Volume 24 Number 2
Wound biofilm: a bacterial success story
Ewa K Stuermer
Keywords wound biofilm, antimicrobials, wound dressings, physical wound therapy, biofilm diagnostics
For referencing Stuermer EK. Wound biofilm: a bacterial success story. Journal of Wound Management 2023;24(2):6-13.
DOI
https://doi.org/10.35279/jowm2023.24.02.04
Submitted 13 February 2023
Abstract
Background Wound biofilm is one of the greatest challenges in the local therapy of chronic wounds. In clinical practice, it becomes apparent that there are significant differences in the efficacy of wound therapies, although the corresponding guidelines often classify them – due to lack of evidence and knowledge – as “equivalent”.
Hypothesis Since (almost) no randomised controlled clinical trials (RCT) on antimicrobial local therapies exist, translational research using human biofilm models can further generate information to demonstrate difference and equivalence of topical and physical wound therapies.
Methods This narrative, but also scientific, review addresses several anti-biofilm therapies that have been validated in the translational, human biofilm model based on blood plasma, buffy coat, and various bacterial specimens. Bacterial colonisation patterns of Pseudomonas aeruginosa and methicillin-resistant Staphylococcus aureus (MRSA) of patients and ex vivo biofilm models were compared.
Results Substantial differences in the anti-biofilm efficacy of antimicrobials, which were often evaluated equally in guidelines, are shown. Octenidine dihydrochloride-phenoxyethanol, polyhexanide (PHMB) and cardexomer iodine perform against biofilm with delay but well. Many antimicrobials fail because of the extrapolymeric substance (EPS) of biofilm, a bacterial product of polysaccharides and (lipo)proteins, acting like a protecting shield. Physical therapies also have their limitations here.
Conclusions The persistence of biofilm makes wound healing stagnate. Multiple surgical debridement is not the optimal therapy for aged, critically ill patients. Wound biofilm requires a combined topical therapy because it consists of several ‘components’. Currently, effective anti-biofilm strategies sustainably removing it from chronic wounds are still lacking. Researchers and manufactures are challenged, because the understanding of wound biofilm is already considerable, promising combined therapies are conceivable, only implementation is still missing.
Key messages
- Considering the potential presence of bacterial biofilm on a wound is the first step in its therapy.
- After debridement, antimicrobials and antimicrobial dressings can better perform their efficacy against pathogens in biofilms and wounds.
- To date, no local sustainable therapy exists to eliminate biofilm on chronic wounds except multiple sharp and/or surgical debridement.
Introduction
Wounds colonised with biofilms are one of the greatest challenges in chronic wound care1,2. An estimated 2% of the population in central Europe suffer from chronic wounds, with risk increasing with age3. Diagnostics and treatment of the underlying disease, usually peripheral arterial disease (PAD), venous insufficiency (CVI), and diabetes mellitus type I or II, or even immunological disease, are the first steps of a successful treatment4. Recurring (local) infections and persistent wound biofilm prolong the healing process. This usually takes months and also requires cross-sectoral treatment (hospital, outpatient clinic, general practitioner or specialist, nursing)5. In addition, a closed wound remains at risk of re-opening. Accordingly, the term ‘wound remission’ seems to be more appropriate than ‘wound healing’ for patients with chronic wound healing disorders.
According to a meta-analysis, about 78% of all chronic wounds are colonised with pathogenic microorganisms in the form of biofilms6. These are responsible for the persistence of a wound, provided the best therapy of the underlying disease has been implemented2. Wound biofilm is defined as follows:
Biofilm is a structured community of microbes with genetic diversity and variable gene expression (phenotype), which creates behaviors and defenses used to produce unique infections (chronic infection) with characteristics of significant tolerance to antibiotics and biocides whilst also being protected from host immunity – Consensus Delphi Process, IWII (05/2016).
Every wound, even an acute one, becomes contaminated within a few hours by microorganisms of the skin microbiome, which may also contain pathogenic species. The wound bed constitutes a good ‘nutrient medium’. At this stage, it is referred to as ‘wound contamination’. Subsequently, bacteria replicate in the wound and bacterial colonies are formed; it is now referred to as ‘colonisation’. External wound cleansing can reduce bacterial burden. The body’s immune system also works against the spread of bacteria in the wound. If these processes do not occur or if the local immune cells do not function efficiently, more and more bacteria colonise the wound (Figure 1) and begin to organise themselves.
