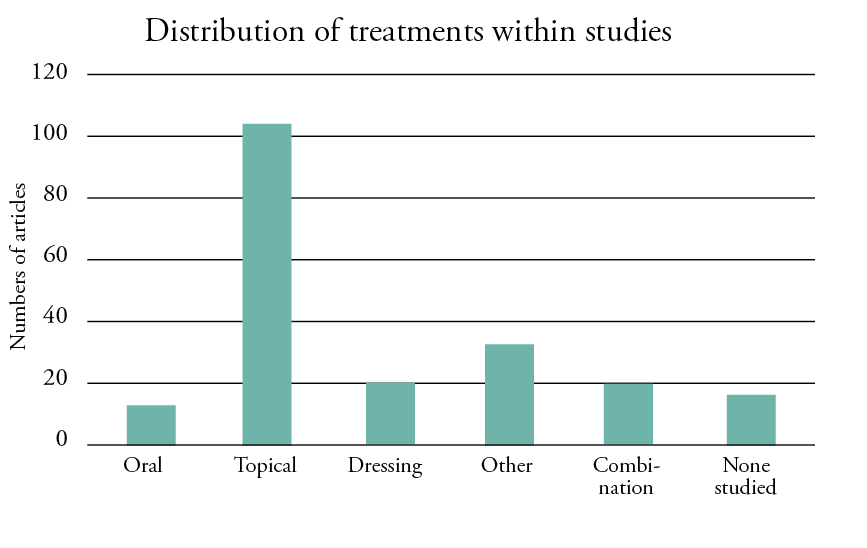
Figure 3: Distribution of treatments within the included studies
Twenty-four sources of evidence (12%) measured pain using a visual analog scale (VAS) for breast cancer patients, 26 (13%) applied a different measurement tool or a self-administrated questionnaire, three (2%) did not specify and 56 (29%) did not assess pain. In the head and neck cancer population, seven sources of evidence (4%) used a VAS scale; 11 (6%) applied a different measurement tool, such as a numerical grading scale; or collected qualitative data. One source of evidence (0.5%) did not specify the tool used, and 26 (13%) did not measure pain at all. The pain felt by patients secondary to radiotherapy treatment for head and neck and breast cancer was weak (18). Pain was mostly represented by the concept of quality of life.
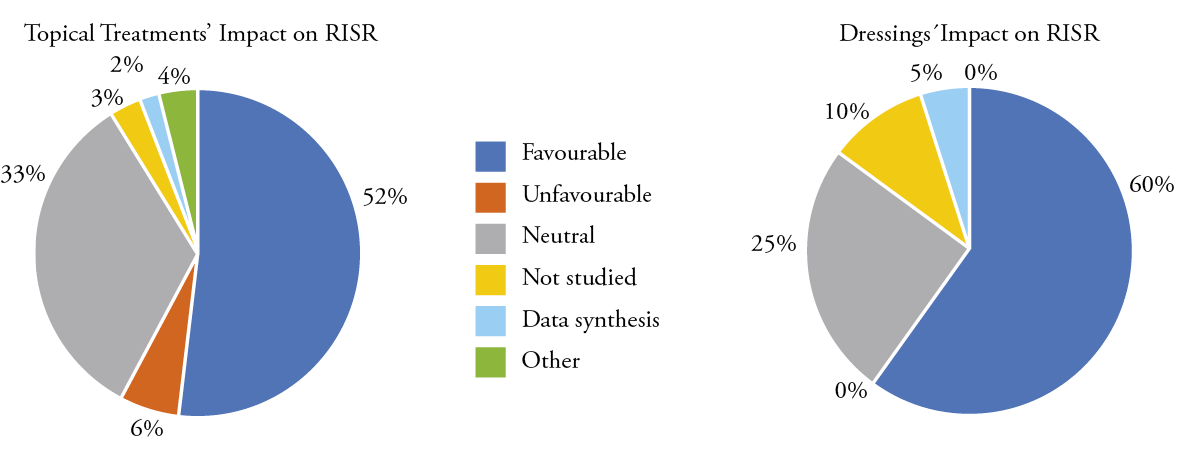
Figure 5: Topical agents and dressings’ impact on radiodermatitis
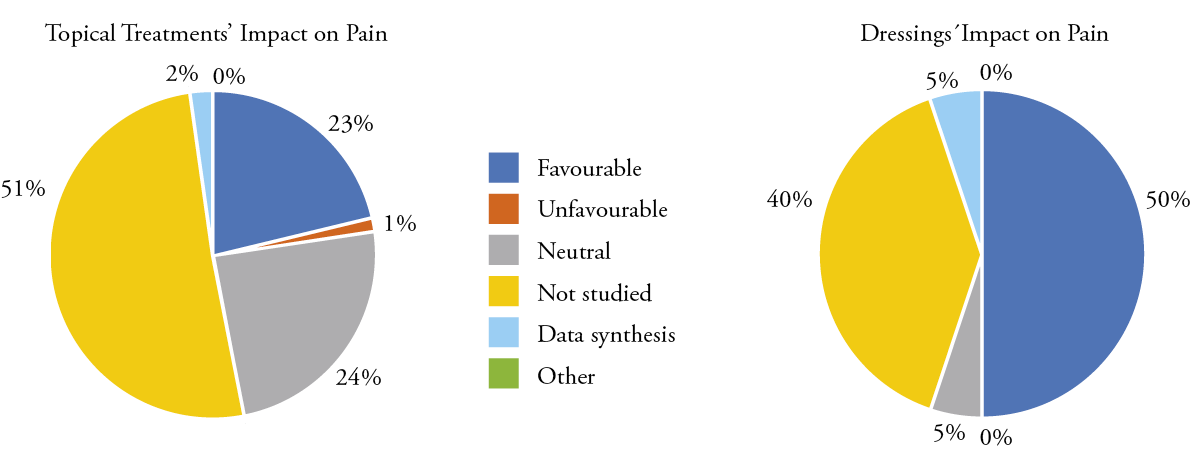
Figure 6: Topical agents and dressings’ impact on pain
Treatment modalities
Different treatment modalities were reported. Most (52%, n = 102) of the studies described either the application of creams, lotions or ointments. The use of different wound dressings was used in 10% (n = 20), and 6% (n = 11) of the studies reported the administration of oral treatments. Different treatments, such as hyperbaric oxygen, photo-biomodulation or hypno-sedation, were reported in 16% (n = 32) of the studies, and 10% (n = 19) used a combination of treatments. Seven percent (n = 14) were systematic or non-systematic reviews and did not address a specific treatment.
