Volume 23 Number 3
Clinical significance of a novel imaging device to evaluate infection on wounds: Performance comparison with culture method and metagenome sequencing
Rajesh Kesavan, Changam Sheela Sasikumar
Keywords diabetic foot ulcers, Autofluorescence, machine learning, pathogens, metagenome sequencing, point-of-care device
DOI 10.35279/jowm2022.23.03.06
Abstract
Background Diabetic foot and lower limb complications affect 40–60 million people globally. A rapid method is needed to understand the bioburden and type of infecting bacteria on diabetic foot ulcers.
Aim To compare the accuracy and efficacy of a novel multispectral imaging device, against the standard culture method and correlate bacterial bioburden levels with metagenome sequencing.
Method A clinical study was conducted on diabetic foot ulcer patients. Wounds, post-debridement, were imaged using the multispectral imaging device and the report, containing spatially mapped regions of bacterial burden along with their bacterial gram type, was compared with the culture sensitivity report. Additionally, metagenome sequencing was done on a subset of the patient samples.
Results A total of 157 patients were imaged, and 177 deep tissue biopsies were taken from colour-coded regions (Gram Positive/Gram Negative infected) identified by the machine learning algorithm. The class-averaged accuracy of the device was found to be 90.4% for gram-positive, -negative, polymicrobial and for no bacterial burden. A total of 26 biopsies were also sent for 16S rRNA sequencing. Of these, cultures were positive in 17 and correlated with the species identified through 16S rRNA results in 16 cases. In five cases where there was no growth in culture, the multispectral imaging device could still detect the presence of bacteria as confirmed by 16S rRNA results (using 50 reads as a cut-off) and thus be used as an adjunct for monitoring wounds’ bacterial burden over time.
Conclusions The novel multispectral imaging device can be used to effectively understand the bioburden in a wound of clinical significance and the gram type of infecting bacteria.
Implications for clinical practice The autofluorescence imaging device can assist doctors in evaluating wounds’ bacterial burden level, spatial bioburden extent and the gram type of bacteria present, thus aiding in effective debridement and proper wound-management protocols.
INTRODUCTION
Diabetes, one of the most prevalent chronic diseases, is projected to affect more than 700 million people globally by 2050 according to the International Diabetes Federation (1). People in underdeveloped and low-income countries are more prone to develop diabetes, and about 12–25% of those are at risk of developing diabetic foot ulcers as well, which can further lead to chronic wounds, amputation and even death (2-6). Wound bacterial burdens are known to be initially composed predominantly of gram-positive bacteria (such as Methicillin-susceptible/resistant Staphylococcus aureus, Streptococcus), but as the infection progresses, they become colonised by gram-negative bacteria (such as Pseudomonas aeruginosa, Klebsiella species) (7-12).
Wounds frequently differ in their clinical characteristics, making the diagnosis of infections through signs and symptoms alone difficult (13). In hospital settings, the current standard method for detecting bacterial burden requires a swab or deep tissue biopsy for culturing microorganisms, followed by biochemical methods for species recognition and antibiotic susceptibility testing, which usually takes 3–5 days. In short, these methods fail to provide the immediate clinical information (such as the presence/absence of bacterial burden and gram type of bacteria) required for first-line treatment (14). In addition, the culture sensitivity methodology has other limitations, including a bias to certain species, depending on the choice of growth medium and incubating conditions; a requirement for special apparatus for anaerobic bacteria; and an inability to pick up the full diversity of infecting organisms (2). Accurate identification of polymicrobial species infecting the wound can only be achieved using genotypic methods such as16s rRNA sequencing, shotgun sequencing and so on (15-16). However, these methods are cumbersome, costly and have yet to be adopted in mainstream clinical practice (14).
The ability to offer targeted treatment during a first consultation offers a tremendous advantage for timely wound healing. With proper and timely management of a wound, clinicians can achieve resolution in >90% of mild to moderate soft tissue infections (7); therefore, there is a significant need for a modality that focuses on the early assessment and classification of pathogenic gram types infecting a wound.
Multispectral imaging, which involves shining multiple wavelengths of light and collecting the emission response, has proven to be a useful technique for identifying and classifying bacteria based on their autofluorescence (19). In addition, autofluorescence imaging is label-free, which is advantageous in terms of reduced complexity and cost. Studies have shown that bacteria have characteristic emission fluorescence when excited in the UV and blue regions of light, contributed by metabolic and infectious markers such as NAD(P)H, flavins, porphyrins and pyoverdine (in the case of Pseudomonas) and so on (19-20). Previous studies have also shown that gram-positive bacteria typically have more fluorescence intensity in the red region, due to an increase in the release of porphyrins, compared to gram-negative bacteria. Similarly, some gram-negative bacteria have increased NAD(P)H and flavins, when compared to gram-positive bacteria (21-22). In addition, Pseudomonas aeruginosa, which is one of the most predominant pathogens present in diabetic foot infections (21), has clearly distinguishable autofluorescence contributed by pyoverdine. These key differences in the relative concentration of biomarkers and autofluorescence in various spectral bands can potentially be used to design a rapid, non-invasive diagnostic technique for the presence/absence of bacterial burden and the classification of the gram type of the bacteria infecting the wounds. Fluorescence imaging has emerged as a promising point-of-care technique for monitoring bacterial burden in wounds (21,22). Fluorescence imaging, in combination with clinical signs and symptoms, has been used to effectively monitor wounds with moderate-to-heavy bacterial loads (23). Similarly, surgical sites with a high prevalence of bacterial burden have been assessed with point-of-care fluorescence imaging devices (24). These results show that when the standard clinical signs and symptoms are assessed in conjunction with the inputs from fluorescence imaging, bacterial burden diagnostic accuracy improves. A bacterial fluorescence imaging system was also used for effective wound debridement, which results in accelerated wound healing (25). Further studies have demonstrated that the use of fluorescence imaging to monitor wounds results in reduced antibiotic use (>30%), a reduction in antimicrobial wound dressings (>45%) and improved wound healing rates (>20%) over 12 weeks (26). Overall, the improved healing rates are projected to reduce annual wound care costs by 10% in National Healthcare System, United Kingdom. Recently, guidelines were evolved for the detection of bacterial burden in wounds using fluorescence imaging based on the Delphi consensus protocol (27).
The current point-of-care devices for fluorescence imaging require careful interpretation. In addition to autofluorescence from bacterial bioburden, human tissue has its own autofluorescence, contributed to, for example, by collagen or elastin, thus it is imperative to distinguish pathogenic autofluorescence from this background noise. Advanced machine learning techniques trained on data from skin and wound regions can overcome the above limitations by providing correction for these interfering factors.