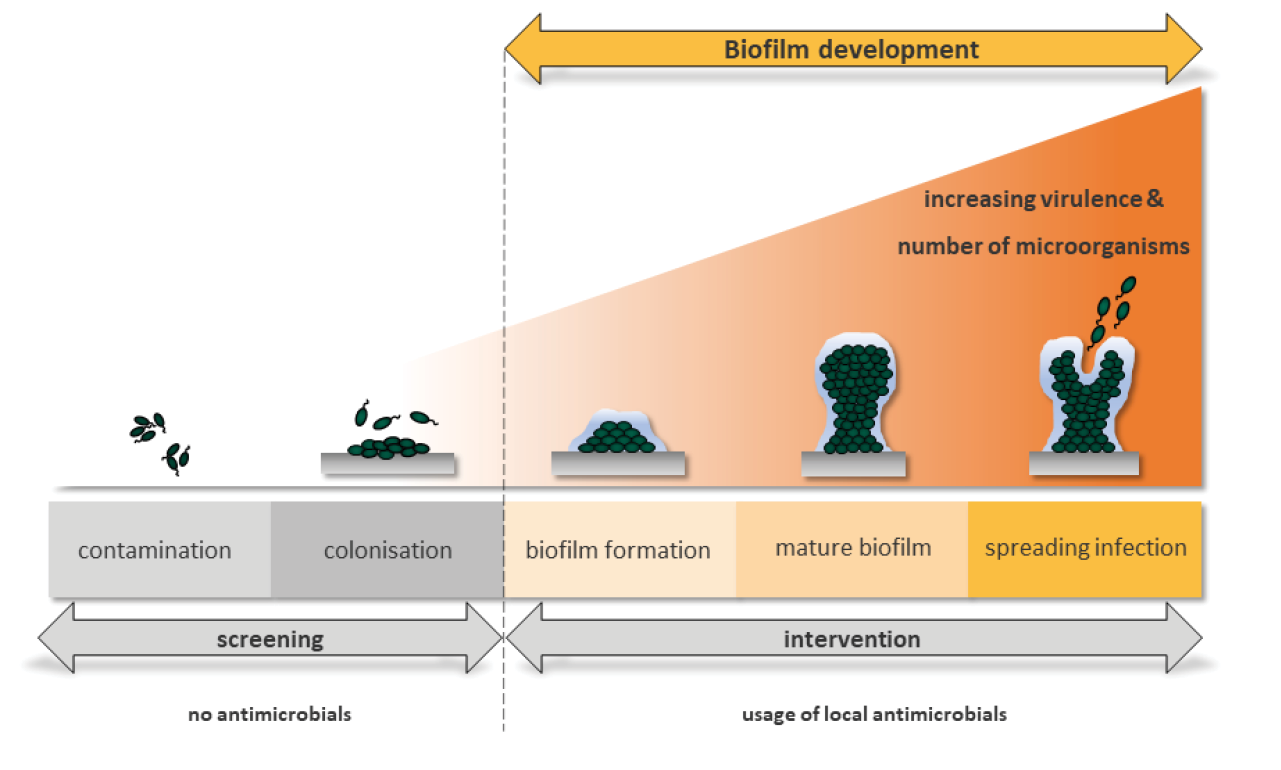
Figure 1. Schematic illustration of the infection continuum and biofilm formation in the time course. At the beginning exists a contamination with the presence of non-proliferating microbes at a level that does not evoke a host response. This is followed by a colonisation where microorganisms undergo limited proliferation without evoking a host reaction. Both stages require no antimicrobial cleansing. From these colonies, a biofilm is formed, which is referred to as structured or ‘mature biofilm’ after 24 hours. In these stages it is recommended to use antimicrobial substances for wound cleaning and in wound dressings. As biofilm formation progresses, mostly the amount of wound exudate increases and seeding of the biofilm bacteria in other areas of the wound or the wound surrounding takes place. Spreading infection describes the invasion of the surrounding tissue by infective organisms that have spread from a wound.
The so-called ‘immature biofilm’ can form as quickly as within only 24 hours. In the case of all wounds, it does not consist of only one microbial species (for example as implant biofilms in orthopaedic surgery), but contains many different bacterial or fungiform species, forming a multi-species biofilm. The leading species in wound biofilms, here illustrated by the example of leg ulcers, are Staphylococcus aureus, its methicillin-resistant strain (MRSA), Pseudomonas aeruginosa and Enterobacteria7.
These bacteria – less frequently fungi are also involved – begin to wrap themselves in the so-called extrapolymeric substance (EPS), almost ‘walling’ themselves in it. The EPS consists mainly of polysaccharides (e.g. alginate, cellulose, dextran) and a variety of proteins, lipids, glycoproteins, glycolipids8, i.e. sugars and proteins, which make the biofilm adhere firmly to the wound bed. After 2–4 days, it is called a ‘mature biofilm’ (Figure 1). It becomes up to 2mm thick, three-dimensional and sometimes visible with the naked eye (Figure 2A/B). Using a curette, it can be partly removed from the wound surface in a blood-dry manner (Figure 2B). At this stage, the biofilm is able to release planktonic bacteria within the wound exudate, which is produced extensively due to local immune response, and these bacteria can colonise in the wound environment or in other wounds9. Then, the cycle of biofilm formation starts again.

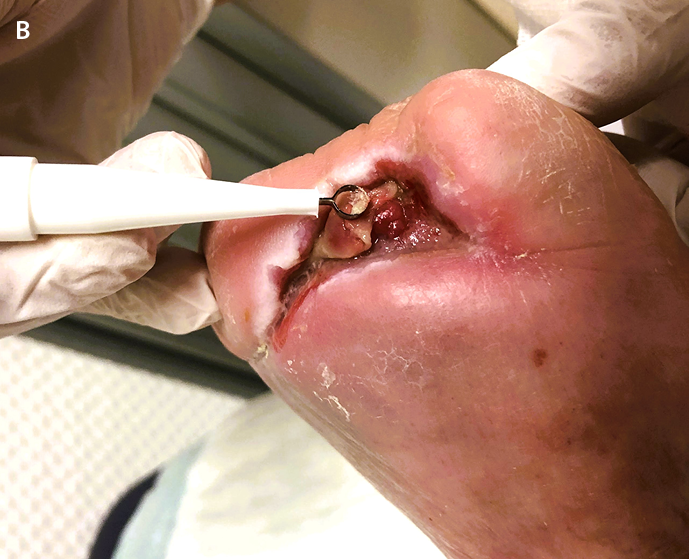
Figure 2. Postoperative wound healing disorder at the amputation stump after moist forefoot gangrene due to advanced PAD.
A: Identification of the dense, carpet-like wound biofilm attaching strongly to the wound bed (centre of the wound)
B: Significant reduction of it by curettage without bleeding
The following narrative review article on wound biofilm includes data from current translational research that use three-dimensional human biofilm models. These are based on blood plasma, buffy coats and various bacterial or fungal species10. Even though the analysed models cannot be transferred directly into clinical practice, they have a special significance due to the resemblance of interactions between the bacteria in the biofilm and the human immune defence. Taking into account that there are few randomised controlled trials (RCTs) comparing local wound biofilm therapy, as reviewed in the EWMA position document Antimicrobials and non-healing wounds: an update11, the presented results may close a gap between the claimed or predescribed anti-biofilm performance, probably obtained during the approval processes of antimicrobial wound therapies, and the real clinical findings.
Materials and methods
Search method
The literature search for this review revolved around biofilm models in the context of anti-biofilm treatment published within the past 10 years from major journals in the field. Medline MBASE was searched using the combination of following keywords – wound biofilm models, antimicrobials, dressings, physical wound therapy, biofilm diagnostics. This information was supplemented with own research and clinical experience in chronic wound care.