Effect of topical interventions
Topical treatments represented 52% (n = 102) of the therapies for radiodermatitis and radiation-induced pain. Out of these, 52% (n = 53) reported favourable outcomes on radiodermatitis, and 33% (n = 34) showed no effect. Of the 102 studies reporting topical treatments, 49% (n = 50) reported radiation-induced pain. The application of a topical treatment was demonstrated in 22% (n = 22) of the studies on pain relief, and 24% (n = 24) reported topical treatments as having no effect.
Effect of dressings
Advanced wound dressings, such as foam dressings, were used in 10% (n = 20) of the included studies; out of these, 60% (n = 12) showed a positive effect on radiodermatitis, while 25% (n = 5) were neutral or even had a negative impact on radiodermatitis. With the application of a dressing in 50% (n = 10) of the studies, pain was relived, whereas in 5% (n = 1) no effect was reported and 25% (n = 5) did not assess pain.
Effect of oral treatments
Of all the included studies, only 6% (n = 11) assessed oral treatments for radiodermatitis or pain, and among these, only 36% (n = 4) reported favourable results for radiodermatitis and/or pain.
DISCUSSION
The purpose of this scoping review was to provide an overview of treatment options for head and neck as well as breast cancer patients with radiodermatitis, to report pain felt by patients after a radiotherapy treatment and to guide clinical practice to enhance patient outcomes.