Innovation
To the best of our knowledge, lluminate® is the first device to use multispectral autofluorescence imaging integrated with machine learning to provide spatial–temporal information on the presence/absence of bacterial burden and gram type of bacteria infecting a wound and to provide the ability to monitor a wound continuously (Figure 1). We conducted a clinical study on diabetic foot ulcer patients to understand the clinical significance of this device for bacterial burden identification and accuracy of the gram type classification of bacteria in wounds, to aid doctors in administering first-line treatment.
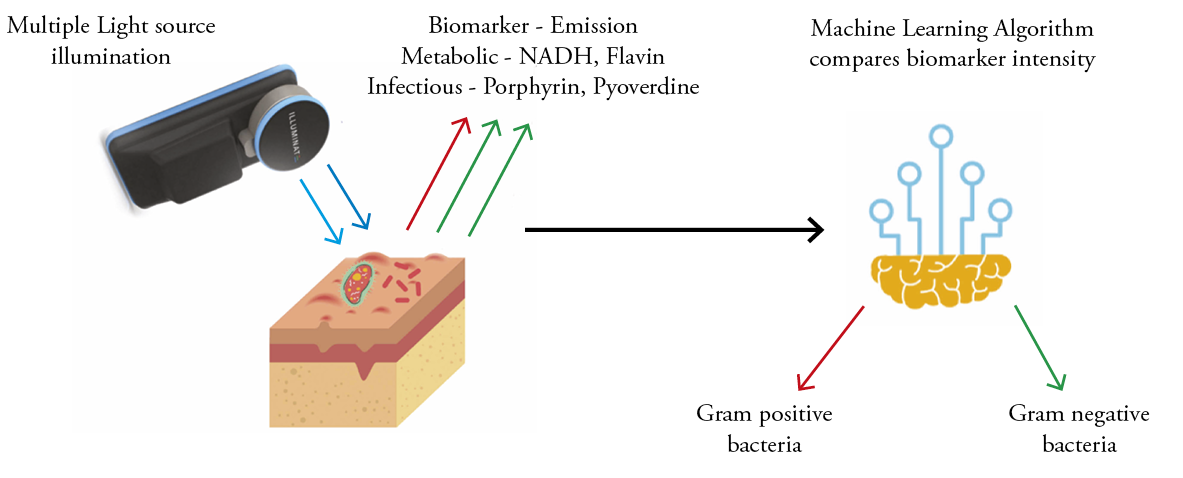
Figure 1: Overview of Illuminate multi-spectral autofluorescence imaging platform for pathogen detection and gram-type classification
Clinical problem addressed
Currently, the assessment of wounds for the presence or absence of bacterial burden based on visual characteristics alone is subjective and can be erroneous. In addition, information on the gram type of bacteria infecting the wound using standard microscopy and culture tests is both time-consuming and reagent-intensive. Understanding the bacterial burden and infection status and monitoring these infections in a wound over time is of the utmost importance for enabling wound closure. Further, identifying bacterial burdens that are of clinical relevance is equally important, as wounds have high loads of commensals present. A bacterial load >104 CFU/g is usually considered to be of clinical significance, and earlier fluorescence imaging methods have been able to detect bacterial burden above this value effectively (23). With the imaging device, the algorithm thresholds are adjusted to detect a bacterial load >104 CFU/g.
MATERIALS AND METHODS
Study design
An interventional single-arm comparative study was carried out at a tertiary care centre in Chennai, India after obtaining institutional ethics committee approval (IEC 031#HYC/IEC/2018). The study was registered with the Clinical Trial Registry of India (Reg. No. CTRI/2018/10/016147). Adults (>18 years) diagnosed with diabetic foot ulcers and who were willing to give their consent were included in the study and imaged on their first visit, and during any follow-up visits to the hospital. Patients’ diabetic status was confirmed from various standard biochemical tests that measure blood sugar levels. Patients with infections under intact skin and/or with pre-existing skin conditions, such as eczema or psoriasis in areas close to the wounds, were excluded from the study.
Imaging procedure with the multispectral autofluorescence imaging device
All multispectral images were collected using the device in either a dark room, or by covering the wound with a black hood to minimise interference due to ambient light. Patient details were entered into the device, and the wounds were cleaned with normal saline solution before imaging. The device was held 10–12 cm from the wound region. A guide icon on top of the application page, which uses information from the distance sensor integrated into the device, ensured that the required distance of ~10 cm was maintained during imaging. The capture button was then pressed, to record a series of >15 multispectral autofluorescence images in <20 seconds. Upon successful imaging, the user was prompted with a white light clinical image to ‘lasso’ the region of interest to assess the bacterial burden. The built-in image processing (to compensate for the slight movement of patients during the imaging and identify regions of interest) and an edge-inferencing machine learning algorithm (to detect and classify bacteria causing infections) then displayed a colour-coded overlaid image of the wound, along with the infected region (if any) and the gram type of bacteria. The device also displayed the length, breadth and area of the wound based on proprietary wound segmentation algorithms. A guided swab/tissue sample was taken from the infected region predicted by the device and sent for microbial culture, and the presence/absence of the bacterial burden, along with the gram type, were compared with the report generated from the device. Patients were imaged progressively, and a progressive report tracking/monitoring the wound was also displayed to get a quick understanding of the wound status during follow-up visits.
Image processing and machine learning algorithms
After collecting multispectral autofluorescence images, thresholding was applied to identify the regions exhibiting higher autofluorescence, and spectral intensity features were extracted from each of the threshold images and sent for machine learning. The algorithm’s image-processing thresholds were adjusted to detect bacterial loads, typically >104 CFU/g. The machine learning algorithm based on random forests (a highly accurate ensemble classifier) was trained using an 80:20 data split ratio. The hyperparameters, such as the number of trees, tree depth, split criterion and so on, were optimised for maximum accuracy. A five-fold cross-validation was done to assess the accuracy of pathogen detection and gram type classification.