Human biofilm models
The analyses described below were performed using solely the human biofilm models hpBIOM (human plasma biofilm model) and lhBIOM (leucocyte depleted human plasma biofilm model), which consisted of human blood products and human pathogenic bacterial species. During biofilm development, microorganisms attach to and invade the wound bed and margin, proliferate and reorganise the surrounding milieu to produce the EPS12,13. To mimic these conditions, human fresh frozen plasma (FFP) including the individual buffy coat (hpBIOM) or a buffy coat originating from the LRS® chamber of leukocyte apheresis (platelet donation), which contain the cellular immune competence of the donor (IhBIOM), were used. For preparation of the biofilm models, buffy coats were centrifuged at 1610g at room temperature (30 minutes) to remove residual erythrocytes. In the next step, FFP and buffy coat were mixed in a sterile glass bottle and a solution (1.5x106 CFU/ml) with different bacterial species (P. aeruginosa, S. aureus, MRSA) was added. To induce coagulation, 18.26µL (500mM) CaCl2/ml suspension was added to the still liquid biofilm mix. This biofilm base was immediately transferred into 12-well plates (1.5ml/model and well). The well plates were incubated for 12–18 hours on a rotation shaker at 60rpm and 37.0˚C to polymerise and forming the bacterial EPS, finally yielding in stable biofilm discs with integrated test organisms. The results presented refer only to mono species biofilm models of P. aeruginosa, S. aureus and MRSA.
Local anti-biofilm therapies
The efficacy of a wide range of antimicrobial and physical anti-biofilm therapies were analysed using the aforementioned human biofilm models. For testing antimicrobial wound irrigation solutions 0.1% octenidine-dihydrochloride/phenoxyethanol [OCT/PE; Octenisept®, Schülke&Mayr, Germany[, 0.04% polyhexanide [PHMB; Lavasorb®, Fresenius Kabi, Switzerland] and three chlorine-based and -releasing antimicrobial wound irrigation solutions were used. The latter included ActiMaris® forte (0.2% sodiumhypochlorite (NaClO)/3% sal maris [Chemomedica Medizintechnik & Arzneimittel Vertriebsgesellschaft mbH, Germany]), Lavanox® (<0.08% NaClO [Serag&Wiessner GmbH, Germany]) and Kerrasol® (<0.08% NaClO [3M GmbH, USA]). In terms of anti-biofilm efficacy, the antimicrobial dressings containing either octenidine di-hydochloride (Sorelex® [Contipro C, Czech Republic]), polyhexanide (Suprasorb®P+PHMB [Lohmann & Rauscher GmbH, Germany]), cadexomer-iodine (Iodosorb® dressing [Smith&Nephew GmbH, England]) or nanocrystalline silver (UrgoClean®Ag [URGO GmbH, France]) were evaluated and compared to an agent-free control dressing (UrgoClean® [URGO GmbH, France]). All antimicrobial dressings release the active ingredient contained (medical device class IIb or III).
As physical, potentially anti-biofilm therapies, laser and atmospheric cold plasma (CAP) were investigated. The following devices were used: a pulsed low level laser device with a wavelength of 904nm and a frequency of 3.200Hz [IDL MP 2510, Biomedical Systems, Germany] and a CAP source called kINPen® MED [neoplas med GmbH, Germany], a German class IIa approved medical device, which generates a plasma beam whose temperature is below 40˚C and with an argon gas flow of 4.5l/min.
Clinical insights and impact on swabbing and debridement
Chronic wounds of 20 patients were analysed. Colonisation patterns of S. aureus and P. aeruginosa were detected with the UV-near light of 405nm wavelength. The obtained qualitative and visual results were compared to immunohistochemical data on the human full-thickness skin biofilm wound models, containing S. aureus and P. aeruginosa. Subsequently, implications for swabbing techniques and debridement were reported.
Results
Identification of biofilm on and in wound surfaces
The first and most important step is the clinical diagnosis is to think about the possibility of biofilm colonisation of the (chronic) wound as an interfering factor in the healing process. The mature biofilm can be identified and often verified by wound (edge) exploration using forceps or curettes (Figure 2B). However, the bacteria are not only located on the wound surface, but instead they penetrate the wound bed or wound margin. For example, human ex vivo wound biofilm models with MRSA or P. aeruginosa showed bacteria-specific differences with respect to their localisation within the full-thickness skin tissue14, where MRSA was less destructive compared to P. aeruginosa. Large numbers of MRSA colonised the wound bed only superficially. P. aeruginosa was significantly more destructive in the tissue and infected predominantly the epidermis of the wound margin, resulting in complete loss of tissue integrity. P. aeruginosa hardly invaded the wound bed, but instead concentrated in and on the wound margins, where it colonised between the keratinocytes of the stratum granulosum and the stratum corneum, and between the stratum granulosum and the stratum spinosum14.
In chronic wounds with resting biofilms, beyond failure to heal, usually no significant (tissue) damage results. It is due to the fact that the various microbial species in the biofilm exist symbiotically with reduced metabolism and proliferation, obtaining nourishment from (human) wound exudate and tissue. Signalling molecules also allow microorganisms to communicate with each other and, as a result, change their level of metabolic activity10. A lower metabolic activity of specific bacteria, called persister cells, located in the depth of the biofilm, as well as the interaction of different microorganisms, e.g. by lateral resistance gene transfer, are further issues contributing to the high resilience of biofilms15. This can be assumed as ‘quorum sensing’ utilised by both Gram-negative and Gram-positive bacteria to coordinate processes in the bacterial community in a limited space16,17. Quorum sensing coordinates behaviours such as abilities for symbiosis, virulence, motility and biofilm formation. The essential signalling molecules are grouped together as autoinducers that alter cell population density. They are also used for communication between different bacterial species. Consequently, highly aggressive biofilms can be formed, e.g. those dominated by P. aeruginosa. They are associated with strong exudation and damage to the wound as well as the surrounding skin, often visible as the swollen wound margin, in turn, leading to wound enlargement. An accompanying strong wound odour is frequent.