The designs of the included studies varied from descriptive to experimental, including randomised controlled trials and guidelines. There was a variation in sample size, study duration, treatment modalities and their effects. Most of the literature came from small, single-institution studies with limited power to detect differences between treatment and control arms. Our results demonstrate that there is a heterogeneity of reported outcomes using mostly low levels of evidence. There is a gap between theory and clinical practice on the treatment of radiodermatitis; however, the included study protocols did use experimental designs, therefore they contribute to the body of knowledge suitable for guiding clinical practice.
This scoping review demonstrates a variety of treatment options for breast and head and neck cancer patients with radiodermatitis, even though different dosages were applied. Radiodermatitis was mostly treated using creams, lotions or ointments. The treatments were most often compared using no group control, thus comparisons across trials were difficult. An additional difficulty was the use of different dermatitis assessment tools and the inconsistent selection of study endpoints. Pain was assessed in only 24% of the included studies, and done so using different assessment tools. The VAS was the most commonly used tool. Clinical practice demonstrates that pain is a common side effect when undergoing radiotherapy (32), but it is mostly not included as an outcome in head and neck and breast radiotherapy studies. This can lead to a lack of consensus on what constitutes standard of care for radiodermatitis.
Strengths and limitations
This scoping review included a wide range of sources of evidence based on different designs and provided a wide overview of treatment options for head and neck as well as breast cancer patients with radiodermatitis. Additionally, we reported how pain was assessed. We included various sources and different levels of evidence. Studies on radiodermatitis treatment are mostly studies with higher evidence levels from single-institution studies with limited power. As we did not assess the studies’ methodological quality, no recommendations can be made.
CONCLUSION AND IMPLICATIONS FOR CLINICAL PRACTICE
This scoping review presents an overview of treatment options for head and neck and breast cancer patients with radiodermatitis, as well the pain felt by patients after radiotherapy treatment. The available literature has not clearly defined optimal treatment possibilities. We, therefore, suggest increasing the investigation of valid treatment options to guide clinical practice.
Acknowledgments
Chanie Lafrance-Veillette, B.Sc. in Nursing, MD. Faculty of Medicine, University of Montreal. Province of Quebec, Canada. Chanie Lafrance-Veillette created Table 1 with the exhaustive list of keywords and completed all of the literature searches of the databases.
Key messages
- Clinicians must, at minimum, follow the best guidelines on wound care treatment, so as not to cause any harm or negative interactions with the radiotherapy treatment.
- The literature is heterogeneous and has not clearly defined optimal treatment options for radiodermatitis. Sadly, no consensus on the treatment of radiodermatitis.
- There is a gap between theory and clinical practice on the treatment of radiodermatitis and the importance of developing research.
Author(s)
Maryse Beaumier, RN, PhD, Professor, Health Care Science Department, Université du Québec at Rimouski, Province of Quebec, Canada
Sebastian Probst, RN, DClinPrac, MNS, BNS, Professor of Tissue Viability and Wound Care, Geneva School of Health Sciences, HES-SO, University of Applied Sciences and Arts Western Switzerland, Geneva School of Health Sciences, Geneva, Switzerland. Care Directorate, University Hospital Geneva, Geneva Switzerland
Faculty of Medicine Nursing and Health Sciences, Monash University, Melbourne, Australia
Dr Mathieu Chamberland, MD, Faculty of Medicine, Université Laval, Province of Quebec, Canada
Antony Bertrand-Grenier, Medical Physicist, PhD, Centre intégré universitaire de santé et des services sociaux de la Mauricie Centre-du-Québec
(CIUSSS-MCQ), Province of Quebec, Canada
Dr Anne Dagnault, MD, PhD, FRCPC, Radiation Oncologist, Faculty of Medicine, Université Laval, Province of Quebec, Canada
Jérémy Laroche, Marie-Ève Daigle, Aude Jalbert-Drouin, Annabelle Prud’homme, Élyse Ménard, all Medical students, Faculty of Medicine,
Université Laval, Province of Quebec, Canada
Hind Sadiqi, B.Sc. Nutrition, Medical student, Dania Sakr, B.Sc., M.Sc. Pharmacy, Faculty of Medicine, Université Laval, Province of Quebec, Canada
Correspondence: Maryse Beaumier, Maryse_Beaumier@uqar.ca
Conflict of interest: None
References
- Sung, H., Ferlay, J., Siegel, R. L., Laversanne, M., Soerjomataram, I., Jemal, A., & Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Cancer J Clin 2021; 71(3):209-9.