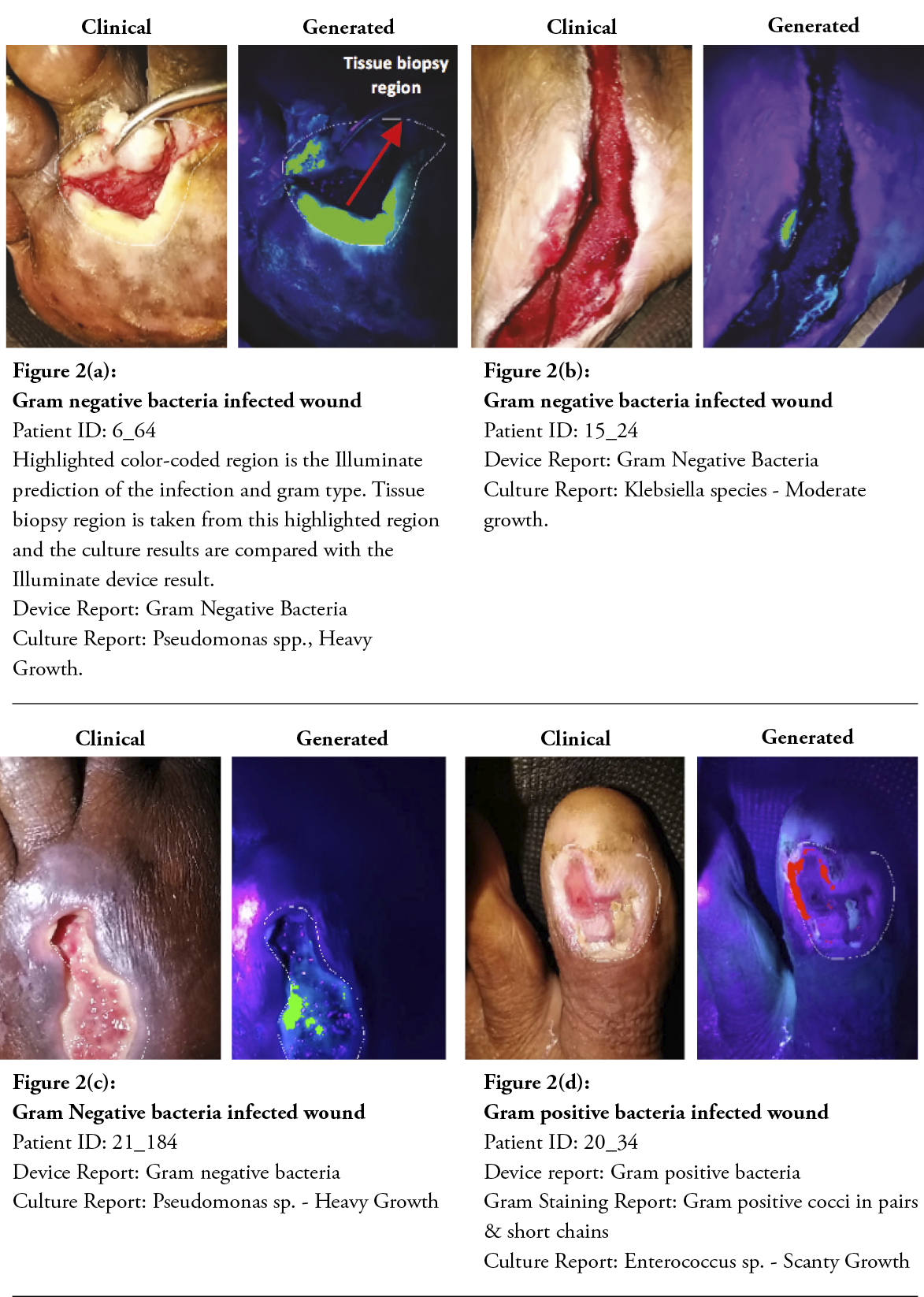
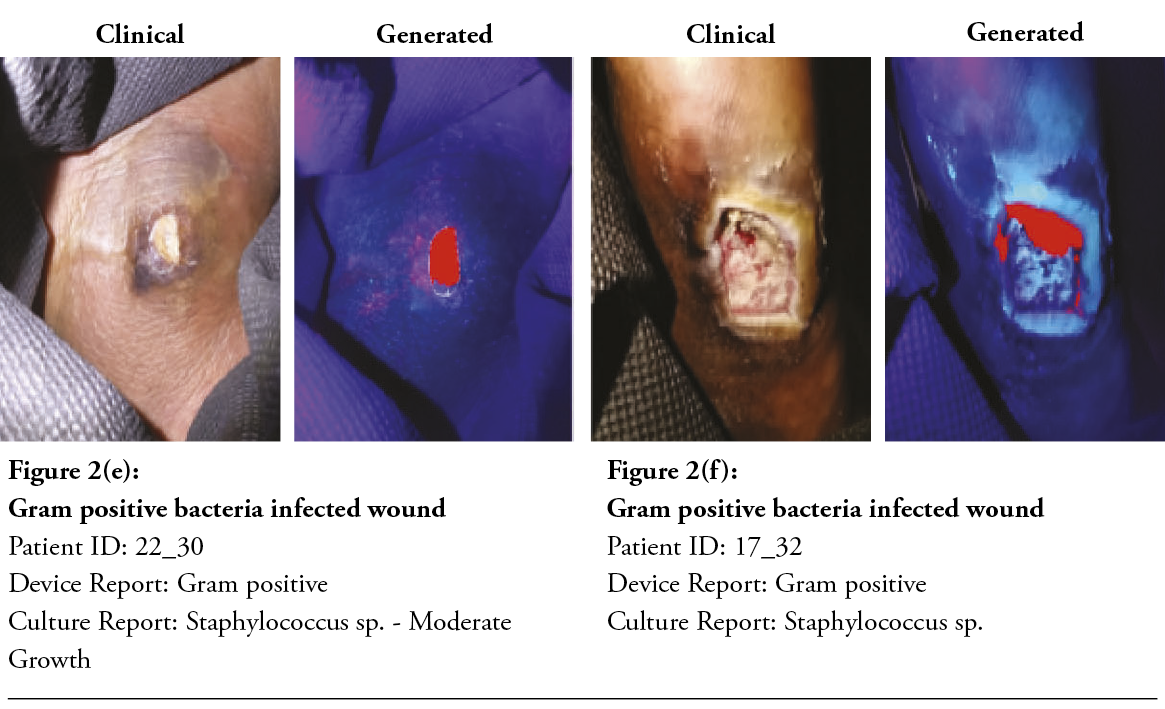
After two minutes, the machine learning algorithm could detect and classify the gram type of bacteria, and the built-in application displayed a wound report, as shown in Figure 2a–f. Both clinical and device’s post-processed wound images were displayed for easier comparison. Gram-positive bacteria are colour-coded in red, and gram-negative bacteria are in green.