A wound biofilm and/or a severe bacterial colonisation of the wound (>104CFU/mm2) can be macroscopically visible using the UV-near light (e.g. 405nm, MolecuLight®) (Figure 3A/B). With this method, areas of high bacterial metabolic activity can be detected by a red fluorescence of the deposited bacterial metabolic products, such as porphyrins (from e.g. Staphylococcus Spp. or Enterobacteriacea) or by the cyan-blue fluorescence of pyoverdine secreted by Pseudomonas Spp. P. aeruginosa predominantly colonises and infiltrates the wound margin, whereas S. aureus is more likely to be found in the wound bed14.
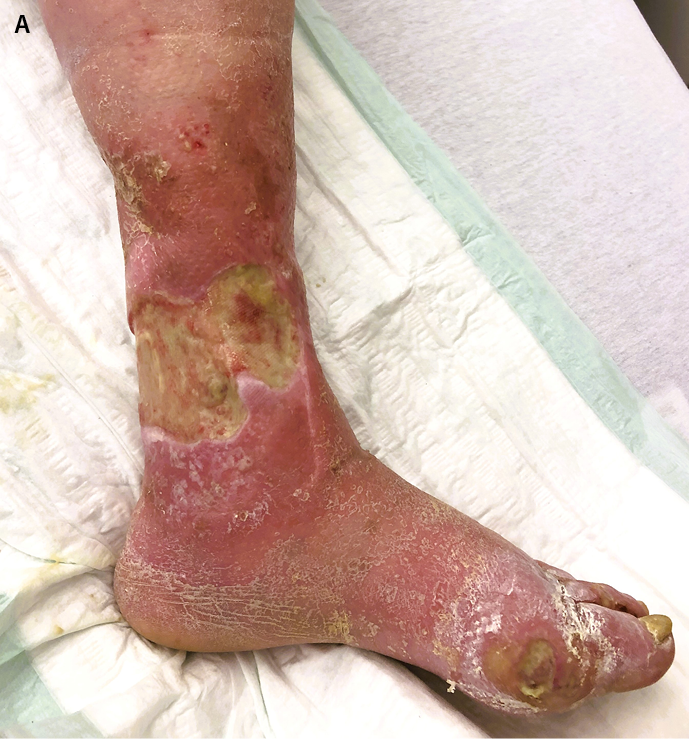
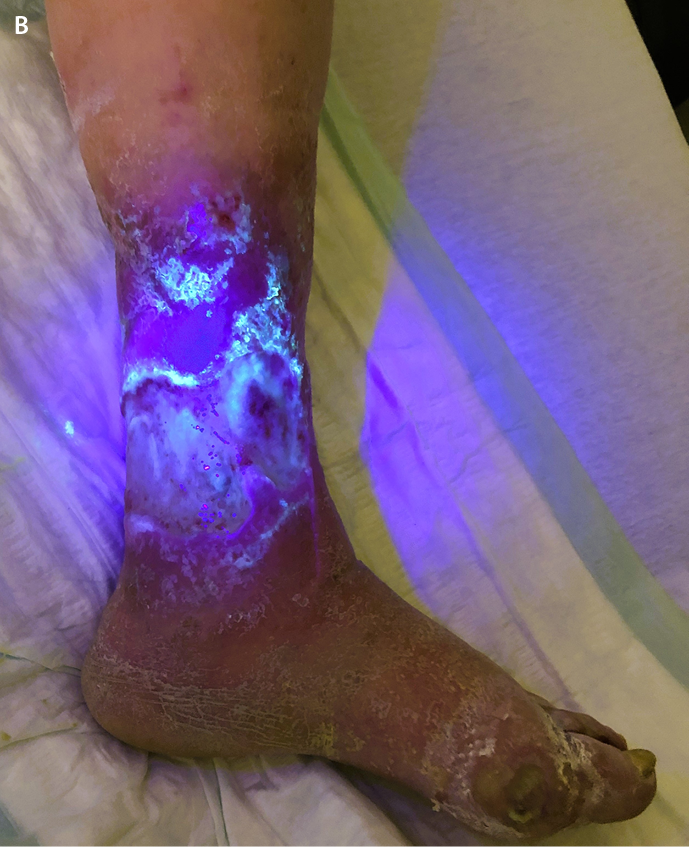
Figure 3. Leg ulcer of a patient suffering from diabetes and PAD
A: Extensive sludge on the wound and dermatitis of the wound environment due to severe exudation
B: Visualisation in bacterial density (>104 CFU/mm2) by UV-near light, which makes the self-fluorescence of bacterial metabolic products visible to the human eye. Cyan-blue reflection at the wound margin, which is typical for
P. aeruginosa
Efficacy of antimicrobial wound irrigation solutions against biofilm
The symbiotic, interspecies ‘society’ of wound biofilms is formed by producing a protective, so-called EPS that on one hand works as a biochemical barrier against the host immune system and on the other hand against systemic antibiotics or externally applied antimicrobials18,19. Therefore, the latter often fail in biofilm eradication6,14,20. The ‘tolerance’ of biofilms to antimicrobials is high because antimicrobials do not have the chemical composition to split the polysaccharides and (lipo)proteins of the EPS. Therefore, they cannot penetrate it to kill the embedded bacteria10,15,21. This was visualised in the human biofilm models hpBIOM and lhBIOM in SEM images10,22–24. Thus, antimicrobials do not lose their efficacy against bacteria in the biofilm, they just do not break through the bacterial ‘shield’ EPS. ‘Bacterial resistance’ means that bacteria develop specific mechanisms, such as efflux pumps, target mutations or post-translational or post-transcriptional modifications that enable them to attenuate or completely neutralise the effect of antimicrobial-active substances. ‘Bacterial tolerance’ of microbes embedded in biofilm refers to the fact that they benefit from the protective matrix environment (EPS) and thus have a greatly reduced susceptibility to antimicrobials, antibiotics, and even the human immune system.