- Feng, Y., Spezia, M., Huang, S., Yuan, C., Zeng, Z., Zhang, L., . . . Luo, W. Breast cancer development and progression: Risk factors, cancer stem cells, signaling pathways, genomics, and molecular pathogenesis. Genes Dis 2018; 5(2):77-106.
- Haussmann, J., Corradini, S., Nestle-Kraemling, C., Bölke, E., Njanang, F. J. D., Tamaskovics, B., . . . Mohrmann, S. Recent advances in radiotherapy of breast cancer. Radiat Oncol 2020; 15(1):1-10.
- Singh, M., Alavi, A., Wong, R., & Akita, S. Radiodermatitis: A review of our current understanding. Am J Clin Dermatol 2016; 17(3):277-92.
- Garnier, M., Champeaux, E., Laurent, E., Boehm, A., Briard, O., Wachter, T., . . . Machet, L. High-frequency ultrasound quantification of acute radiation dermatitis: Pilot study of patients undergoing radiotherapy for breast cancer. Skin Res Technol 2017; 23(4):602-6.
- Zhang, B., Mo, Z., Du, W., Wang, Y., Liu, L., & Wei, Y. Intensity-modulated radiation therapy versus 2D-RT or 3D-CRT for the treatment of nasopharyngeal carcinoma: A systematic review and meta-analysis. Oral Oncol 2015; 51(11):1041-6.
- Chiavassa, S., Bessieres, I., Edouard, M., Mathot, M., & Moignier, A. Complexity metrics for IMRT and VMAT plans: A review of current literature and applications. Br J Radiol 2019; 92(1102):20190270.
- Böhner, A. M., Koch, D., Schmeel, F. C., Röhner, F., Schoroth, F., Sarria, G. R., . . . Schmeel, L. C. Objective evaluation of risk factors for radiation dermatitis in whole-breast irradiation using the spectrophotometric L* a* b color-space. Cancers 2020; 12(9):2444.
- McQuestion M. Evidence-based skin care management in radiation therapy: Clinical update. Semin Oncol Nurs 2011; 27(2): e1-e17.
- Bolderston, A., Cashell, A., McQuestion, M., Cardoso, M., Summers, C., & Harris, R. A Canadian survey of the management of radiation-induced skin reactions. J Med Imaging Radiat Sci 2018; 49(2):164-72.
- Campbell IR, Illingworth MH. Can patients wash during radiotherapy to the breast or chest wall? A randomized controlled trial. Clin Oncol 1992; 4(2):78-82.
- Elliott, E. A., Wright, J. R., Swann, R. S., Nguyen-Tân, F., Takita, C., Bucci, M. K., . . . Ryu, J. Phase III trial of an emulsion containing trolamine for the prevention of radiation dermatitis in patients with advanced squamous cell carcinoma of the head and neck: Results of Radiation Therapy Oncology Group Trial 99-13. J Clin Oncol 2006; 24(13):2092-7.
- Jensen, J.-M., Gau, T., Schultze, J., Lemmnitz, G., Fölster-Holst, R., May, T., . . . Proksch, E. Treatment of acute radiodermatitis with an oil-in-water emulsion following radiation therapy for breast cancer: A controlled, randomized trial. Strahlenther Onkol 2011; 187(6):378-84.
- Maiche A, Isokangas OP, Gröhn P. Skin protection by sucralfate cream during electron beam therapy. Acta Oncol 1994; 33(2):201-3.
- Olsen, D. L., Raub, W., Jr., Bradley, C., Johnson, M., Macias, J. L., Love, V., & Markoe, A. The effect of aloe vera gel/mild soap versus mild soap alone in preventing skin reactions in patients undergoing radiation therapy. Oncol Nurs Forum 2001; 28(3):543-7.
- Roy I, Fortin A, Larochelle M. The impact of skin washing with water and soap during breast irradiation: A randomized study. Radiother Oncol 2001; 58(3):333-9.