Specimen collection
Following debridement, a deep-tissue biopsy was taken from the colour-coded region of the wound area, as indicated by imaging device. Specimens were placed in sterile transport containers and delivered to the certified microbiology laboratory for gram staining, culture and antimicrobial susceptibility testing. The laboratory personnel were blind to the findings of the device. Some samples were also sent for 16s rRNA sequencing, for comparison of the culture results for bacterial species identification. Samples were also taken from regions of no fluorescence, to serve as controls. No antimicrobial agents were administered into the wounds before specimen collection.
Cultures
All tissue biopsies obtained post debridement were cultured at a National Accreditation Board for Testing and Calibration Laboratories-accredited laboratory and analysed initially by gram-staining and conventional aerobic methods by plating into various enriched and differential growth medias. The colonies were then sent for biochemical assay for species recognition. Bacterial load was estimated by a plate count semi-quantitative analysis and then graded as scanty, light, moderate or heavy (1+, 2+, 3+ or 4+), wherein moderate and heavy growth indicated a significant bacterial load (i.e., greater than 100,000 per gram of tissue). An antibiotics susceptibility profile was then obtained using the disk diffusion method, as per Clinical and Laboratory Standards Institute guidelines.
16S rRNA sequencing
16S rRNA gene amplicon sequencing was performed on a subset of 26 patient samples at a certified laboratory. DNA was isolated from the tissue samples using the EXpure microbial DNA isolation kit. PCR was used for selective amplification using 27F and 1492R primers targeting the V3 and V4 regions. Unincorporated PCR primers and dNTPs from the PCR products were removed using the Montage PCR cleanup kit. The quality, quantity (~100 ng/μl) and formulation of the PCR product was checked using a Qubit Fluorometer 3.0. Nanopore sequencing was subsequently used on a 1 µg DNA template, and the results were analysed using the EPI2ME 16S analysis workflow. Finally, 16S rRNA sequencing results were compared with the results obtained from the culture method and the fluorescence imaging device.
Statistical analysis
The sample size was calculated to be 164 at a 99% confidence level, with a 10% margin of error. Correlation between the gram type obtained from fluorescence imaging device and the tissue biopsy culture was assessed using Cohen’s Kappa method in IBM’s SPSS software. The results from the device were compared with the culture results for gram type identification, and the multi-class performance metrics, such as accuracy, sensitivity and specificity were calculated using standard formulas (28).
RESULTS
Clinical characteristics of the subjects
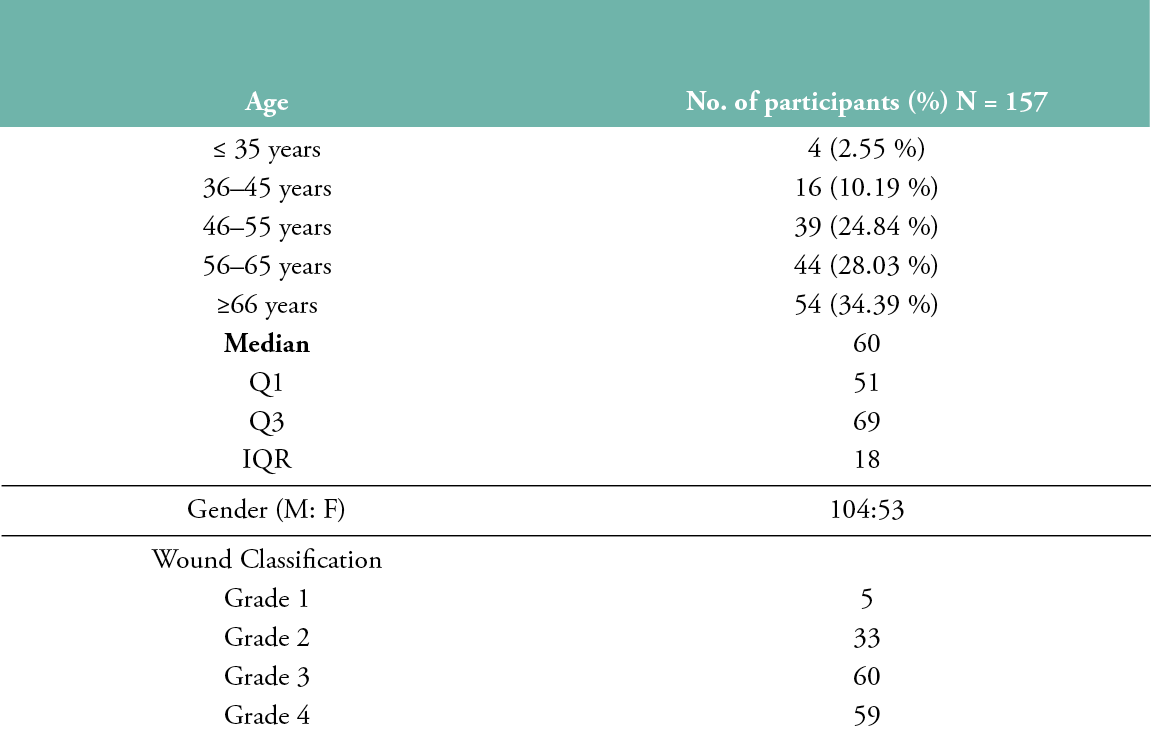
A total of 157 diabetic foot ulcer patients (104 males, 53 females) with a median age of 60 years were enrolled in the study, and 177 tissue samples were obtained from these patients. Nine wounds were present in the distal phalanges, 34 in the dorsal regions, 8 in the lateral heel region, 28 in the medial aspect of the heel, 67 in the plantar region, 10 in the posterior regions and 1 in the anterior tibia. In terms of the Wagner classification scoring system, 5 patients had a Grade 1 ulcer, 33 had a Grade 2 ulcer, 60 had Grade 3 ulcer and 59 had a Grade 4 ulcer (Table 1).