Evidence-based recommendations for the ‘first choice’ antimicrobial wound irrigation solutions regarding potential biofilm eradication do not exist due to the lack of RCTs. Because of the recent renaissance of hypochlorous and chloride-based wound irrigation solutions, it was of particular interest how efficient they were compared to established wound antiseptics like PHMB, octenidine dihydrochloride-phenoxyethanol (Octenisept®) or Iodine. In translational tests with the above-mentioned biofilm model, eradication of the bacteria (P. aeruginosa, S. aureus – including MRSA) did not occur after application of three potent hypochlorous and chloride-based solutions after 24 to 72 hours and a slow bacterial growth was observed instead23. PHMB and Octenisept® performed better, failing to completely kill the bacteria in the biofilm, unlike planktonic bacteria, which are eradicated in a few seconds or minutes6,22,23. These results show that the antimicrobials are nearly unable to penetrate the EPS composed of proteins and polysaccharides. Bacteria in the biofilm are thus more ‘tolerant’ to them. However, this does not mean that antimicrobial resistance – similar to the feared antibiotic resistance – is developed in biofilm bacteria.
Efficacy of antimicrobial wound dressings against P. aeruginosa biofilm
Similarly poor evidence is found in the literature for the use of antimicrobial dressings in biofilm, local or spreading wound infection. RCTs are lacking, so it was pertinent to test antimicrobial wound dressings (only drug-releasing products) in translational, human wound biofilm models produced by P. aeruginosa, which is considered one of the most aggressive biofilm-formers24. As expected, the iodine-, silver-, and polyhexanide-containing wound dressings showed a high bactericidal effect within the first 24 hours, which was, however, only sustained in the case of cadexomer iodine. The PHMB wound dressing induced a kind of ‘bacteriostatic effect’ throughout the test period of 6 days holding on a low but constant 2–3log10 step reduction – only a log10 step reduction >5 (reduction of 99.999% bacteria) is considered as eradication. The silver dressing lost its initial significant antibacterial effect on biofilm, which is equivalent to a failure at the EPS, as the silver molecules released from the dressing are not able to penetrate the depth of the EPS. It remains open whether changing the dressings every 48 hours would induce a stronger and sustainable antimicrobial efficacy in the PHMB and the silver dressing. In the case of the cadexomer iodine dressing, a significant and continuous antimicrobial effect with successive microbial reduction within 6 days was confirmed in previous in vitro and clinical studies25,26. It appears that the cadexomer component in the wound dressing dehydrates the biofilm matrix (EPS), which, in turn, causes its degeneration. This provides a space for iodine to penetrate the biofilm and eradicate the embedded P. aeruginosa.
In summary, there is often a gap between the anti-wound biofilm efficacy claimed by manufacturers and the real bacterial elimination in the biofilm (models). The previously described human biofilm model – even if it consists of ‘waste products’ of the blood bank – is not usable for comprehensive testing as part of the approval processes of medical devices in accredited labs. For this purpose, a wound biofilm model based on sheep blood was developed22, which was shown to accurately mimic the human wound biofilm and – once established – may also reduce the need for animal testing.
Efficacy of antimicrobial physical wound therapies against biofilm
The available physical wound therapies include atmospheric cold plasma (CAP), ultrasound, blue light (high-intensity laser, HIL) or photobiomodulation (low-intensity laser, LILT). Their potential additive efficacy on wound biofilm was tested in the human wound biofilm model lhBIOM. Recently, several clinical trials have confirmed the benefits and efficacy of CAP in chronic wounds27. It is still to unravel whether its antimicrobial potential or rather the (wound) cell stimulation is the driving factor. In the translational setting, planktonic S. aureus was significantly reduced after 120 minutes following the CAP application, whereas in the human biofilm model no significant decrease in the bacterial count was observed regardless of the CAP application mode and its duration28. Electron-microscopically, the structure of the biofilm model was loosened, but this was not reflected in the antimicrobial efficacy. Cautiously applied in the clinical practice, this would imply that CAP application may be more effective in supporting wound healing if the wound biofilm was removed (debrided) first.
Medical lasers are divided into two types – high-intensity laser therapy (HILT) and low-intensity laser therapy. Both types of lasers differ in three main characteristics that influence the therapeutic effects: thermal features, which depend mainly on the tissue chromophores that absorb a particular wavelength; biophysical features, such as the wavelength; and the mode of application, in the form of continuous or pulsed waves. HILT, sometimes called blue light irradiation, is highly efficient against Gram-positive bacteria, Gram-negative bacteria, mycobacteria, moulds, yeasts and dermatophytes29. It is thought to be due to photoexcitation of endogenously produced porphyrins from, for example, P. aeruginosa, Acinetobacter baumannii and Candida albicans. Using the human biofilm model hpBIOM, it was confirmed that the highest antibacterial efficacy results after application of the wavelengths ranging between 402–420nm, complemented by the findings of an efficiency at 455nm and at 480nm, which are in fact limited to P. aeruginosa, Escherichia coli and Staphylococcus epidermidis strains30. In particular, P. aeruginosa appears to be very sensitive throughout the violet/blue spectrum. In contrary to the results of other research groups, no antimicrobial activity against S. aureus was demonstrated by blue light of 455nm and 480nm. In summary, the results indicate that there seems to be a ‘therapeutic window’ within the blue light spectrum (455nm and 480nm) where pathogenic microbes are inactivated while skin cells (here: human primary fibroblasts), remain vital.
Other studies also showed a kind of resistance of keratinocytes (HaCaTs) to blue light frequencies above 415nm. The onset of re-epithelialisation of wounds occurs through the fibroblast-mediated granulation phase, meaning that blue light may delay the healing process despite its good antibacterial potential. Nevertheless, HILT may be useful as a sophisticated therapy (wavelength, dose and irradiance, frequency of application) in individual patients with wounds infected by multidrug-resistant bacteria.