- Schmuth, M., Wimmer, M. A., Hofer, S., Sztankay, A., Weinlich, G., Linder, D. M., . . . Fritsch, E. Topical corticosteroid therapy for acute radiation dermatitis: A prospective, randomized, double-blind study. Br J Dermatol 2002; 146(6):983-91.
- Zhong, W.-H., Tang, Q.-F., Hu, L.-Y., & Feng, H.-X. Mepilex lite dressings for managing acute radiation dermatitis in nasopharyngeal carcinoma patients: A systematic controlled clinical trial. Med Oncol 2013; 30(4):761-761.
- Chan, R. J., Webster, J., Chung, B., Marquart, L., Ahmed, M., & Garantziotis, S. Prevention and treatment of acute radiation-induced skin reactions: A systematic review and meta-analysis of randomized controlled trials. BMC Cancer 2014; 14:53-53.
- Zhang Y, Zhang S, Shao X. Topical agent therapy for prevention and treatment of radiodermatitis: A meta-analysis. Support Care Cancer 2013; 21(4):1025-31.
- Chargari C, Fromantin I, Kirova Y. Intérêt des applications cutanées en cours de radiothérapie pour la prévention et le traitement des épithéliites radio-induites. Cancer/Radiothérapie 2009; 13(4):259-66.
- Ferreira EB Reis PE. Assessing the effectiveness of urea cream as a prophylactic agent for radiation dermatitis. Cancer Nursing, Conference: international conference on cancer nursing, ICCN. 2017. United states 40(6 Supplement 1), E14.
- Iacovelli, N. A., Galaverni, M., Cavallo, A., Naimo, S., Facchinetti, N., Iotti, C., . . . Orlandi, E. Prevention and treatment of radiation-induced acute dermatitis in head and neck cancer patients: A systematic review. Future Oncol 2017; . 14(3), 291-305.
- Trueman, E. The development of national guidelines for the management of radiotherapy-induced skin reactions. Int J Palliat Nurs 2014; 20(5):214-214.
- Orsted, H., Keast, D., Forest-Lalande, L., Kuhnke, J., L., O’Sullivan-Drombolis, D., Jin, S., . . . Evans, R. Foundations of Best Practice for Skin and Wound Management: Best Practice Recommendations for the Prevention and Management of Wounds. Wounds Canada, 1-74.
- Sibbald, R. G., Goodman, L., Woo, K., Krasner, D. L., Smart, H., Tariq, G., . . . Salcido, R. S. Special considerations in wound bed preparation 2011: An update. Adv Skin Wound Care 2011; 24(9):415-36.
- Winter GD. Formation of scab and the rate of epithelialisation of superficial wounds in the skin of the young domestic pig. Nature 1962; 193(293-294).
- Seité S, Bensadoun R-J, Mazer J-M. Prevention and treatment of acute and chronic radiodermatitis. Breast Cancer 2017; 9:551-7.
- de Andrade Fuzissaki, M., Paiva, C. E., de Oliveira, M. A., Canto, P. P. L., & de Paiva Maia, Y. C. The impact of radiodermatitis on breast cancer patients’ quality of life during radiotherapy: A prospective cohort study. J Pain Symptom Manage 2019; 58(1):92-9.e1.
- Tricco, A. C., Lillie, E., Zarin, W., O’Brien, K. K., Colquhoun, H., Levac, D., . . . Straus, S. E. PRISMA extension for scoping reviews (PRISMA-ScR): Checklist and explanation. Ann Intern Med 2018; 169(7):467-73.
- Peters, M. D., Marnie, C., Tricco, A. C., Pollock, D., Munn, Z., Alexander, L., . . . Khalil, H. (2020). Updated methodological guidance for the conduct of scoping reviews. JBI evidence synthesis, 18(10), 2119-2126.
- Holländer-Mieritz, C., Johansen, J., Johansen, C., Vogelius, I. R., Kristensen, C. A., & Pappot, H. Comparing the patients’ subjective experiences of acute side effects during radiotherapy for head and neck cancer with four different patient-reported outcomes questionnaires. Acta Oncol 2019; 58(5):603-9.