Table 1: Age, gender, and ulcer grade distribution
Organisms isolated in culture
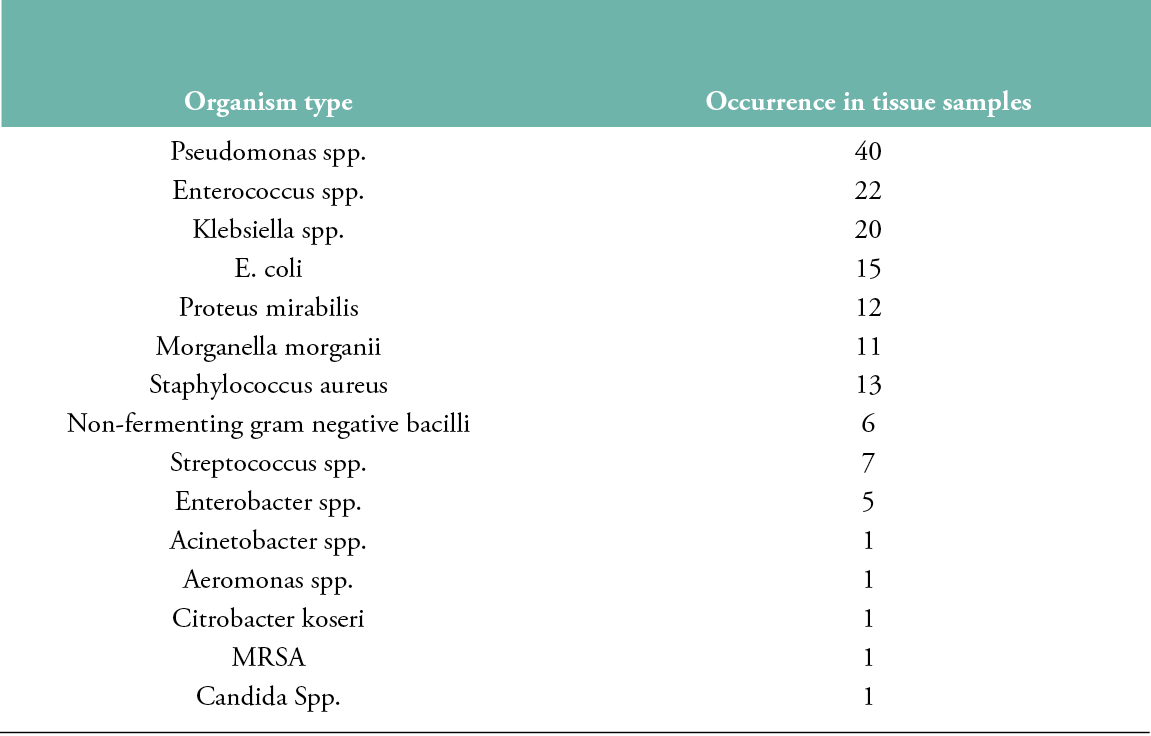
Fifteen pathogens were isolated from the 177 tissue biopsies collected (Table 2): 112 gram-negative, 43 gram-positive and 1 fungus; 42 reports came back showing ‘no growth of bacteria’. Pseudomonas species has the highest occurrence (40 samples), followed by Enterococcus (22) and Klebsiella (20) species.
Table 2: Organisms isolated from tissue biopsy samples
Inferences
The device picked up 111 wounds with gram-negative bacteria, 25 with gram-positive bacteria and 15 wounds with both gram-positive and -negative bacteria. A further 26 wounds had no bacterial burden.
Statistical data
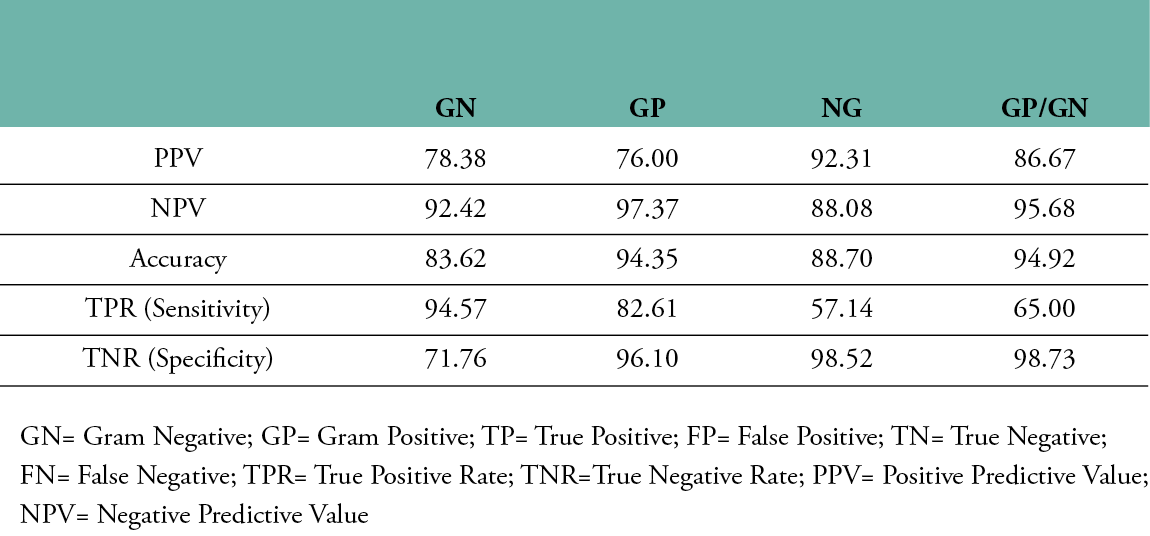
Cohen’s kappa was used to determine the probability of an agreement between gram type results obtained from both the semi-quantitative culture test and the multispectral imaging device in 177 tissue samples. A good agreement was found between both methods, ĸ = .774, 95% CI, [-.0359 to .121], p< .001. The results of the machine learning algorithm are summarised in the confusion matrix provided in Table 3. The class-averaged accuracy of the device was found to be 90.4%, with a positive predictive value of 78.38% for detecting gram-negative bacteria, 76.00% for gram-positive bacteria, 86.67% for polymicrobial (GP&GN) bacterial burden and 92.31% for no significant bacteria burden (no growth). The negative predictive value was found to be 92.42% for gram-negative bacteria, 97.37% for gram-positive bacteria, 95.68% for polymicrobial bacterial burden and 88.08% for no growth. The sensitivity for gram-negative bacteria was 94.57%, 82.61% for gram-positive, 65% for polymicrobial and 57.14% for no growth. Specificity for gram-negative was 71.76%, 96.10% for gram-positive, 98.73% for polymicrobial and 98.52% for no growth. It should be emphasised that, since the accuracy of the gold standard culture method is not 100%, the accuracy of the fluorescence imaging device could be potentially much higher. The device’s imaging results and comparisons with the culture report for various wounds with gram-positive bacteria, gram-negative bacteria and no growth are shown in Figure 2.
