Volume 25 Number 2
Efficacy and safety of EHO-85-based hydrogel for anal fissure treatment: a pilot open-label clinical trial
Diana Petrisor, Zlatka Etropolska, Mariya Armova, Stefan Dimitrov, Petar Yanev, Félix Berrocal
Keywords anal fissure, EHO-85, Olea europaea leaf extract-based hydrogel
For referencing Petrisor D et al. Efficacy and safety of EHO-85-based hydrogel for anal fissure treatment: a pilot open-label clinical trial. Journal of Wound Management. 2024;25(2):64-71.
DOI
10.35279/jowm2024.25.02.05
Submitted 22 November 2023
Accepted 11 March 2024
Abstract
Background Anal fissure (AF) is the most common cause of severe anorectal pain in adults, with an overall annual incidence of 0.1%. The primary objective of this pilot trial was to evaluate the safety and efficacy of an Olea europaea leaf extract (OELE)-based hydrogel (EHO-85), as a supportive treatment for acute AF episodes.
Methods Prospective, open-label, pilot study (May to October 2022). Adult patients with AF were recruited from three centers in Bulgaria and one in Romania. Patients were allocated 1:1 to each treatment using a computer-generated-based system and received either EHO-85 or customary supportive therapy (CST) for 60 days. The primary endpoint was the time to bleeding disappearance.
Results 42 patients were included by web-based allocation to receive EHO-85 (n=21) or CST (n=21). All patients completed the 60 days’ study. Patients reporting bleeding absence were significantly higher in the EHO-85 group at days 7 (p=0.032) and 15 (p=0.006). From day 29 or 36, no bleeding at defecation was reported in the EHO-85 and CST groups, respectively. On day 60, all subjects were bleeding-free. No adverse events were reported.
Conclusion EHO-85 was feasible to deliver and showed efficacy, safety, and apparent superiority in promoting and speeding up the healing process. In a future definitive trial, the sample size and diversity may be increased, and new objective methods may be considered.
Implications in clinical practice It highlights the combined wound-healing properties of EHO-85 as a promising noninvasive, cost-effective, and easy-to-apply alternative to current CSTs for AF wound repair.
Key messages
- OELE-based hydrogel (EHO-85)-combined antioxidant, moisturizing, and protective properties have proven useful in speeding up the wound-healing process.
- The study aimed to evaluate the safety and efficacy of EHO-85 annal fissure management.
- EHO-85 reduced the time to complete healing and decreased the levels of pain and discomfort at several time points when compared with the standard therapy.
- EHO-85 presents as a promising alternative to current AF treatments, allowing a more focused, faster, and efficient AF-wound healing.
Introduction
An anal fissure (AF) is a split or cut in the anal canal that starts below the dentate line extending to the anal verge, that can be linear or oval-shaped.1 In adults, it is the most common cause of severe anorectal pain.2 The typical symptoms are severe tearing pain with defecation frequently accompanied by hematochezia found on the stool or toilet paper.3 The anal pain, present in 90.8% of patients,4 may appear exclusively during defecation or it can last for several minutes to hours afterwards.1 When AFs fail to heal within six to eight weeks, they are considered chronic (CAFs).1 Usually, CAFs feature exposed fibers of internal anal sphincters at the base, together with hypertrophied anal papilla proximally, and a skin tag distally, known as sentinel pile.1,3 The overall annual incidence of AFs is 0.1%,5 with a similar frequency in both sexes,1 and it is estimated that around 10% of new patients referred to coloproctology clinics have CAFs.2
Although AF pathophysiology is not entirely clear, trauma to the anal canal, often resulting from the passage of hard stool due to constipation, seems to be an important factor.1 Other elements that may cause trauma are prolonged diarrhea, vaginal delivery, or anoreceptive intercourse.1,3
It is believed that acute injury leads to local pain and spasms of the internal anal sphincter (IAS).3 Moreover, the spasms typically entail an increase in the IAS pressure.1,3 In fact, resting pressures of the IAS have been found to be higher in patients with AFs than in normal controls.2 This increase in pressure further leads to a decrease in blood flow, ischaemia, and poor healing.3 It is proposed that if this cycle of recurring anal pain and bleeding remains unbroken, the fissure will persist, becoming chronic in up to 40% of patients.3,6 Moreover, wound chronicity has been associated with oxidative stress.7 Although the production of reactive oxygen species (ROS) is crucial to start wound repair, excessive amounts of ROS may cause oxidative damage, which is detrimental to wound healing.8
The initial approach to treat AFs, known as the conservative treatment, is based on the hypothesis of constipation, thus sitz baths and fiber supplementation are the core therapy, which can be complemented with topical steroids or topical anesthetics.9,10 If AFs persist, there are surgical options (including lateral sphincterotomy) and nonsurgical alternatives. The non-surgical options include pharmacological applications; e.g., topical minoxidil, isosorbide dinitrite 1% or botulinum.11-13 However, these treatments are not free from side effects.2,3,9,14,15 Consequently, it is reasonable to seek new non-invasive therapies with good tolerance that could be used as a supportive first-step approach to help overcome acute episodes of AFs, to reduce the need for pharmacological and surgical alternatives.