In contrast, the primary goal of LILT or photobiomodulation therapy is to stimulate the healing of chronic wounds, which has been demonstrated in several in vitro as well as in vivo studies31–33. Above a frequency of 635nm, there seem to be predominantly positive effects on skin cells and their healing potential (cell differentiation, cell proliferation, metabolic activity, etc.). However, only a few research groups report antibacterial efficacy of LILT34,35. In a translational in vitro study, pulsed LILT application with a wavelength of 904nm at frequencies of 960Hz–50%, 3200Hz, and 3200Hz±50% showed no antimicrobial effect on S. aureus, P. aeruginosa, Enterococcus faecium and C. albicans in a standardised human biofilm model36. However, unlike the skin cells, the microorganisms were not stimulated to grow. Therefore, this result can be evaluated as quite positive, and it can be summarised that LILT positively influences wound healing avoiding bacterial stimulation.
Efficacy of (sharp) debridement against biofilm
Currently, the only effective and sustainable therapy for wound biofilm recommended by expert consensus is a consequent sharp or surgical debridement6,37. It is often, however, not a feasible option, e.g., in the home-care setting (limited hygiene, therapeutically anticoagulated patients) or for critically ill patients. Moreover, a single radical surgical debridement is usually not sufficient. Instead, repetitive interventions in short time intervals have proven to be successful and sustainable in the long run37,38. The use of this comparatively aggressive local therapy even in ‘resting’ wounds with biofilm (Figure 2) tends to require a great amount of perseverance from a practitioner; after removal of the dressing, the wound should be visually inspected for potential biofilm colonisation or with the aid of the above-mentioned UV near light14. Only after sustainable sharp reduction of the biofilm, antimicrobial solutions and dressings can be efficient against the remaining pathogens.
Surgical debridement is often indicated when not only the adherent wound biofilm but also large areas of necrotic tissue are to be removed38,39. It is performed under spinal or intubation anaesthesia. Compared with sharp debridement, it is more invasive and also removes parts of the wound margin and wound bed using a scalpel and shaver. Accordingly, the wound size increases first. Another option for removing the wound biofilm is chemical debridement, which effectively removes the bacteria but also infiltrates the wound bed and wound margin. Here, deep-seated bacteria are killed, but human cells are also involved, so that a primary wound enlargement occurs as well40,41.
Discussion
This narrative review summarises translational, in vitro and ex vivo studies of antimicrobial therapies of chemical and physical nature against bacterial biofilm and reflects the results in clinical findings. These analyses were based on human blood-based biofilm models, each with one bacterial, human-pathogenic species. This constitutes a limitation to the conclusions as the wound biofilms in clinical practice are always multi-species. However, it is also known that dominant and less dominant species exist in these biofilms (reviewed in42). Thus, this model is at least more suitable than the existing ones, mostly two-dimensional or liquid43–45. To date, only two other 3D biofilm models exist: a liquid-filled chamber with bacterial species inside46–48; and a collagen gel matrix with serum proteins containing up to two bacterial species49. With regard to biofilm in chronic wounds, the comparability and durability of both models are low. However, in most biofilm analyses, only the biofilm-conditioned medium (BCM) is used in order to avoid direct contact between bacteria and (human) cells50–52. In summary, the unique composition of the hpBIOM and lhBIOM mimics the wound milieu and includes the individual immune competence, which represents an improvement of biofilm models with regard to the clinical translation of results. This is of relevance because, to the author’s knowledge, there are no evidence-generating statements and RCTs on how effective antimicrobial agents, dressings and techniques against biofilm-forming bacteria really perform in clinical practice.
Descriptively, the efficacy of antimicrobial agents is broken down to the value of the so-called ‘log10-step reduction’, i.e. to logarithms of 10. Orientating, bacteriostatic effects correspond to a log10 reduction to ≤3 (to 99.9%), and bacteritoxic effects to a log10 reduction ≥5 (to 99.999%) are used. This topic was scrutinised earlier in this review.
From the translational in vitro, ex vivo and clinical results shown, it is evident that the biofilm matrix, the so-called EPS, plays a crucial role in the resilience of the wound biofilm and its defence against antimicrobials. It acts as a ‘protective shield’, where those highly proficient at making antibacterial therapies tend to fail. This predominantly bacterial construct of polysaccharides, a variety of proteins, lipids, phospholipids, glycoproteins, glycolipids and/or lipopolysaccharides (LPS)53, cannot be penetrated or cracked by the antimicrobials, which are, indeed, made solely for the purpose of destruction of the bacterial surface or their metabolism. This conglomerate of matrix and microorganisms (fungi can also be present in the biofilms) requires the use of combined therapies containing antimicrobials and other ingredients that attack and break down the EPS in a first step. The products containing octenidine dihydrochloride-phenoxyethanol and cadexomer iodine make this apparent.
Conclusion
Wound biofilm requires a combined topical therapy because it consists of several ‘components’. It is important to realise that it can be found on more than 75% of all chronic wounds and has to be removed. Wound cleansing with irrigation solutions and compresses alone is not sufficient; it does not sustainably reduce the microbial load in biofilm infiltrated wounds. The persistence of biofilm causes wound healing to stagnate. In the translational testing of the most established antimicrobials, only octenidine dihydrochloride-phenoxyethanol and PHMB showed (delayed) efficacy against it. The wound dressing containing cadexomer iodine exhibited a very good anti-biofilm effect. Regardless of the topical therapy, however, the underlying disease that leads to the persistence of the wound has always to be treated primarily. Accordingly, a biofilm-covered, severely exuding wound is also not a contraindication for medically indicated compression therapy (e.g. in CVI, lymphoedema or lipedoema).
Acknowledgements
I would like to thank all the scientists and technical assistants who have contributed to the various translational projects summarised here. The names of persons involved in each project are listed in the respective publications of the research group under References.
Conflict of interest
The authors declare no conflicts of interest.
Funding
The presented work was partly funded by the German Initiative Chronische Wunden e. V. (ICW) and by the German Research Foundation DFG (OP 207/11-1).
Author(s)
Ewa K Stuermer* Prof., MD
Department of Vascular Medicine, University Medical Center Hamburg-Eppendorf (UKE), Martini Street 52, 20246 Hamburg, Germany
*Corresponding author email e.stuermer@uke.de
References
- Noskin GA, Rubin RJ, Schentag JJ, Kluytmans J, Hedblom EC, Jacobson C, et al. National trends in Staphylococcus aureus infection rates: impact on economic burden and mortality over a 6-year period (1998–2003). Clin Infect Dis 2007;45(9):1132–1140.