Table 3: Confusion matrix for infection and gram type classification
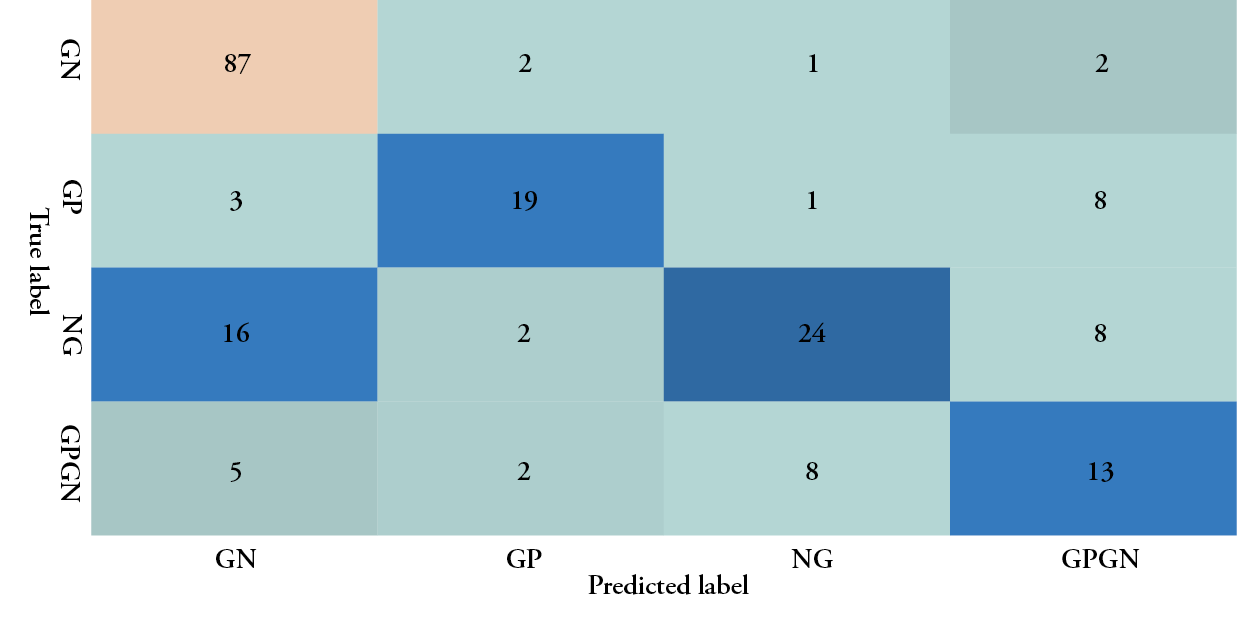
Table 4: Machine learning performance of the Illuminate device
16S rRNA sequencing results
To better assess the performance of the fluorescence imaging device in the detection of bacterial burden, 16S rRNA sequencing was conducted to provide more information on the polymicrobial species present in the wound, as compared to a standard culture test. The results of 16S rRNA sequencing were compared with the results obtained by the fluorescence imaging device and the culture method for the presence/absence of significant bacterial bioburden. Of the 26 biopsies compared with the 16S rRNA sequencing and culture method, the culture method gave positive results in 17 cases. In these 17 cases, the species identified by the culture method correlated with at least one of the species identified by the 16S rRNA sequencing results in 16 cases. The device also showed significant levels of bacterial burden, which correlated with both the culture and 16S rRNA results. However, the culture method showed no growth in several cases (9 cases), where the 16S rRNA results showed significant reads (>50 reads for at least one organism) in five samples. Interestingly, in four of the five cases, the fluorescence imaging device was still able to show the presence of bacterial burden, demonstrating the superior sensitivity of the device, as compared to the culture.
DISCUSSION
Understanding bacterial burden is important for the effective management of diabetic foot ulcers and preventing downstream complications. Earlier studies have shown that the clinical evaluation of signs and symptoms (CSS) has a low accuracy rate for identifying wounds with moderate to heavy bacterial burdens (23). CSS is typically based on the clinical signs of critical colonisation (NERDS) or infection (STONEES) (29). The accuracy results vary widely among the studies, ranging from <30% (23) to <80% (29), hence, a better technique is needed for accurate wound bioburden assessment. It is well known that pathogens emit autofluorescence when excited with UV and violet/blue regions of light during the phase of growth and infection (21-22). This weak autofluorescence is typically studied using standard spectrofluorometers, which are both expensive and bulky, making them difficult to adopt in a point-of-care setting (30). A novel portable and handheld multispectral imaging device was developed for rapid and non-invasive bacterial burden assessment at the bedside in under 2 minutes (31-31). The device detects bacteria that have infected the tissue and are expressing significant metabolic activity, as the device mainly targets NAD(P)H and flavins. The device integrates multiple wavelengths of light both in the UV-A and violet regions and captures weak autofluorescence at different spectral emission bands using high optical density to minimise background autofluorescence. These spectral images are fed to an image processing and machine learning algorithm that compares these images and identifies significant bacterial burden regions based on the differences in autofluorescence intensities at various spectral bands before classifying them based on the gram type.
This study was carried out to detect the accuracy of the device in comparison with the current gold standard culture method and proved that the device is capable of accurately detecting and distinguishing the gram type of bacteria within 2 minutes. Thus, fluorescence imaging device can be used as a first-line screening solution, especially when access to microbiology labs is problematic (33). In addition, the results show that the device has a better sensitivity and specificity in identifying bacterial burden, compared to the standard CSS technique. The device also enables evidence-based continuous monitoring of patients’ wounds during hospitalisation, rather than visual inspection, making wound tracking easier.
At present, it is difficult to accurately assess if infected tissues have been removed completely post debridement using CSS alone. Fluorescence imaging can aid in effective debridement (25), as demonstrated in earlier studies, and the device used in the present study was also able to improve debridement efficiency by providing immediate feedback regarding the status of bacterial burden after debriding each layer. This also saves healthy viable tissue from excessive debridement, improving wound healing. Further, the device can be used to check whether a wound area is free of bacterial burden before tissue grafting.
There are several instances when a wound may appear infected clinically, but the culture report shows no growth. This could be the result of concurrent antibiotic therapy, the ability of culturing to pick only out certain types of bacteria or a defective wound swabbing/biopsy site (34-37). Devices such as the novel multispectral imaging device can guide accurate swabbing by identifying areas with the highest levels of bacterial burden, leading to a better understanding of the overall microbial burden, especially for polymicrobial wounds, for further therapeutic interventions. In addition, the device helps doctors to identify bacterial burdens away from the wound, which can potentially be missed by visual inspection alone.