- James GA, Swogger E, Wolcott R, Pulcini Ed, Secor P, Sestrich J, et al. Biofilms in chronic wounds. Wound Repair Regen 2008;16(1):37–44.
- Heyer K, Herberger K, Protz K, et al. Epidemiology of chronic wounds in Germany: analysis of statutory health insurance data. Wound Repair Regen 2016;24(2):434–442.
- Hachenberg T, Senturk M, Jannasch O, Lippert H. Postoperative wound infections. Pathophysiology, risk factors and preventive concepts. Anaesthesist 2010;59(9):851–866; quiz 867–858.
- Augustin M, Stuermer EK, Dissemond J, Gerber V, Gruber B, Morbach S, et al. Recommendations for the improvement of the health care services for people with chronic wounds in Germany. Wundmanagement 2020;14(1):357–365.
- Malone M, Bjarnsholt T, McBain AJ, James GA, Stoodley P, Leaper D, et al. The prevalence of biofilms in chronic wounds: a systematic review and meta-analysis of published data. J Wound Care 2017;26(1):20–25.
- Jockenhofer F, Gollnick H, Herberger K, Isbary G, Renner R, Stücker M, et al. Bacteriological pathogen spectrum of chronic leg ulcers: results of a multicenter trial in dermatologic wound care centers differentiated by regions. J Dtsch Dermatol Ges 2013;11(11):1057–1063.
- Flemming HC, Wingender J. The biofilm matrix. Nat Rev Microbiol 2010;8(9):623–33.
- Rembe JD, Stuermer EK. Modern wound antiseptics – indications and limitations, between knowledge, desire and doubt. Gefäßchirurgie 2020;25(4):272–276.
- Besser M, Dietrich M, Weber L, Rembe JD, Stuermer EK. Efficacy of antiseptics in a novel 3-dimensional human plasma biofilm model (hpBIOM). Sci Rep 2020;10(3):4792.
- Probst S, Apelqvist J, Bjarnsholt T, Lipsky BA, Ousey K, Peters EJG. Antimicrobials and non-healing wounds: an update. J Wound Management 2022;23 (3 Sup1):1–33.
- Spiliopoulou AI, Kolonitsiou F, Krevvata MI, Leontsinidis M, Wilkinson TS, Mack D, Anastassiou ED. Bacterial adhesion, intracellular survival and cytokine induction upon stimulation of mononuclear cells with planktonic or biofilm phase Staphylococcus epidermidis. FEMS Microbiology Lett 2012;330:56–65.
- James GA, Swogger E, Wolcott R, Pulcini Ed, Secor P, Sestrich J, Costerton JW, Stewart PS. Biofilms in chronic wounds. Wound Repair Regen 2008;16:37–44.
- Stuermer EK, Besser M, Debus ES, Smeets R, Dietrich M. Bacterial infiltration in biofilm-colonized wounds: analyses in the hpBIOM ex vivo wound model and possible impact on swabbing and debridement. J Wound Care 2023; 32:[accepted]
- Williams P, Winzer K, Chan WC, Camara M. Look who’s talking: communication and quorum sensing in the bacterial world. Philos Trans R Soc Lond B Biol Sci 2007;362(1483):1119–1134.
- Eickhoff MJ, Bassler BL. SnapShot: bacterial quorum sensing. Cell 2018;174:1328–1328.e1.
- Mukherjee S, Bassler BL. Bacterial quorum sensing in complex and dynamically changing environments. Nat Rev Microbiol 2019;17:371–382.
- Thurlow LR, Hanke ML, Fritz T, Angle A, Aldrich A, Williams SH, et al. Staphylococcus aureus biofilms prevent macrophage phagocytosis and attenuate inflammation in vivo. J Immunol 2011;186(11):6585–6596.
- Cowan T. Biofilms and their management: from concept to clinical reality. J Wound Care 2011;20(5):220, 222–226.
- Larsen T, Fiehn NE. Resistance of Streptococcus sanguis biofilms to antimicrobial agents. APMIS 1996;104(4):280–284.
- Percival SL, Salisbury AM, Chen R. Silver, biofilms and wounds: resistance revisited. Crit Rev Microbiol 2019;45(2):223–237.
- Stuermer EK, Besser M, Brill FHH, Geffken M, Plattfaut I, Severing AL, et al. Comparative analysis of biofilm models to determine the efficacy of antimicrobials. J Environ Health 2021;234:113744.
- Rembe JD, Huelsboemer L, Besser M, Stuermer EK. Antimicrobial hypochlorous wound irrigation solutions demonstrate lower anti-biofilm efficacy against bacterial biofilm in a complex in vitro human plasma biofilm model (hpBIOM) than common wound antimicrobials. Front Microbiol 2020;11:564513.
- Stuermer EK, Plattfaut I, Dietrich M, Brill FHH, Kampe A, Wienecke V, et al. In vitro activity of antimicrobial wound dressings on P. aeruginosa wound biofilm. Front Microbiol 2021;30;12:664030.
- Fitzgerald DJ, Renick PJ, Forrest EC, Tetens SP, Earnest DN, McMillan J, et al. Cadexomer iodine provides superior efficacy against bacterial wound biofilms in vitro and in vivo. Wound Repair Regen 2017;25:13–24.
- Roche ED, Woodmansey EJ, Yang Q, Gibson DJ, Zhang H, Schultz GS. Cadexomer iodine effectively reduces bacterial biofilm in porcine wounds ex vivo and in vivo. Int Wound J 2019;16:674–683.
- Strohal R, Dietrich S, Mittlböck M, Hämmerle G. Chronic wounds treated with cold atmospheric plasmajet versus best practice wound dressings: a multicenter, randomized, non-inferiority trial. Sci Rep 2022;7;12:3645.
- Plattfaut I, Besser M, Severing AL, Oplaender C, Stuermer EK. Plasma medicine and wound management: evaluation of the antibacterial efficacy of a medically certified cold atmospheric argon plasma jet. Int J Antimicrob Agents 2021;11:106319.