Despite the above, the manual interpretation of multi-spectral autofluorescence images is difficult, subjective and requires significant training. This cumbersome process might pose severe challenges in terms of ensuring the accuracy of decisions. This is where machine learning plays a vital role, as it corrects for the background noise caused by tissue autofluorescence and automatically classifies infected regions as gram-positive or gram-negative. The colour-coding aids in effective spatial understanding of pathogen presence in the wound. Typically, it is assumed that the bacterial burden is present at the centre of the wound, at the site of maximum slough, but the device was frequently able to detect the presence of pathogens, including pseudomonas, away from wound, at the peri wound site, providing hints about the reasons behind delayed wound healing.
Limitations
The device has the following limitations. Bone, tendon and fat regions of wounds have autofluorescence of their own, primarily contributed by collagen. The algorithm may misrepresent these regions as infected, due to high levels of autofluorescence. Hence, the results from the device must be clinically correlated in such scenarios. The presence of betadine and other cleaning solutions that are fluorescent in nature will also interfere with these weak autofluorescence signals; hence, the wound must be thoroughly cleaned with saline solution before imaging. In future, through advanced image processing and machine learning techniques, it may be possible to compensate for the interference from betadine fluorescence and extract the bacterial fluorescence signatures to assess the bioburden, especially at high bacterial loads. Surgical sutures and cotton dressing remaining on wounds might also predict a false bacterial burden, hence one needs to be cautious while imaging.
CONCLUSION
The device is a first-of-its-kind device that uses multispectral autofluorescence imaging combined with machine learning for the rapid detection of bacterial burden and gram type classification on diabetic foot wounds. The device demonstrated >90% class-averaged accuracy, compared to the current gold standard culture method in gram type classification. In addition, the device can pick up bacterial burdens, even in cases the culture report has missed, as confirmed by 16S rRNA sequencing. Thus, the device can guide doctors towards guided debridement, the right first-line treatment protocol and improved wound tracking.
IMPLICATIONS FOR CLINICAL PRACTICE
- Doctors and clinicians can obtain an instant understanding of the bioburden on the wound and gram type of infecting bacteria.
- The device aids in effective wound debridement.
- Doctors can optimise dosages and prescriptions based on the bacterial type and bioburden present on the wound.
Further research
Areas for further research could include:
- Large-scale studies correlating the performance of the device with the results from metagenome studies.
- Quantification of the bioburden using a fluorescence imaging device.
- Longitudinal tracking of bioburden and correlations with wound healing in ulcer patients.
Acknowledgments
The authors thank Vignesh Devaraj, Clinical Research Intern, who was pivotal in data collection and documentation.
Funding
No exclusive funding was provided by Adiuvo Diagnostics or any other entity for the clinical studies.
Ethical statement
The authors are accountable for all aspects of the work, including ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The trial was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was conducted at Hycare Hospital, in Chennai, India, after obtaining institutional ethics committee approval (IEC 031#HYC/IEC/2018). All data were collected with patient consent prior to their participation in the study. The study was registered with the Clinical Trial Registry of India (Reg. No. CTRI/2018/10/016147).
Key messages
- A novel multispectral imaging device is a first of its kind device which uses multispectral autofluorescence imaging combined with machine learning for rapid detection of bacterial burden and gram type classification on the diabetic foot ulcers.
- The device demonstrated >90% class-averaged accuracy as compared to the current gold standard culture method in gram type classification and can pick-up bacterial burden even in cases where the culture method has missed as confirmed by 16S rRNA sequencing.
- It can assist doctors towards right first line treatment protocol, guided debridement, and effective wound tracking.
Author(s)
Rajesh Kesavan1, Changam Sheela Sasikumar2
1 Department of Podiatric Surgery, Hycare Super Specialty Hospital, Chennai, Tamil Nadu, India. Adjunct Faculty, SRM Institute of Science and Technology, Kattankulathur, Tamil Nadu, India.
2 Department of Clinical Research, Hycare Super Specialty Hospital, Chennai, Tamil Nadu, India. Managing Partner - SS Clini Research LLP, Adjunct Faculty, Department of Biochemistry, Saveetha Dental College, Saveetha Institute of Medical & Technical Sciences, Saveetha University Chennai, Tamil Nadu, India.
Correspondence: Dr Rajesh Kesavan, hycareforwound@gmail.com
Conflict of interest: None
References
- IDF diabetes ATLAS 9th edition [Internet]. 2019. [2102 April 6]. Available at https://www.diabetesatlas.org/upload/resources/material/20200302_133351_IDFATLAS9e-final-web.pdf
- Andersen CA, Roukis TS. The diabetic foot. Surgical Clinics of North America. 2007 Oct;87(5):1149–77.
- Singh N, Armstrong DG, Lipsky BA. Preventing foot ulcers in patients with diabetes. JAMA 2005; 292(2):217-28.
- Boulton AJM, Armstrong DG, Hardman MJ, Malone M, Embil JM, Attinger CE, Lipsky BA, Aragón-Sánchez J, Li HK, Schultz G, Kirsner RS. Diagnosis and Management of Diabetic Foot Infections. Arlington (VA): American Diabetes Association; 2020 Jan. PMID: 32105420.
- Zubair M, Ahmad J. Potential risk factors and outcomes of infection with multidrug resistance among diabetic patients having ulcers: 7 years study. Diabetes Metab Syndr. 2019 Jan-Feb;13(1):414-418. doi: 10.1016/j.dsx.2018.10.014. Epub 2018 Oct 13. PMID: 30641735.
- Wu M, Pan H, Leng W, Lei X, Chen L, Liang Z. Distribution of Microbes and Drug Susceptibility in Patients with Diabetic Foot Infections in Southwest China. J Diabetes Res. 2018 Aug 5;2018:9817308. doi: 10.1155/2018/9817308. PMID: 30175153; PMCID: PMC6098928.
- Sadeghpour Heravi F, Zakrzewski M, Vickery K, G Armstrong D, Hu H. Bacterial Diversity of Diabetic Foot Ulcers: Current Status and Future Prospectives. J Clin Med. 2019 Nov 10;8(11):1935. doi: 10.3390/jcm8111935. PMID: 31717640; PMCID: PMC6912738.
- Sankar N, Khaja Moinuddin S, Mohan S, Microbial etiology of diabetic foot ulcers: Swab versus tissue culture. Int J Surg Sci 2020; 4(2):146-8.
- Huang Y, Cao Y, Zou M, Luo X, Jiang Y, Xue Y, et al. A comparison of tissue versus swab culturing of infected diabetic foot wounds. International Journal of Endocrinology. 2016;2016:1–6.
- Ogba OM, Nsan E, Eyam ES. Aerobic bacteria associated with diabetic foot ulcers and their susceptibility pattern. biomed dermatol. 2019 Dec;3(1):1.
- Aragón-Sánchez J, Luis Lázaro-Martínez J, Pulido-Duque J, Maynar M. From the diabetic foot ulcer and beyond: how do foot infections spread in patients with diabetes? Diabetic Foot & Ankle. 2012 Jan;3(1):18693.
- Tascini C, Lipsky BA, Iacopi E, Ripoli A, Sbrana F, Coppelli A, et al. KPC-producing Klebsiella pneumoniae rectal colonization is a risk factor for mortality in patients with diabetic foot infections. Clinical Microbiology and Infection. 2015 Aug;21(8):790.e1-790.e3.
- Hassan MA, Tamer TM, Rageh AA, Abou-Zeid AM, Abd El-Zaher EHF, Kenawy ER. Insight into multidrug-resistant microorganisms from microbial infected diabetic foot ulcers. Diabetes & Metabolic Syndrome: Clinical Research & Reviews. 2019 Mar;13(2):1261–70.
- Miyan Z, Fawwad A, Sabir R, Basit A. Microbiological pattern of diabetic foot infections at a tertiary care center in a developing country. J Pak Med Assoc. 2017 May;67(5):665-669. PMID: 28507348.
- Edwards U, Rogall T, Blöcker H, Emde M, Böttger EC. Isolation and direct complete nucleotide determination of entire genes. Characterization of a gene coding for 16S ribosomal RNA. Nucl Acids Res. 1989;17(19):7843–53.
- Gunasekaran P. Assessment of major microorganisms involved in diabetic foot infection which delays wound healing. IAIM 2017; 4(8):87-90.
- Ramani A, Ramani R, Shivananda PG, Kundaie GN. Bacteriology of diabetic foot ulcers. Indian J Pathol Microbiol 1991; 35(2):81-7.
- Dartnell LR, Roberts TA, Moore G, Ward JM, Muller JP. Fluorescence characterization of clinically-important bacteria. Brody JP, editor. PLoS ONE. 2013 Sep 30;8(9):e75270.
- Leriche F, Bordessoules A, Fayolle K, Karoui R, Laval K, Leblanc L, et al. Alteration of raw-milk cheese by Pseudomonas spp.: Monitoring the sources of contamination using fluorescence spectroscopy and metabolic profiling. J Microbiol Methods 2004 Oct; 59(1):33-41.
- Estes C, Duncan A, Wade B, Lloyd C, Ellis W Jr, Powers L. Reagentless detection of microorganisms by intrinsic fluorescence. Biosens Bioelectron 2003; 18(5-6):511-9.
- Radhakrishnan G, Gupta A, King J, Ganvir D. Rapid handheld screening device to detect skin and soft tissue infections. PROC SPIE 2020; 11211.
- Rennie M, Dunham D, Lindvere-Teene L, Raizman R, Hill R, Linden R. Understanding real-time fluorescence signals from bacteria and wound tissues observed with the moleculight i:xTM. Diagnostics. 2019 Feb 26;9(1):22.
- Serena, TE, Harrell K, Serena L, Yaakov RA. Real-time bacterial fluorescence imaging accurately identifies wounds with moderate-to-heavy bacterial burden. J Wound Care 2019; 28(6):346-57.
- Sandy‐Hodgetts K, Andersen CA, Al‐Jalodi O, Serena L, Teimouri C, Serena TE. Uncovering the high prevalence of bacterial burden in surgical site wounds with point‐of‐care fluorescence imaging. International Wound Journal. 2022 Oct;19(6):1438–48.
- Cole W, Coe S. Use of a bacterial fluorescence imaging system to target wound debridement and accelerate healing: A pilot study. J Wound Care 2020; 29(Sup7):S44-S52.
- Price N. Routine fluorescence imaging to detect wound bacteria reduces antibiotic use and antimicrobial dressing expenditure while improving healing rates: retrospective analysis of 229 foot ulcers. Diagnostics. 2020 Nov 10;10(11):927.
- Oropallo AR, Andersen C, Abdo R, Hurlow J, Kelso M, Melin M, et al. Guidelines for point-of-care fluorescence imaging for detection of wound bacterial burden based on delphi consensus. Diagnostics. 2021 Jul 6;11(7):1219.
- Tharwat A. Classification assessment methods. ACI. 2021 Jan 4;17(1):168–92.
- Woo KY, Sibbald RG. A cross-sectional validation study of using NERDS and STONEES to assess bacterial burden. Ostomy Wound Manage. 2009 Aug 1;55(8):40-8. PMID: 19717855.
- Kumar AA, Hennek JW, Smith BS, Kumar S, Beattie P, Jain S, et al. From the bench to the field in low-cost diagnostics: two case studies. Angew Chem Int Ed. 2015 May 11;54(20):5836–53.
- Radhakrishnan G, Gupta A, King J, Ganvir D. Rapid handheld screening device to detect skin and soft tissue infections. PROC SPIE 2020; 11211.
- Shah BM, Ganvir D, Sharma YK, Mirza SB, Misra RN, Kothari P, et al. Utility of a real-time fluorescence imaging device in guiding antibiotic treatment in superficial skin infections. IJDVL. 2021 Feb 24;88:509–14.
- Lipsky BA, Senneville É, Abbas ZG, Aragón‐Sánchez J, Diggle M, Embil JM, et al. Guidelines on the diagnosis and treatment of foot infection in persons with diabetes (IWGDF 2019 update). Diabetes Metab Res Rev [Internet]. 2020 Mar [cited 2022 Nov 3];36(S1). Available from: https://onlinelibrary.wiley.com/doi/10.1002/dmrr.3280
- Bill TJ, Ratliff CR, Donovan AM, Knox LK, Morgan RF, Rodeheaver GT. Quantitative swab culture versus tissue biopsy: a comparison in chronic wounds. Ostomy Wound Manage. 2001;47(1):34-37.
- Levine NS, Lindberg RB, Mason AD Jr, Pruitt BA Jr. The quantitative swab culture and smear: A quick, simple method for determining the number of viable aerobic bacteria on open wounds. J Trauma. 1976;16(2):89-94.
- Demetriou M, Papanas N, Panopoulou M, Papatheodorou K, Bounovas A, Maltezos E. Tissue and swab culture in diabetic foot infections: neuropathic versus neuroischemic ulcers. The International Journal of Lower Extremity Wounds. 2013 Jun;12(2):87–93.
- Bonham PA. Swab cultures for diagnosing wound infections: a literature review and clinical guideline. Journal of Wound, Ostomy & Continence Nursing. 2009 Jul;36(4):389–95.