- Wang Y, Wang Y, Wang Y, Murray CK, Hamblin MR, Hopper DC, et al. Antimicrobial blue light inactivation of pathogenic microbes: state of the art. Drug Resist Updat 2017;33–35:1–22.
- Plattfaut I, Demir E, Fuchs P, Schiefer JL, Stuermer EK, Bruening AKE, et al. Characterization of blue light treatment for infected wounds: antibacterial efficacy of 420, 455 and 480nm light emitting diodes against common skin pathogens vs. blue light-induced skin cell toxicity. Photobiomodul Photomed Laser Surg 2021;39:339–348.
- Zhou Y, Chia HWA, Tang HWK, Lim SYJ, Toh WY, Lim XL, et al. Efficacy of low-level light therapy for improving healing of diabetic foot ulcers: a systematic review and meta-analysis of randomized controlled trials. Wound Repair Regen 2021;29:34–44.
- de Abreu PTR, de Arruda JAA, Mesquita RA Abreu LG, Diniz IMA, Silva TA. Photobiomodulation effects on keratinocytes cultured in vitro: a critical review. Lasers Med Sci 2019;34:1725–1734.
- Rahbar Layegh E, Fadaei Fathabadi F, Lotfinia M, Zare F, Mohammadi Tofigh A, Abrishami S, et al. Photobiomodulation therapy improves the growth factor and cytokine secretory profile in human type 2 diabetic fibroblasts. J Photochem Photobiol B 2020;210:111962.
- Briggs T, Blunn G, Hislop S, Ramalhete R, Bagley C, McKenna D, et al. Antimicrobial photodynamic therapy – a promising treatment for prosthetic joint infections. Lasers Med Sci 2018;33:523–532.
- Pérez C, Zúñiga T, Palavecino CE. Photodynamic therapy for treatment of Staphylococcus aureus infections. Photodiagnosis Photodyn Ther 2021;34:102285.
- Besser M, Schaeler L, Plattfaut I, Brill FHH, Kampe A, Geffken M, et al. Pulsed low-intensity laser treatment stimulates wound healing without enhancing biofilm development in vitro. J Photobiomod Photomed Laser Surg 2022;233:112504.
- Schultz G, Bjarnsholt T, James GA, Leaper DJ, McBain AJ, Malone M, et al. Consensus guidelines for the identification and treatment of biofilms in chronic nonhealing wounds. Wound Repair Regen 2017;25:744–757.
- Schwartz JA, Goss SG, Facchin F, Avdagic E, Lantis JC. Surgical debridement alone does not adequately reduce planktonic bioburden in chronic lower extremity wounds. J Wound Care 2014;23:S4, S6, S8.
- Dissemond J, Bültemann A, Gerber V, Motzkus M, Muenter KC, Erfurt-Berge C. Position document of the Initiative of Chronic Wounds e. V. (ICW) on the nomenclature of debridement of chronic wounds. Hautarzt 2022;73:369–375.
- Schwarzer S, Radzieta M, Jensen SO, Malone M. Efficacy of a topical wound agent methane-sulfonic acid and dimethylsulfoxide on in vitro biofilms. Int J Mol Sci 2021;22:9471.
- Cogo A, Quint BJ, Bignozzi CA. Restarting the healing process of chronic wounds using a novel desiccant: a prospective case series. Wounds 2021;33:1–8.
- Kadam S, Madhusoodhanan V, Dhekane R, Bhide D, Ugale R, Tikhole U, Kaushik KS. Milieu matters: an in vitro wound milieu to recapitulate key features of, and probe new insights into, mixed-species bacterial biofilms. Biofilm 2021;3:100047.
- Harrison F, Buckling A. Siderophore production and biofilm formation as linked social traits. ISME J 2009;3:632–4.
- Huebner NO, Matthes R, Koban I, Rändler C, Müller G, Bender C, Kindel E, Kocher T, Kramer A. Efficacy of chlorhexidine, polihexanide and tissue-tolerable plasma against Pseudomonas aeruginosa biofilms grown on polystyrene and silicone materials. Skin Pharmacol Physiol 2010;23(suppl 1):28–34.
- Watters CM, Burton T, Kirui DK, Millenbaugh NJ. Enzymatic degradation of in vitro Staphylococcus aureus biofilms supplemented with human plasma. Infect Drug Resist 2016;9:71–8.
- Lipp C, Kirker K, Agostinho A, James G, Stewart P. Testing wound dressings using an in vitro wound model. J Wound Care 2010;19:220–6.
- Agostinho AM, Hartman A, Lipp C, Parker AE, Stewart PS, James GA. An in vitro model for the growth and analysis of chronic wound MRSA biofilms. J Appl Microbiol 2011;111:1275–82.
- Woods J, Boegli L, Kirker KR, Agostinho AM, Durch AM, Delancey Pulcini E, Stewart PS, James GA. Development and application of a polymicrobial, in vitro, wound biofilm model. J Appl Microbiol 2012;112:998–1006.
- Werthén M, Henriksson L, Jensen PØ, Sternberg C, Givskov M, Bjarnsholt T. An in vitro model of bacterial infections in wounds and other soft tissues. APMIS 2010;118:156–64.
- Secor PR, James GA, Fleckman P, Olerud JE, McInnerney K, Stewart PS. Staphylococcus aureus biofilm and planktonic cultures differentially impact gene expression, MAPK phosphorylation, and cytokine production in human keratinocytes. BMC Microbiol 2011;11:143.
- Tankersley A, Frank MB, Bebak M, Brennan R. Early effects of Staphylococcus aureus biofilm secreted products on inflammatory responses of human epithelial keratinocytes. J Inflamm (Lond) 2014;11:17. eCollection 2014.
- Kirker KR, James GA, Fleckman P, Olerud JE, Stewart PS. Differential effects of planktonic and biofilm MRSA on human fibroblasts. Wound Repair Regen 2012;20:253–61.
- Flemming HC, Wingender J. The biofilm matrix. Nat Rev Microbiol 2010;8:623–33.