In this context, Olea europaea leaf extracts (OELE), widely cultivated in Mediterranean countries, but also in the Arabian Peninsula, and throughout the Indian and Asian continents,16 have attracted much attention. This is becuase of their reported, antioxidant, hypoglycemic, antihypertensive, antimicrobial, and anti-atherosclerotic features.17 Particularly, taking advantage from its antioxidant phenolic compounds (oleuropein and hydroxytyrosol), OELE-based hydrogel (EHO-85*)18 has been used for wound healing. Apart from OELE, EHO-85 contains Fucocert®, glycerine, Carbopol 980®, Geogard Ultra®, disodium ethylenediaminetetraacetic acid (EDTA), triethanolamine, and purified water. Fucocert®, which is a polysaccharide, glycerine, and OELE are moisturisers. Moreover, Carbopol 980® provides a protective and isolating barrier for the ulcer bed. All together, these components reduce the alkalinity of the ulcer bed due to the overall slightly acidic pH. This gives EHO-85 the ability to modulate the environment of the cutaneous ulcers, thus promoting and speeding up the healing process. Therefore, it is indicated for pressure ulcers, lower limb ulcers, diabetic foot, and AFs.19
* ULKOX® OLE, Noventure SL, Barcelona, Spain; CE marking #0476
This pilot study aimed to address whether the treatment of AF wounds with EHO-85 was an appropriate and feasible trial design concerning safety and efficacy of EHO-85 in adult patients diagnosed with AFs. The primary objective was to assess the feasibility of conducting a definitive trial in terms of treatment adherence, but also in terms of efficacy of EHO-85 versus Customary Supportive Therapy (CST) in complete healing (defined as bleeding disappearance) associated with AFs. Additionally, the study aimed to evaluate the effectiveness of EHO-85 using the following quantitative objectives: (1) the proportion of patients that experienced bleeding disappearance, and (2) the time to pain reduction. Finally, safety of EHO-85 was also examined by means of adverse events and patient withdrawals.
Methods and materials
Study design
A prospective, open-label, double-arm multicenter clinical pilot study was carried out on adult patients with AF in three centers in Bulgaria. Ten patients were recruited from the Ambulatory Practice for Primary Medical Care Dr. Elenski, 20 from Medical Center Prolet and two from the Medical center Prolet. Ten patients were also recruited from the Medical Clinic Endodigest centre in Romania. The clinics involved were private medical and gastroenterological, having as investigators general practitioners, gastroenterologists, family doctors, and internal medicine specialists.
Forty-two study subjects were screened and enrolled by the above-mentioned investigators during consults at their individual cabinets and/or medical centers, between 15 June and 23 August 2022. All subjects provided written informed consent to participate before being screened for inclusion/exclusion criteria. The inclusion criteria were:
- age >18 years
- diagnosis of acute or chronic AF with an acute episode at the time of inclusion
- willingness to sign the Informed Consent form for data collection and processing
The exclusion criteria were:
- unwillingness to provide a signed Informed Consent for data collection
- other medical condition that does not allow participation in this study (e.g., immunodeficiency, patients under blood pressure medication that can induce a relaxation of the anal sphincter such as calcium channel blockers)
- current treatment with CST
- pregnant or breastfeeding patients
- current treatment that could have the same effect as the one proposed in the study (e.g., nitroglycerin, lidocaine hydrochloride, botulinum toxin, nifedipine, diltiazem)
- hypersensitivity or individual allergy to one or more components of the product
Based on the 84% fissure healing rate reported by Kenny et al, the sample size was estimated to detect half of that rate with a 5% risk of type I error and 80% power. Decreasing the type I error risk would have increased the minimum sample size. Moreover, estimating a lower or higher risk of fissure healing rate would have had an impact on the sample size.20
The enrolment was competitive, and patients were allocated with a 1:1 ratio, using a computer-generated number to indicate the type of treatment they would receive. This was in order to have 21 patients treated with Cicatridina® ointment as the Customary Supportive Therapy (CST) and 21 patients treated with EHO-85 as the experimental group, for the statistical analysis (see Figure 1). The study period per subject lasted for 60 days with treatment ending on day 42. The treatment prescription decision (EHO-85 or CST) was taken before each particpant was invited into the study; Each patient attended three visits during product administration, to collect information related to clinical efficacy and safety, and one follow-up visit at day 60.
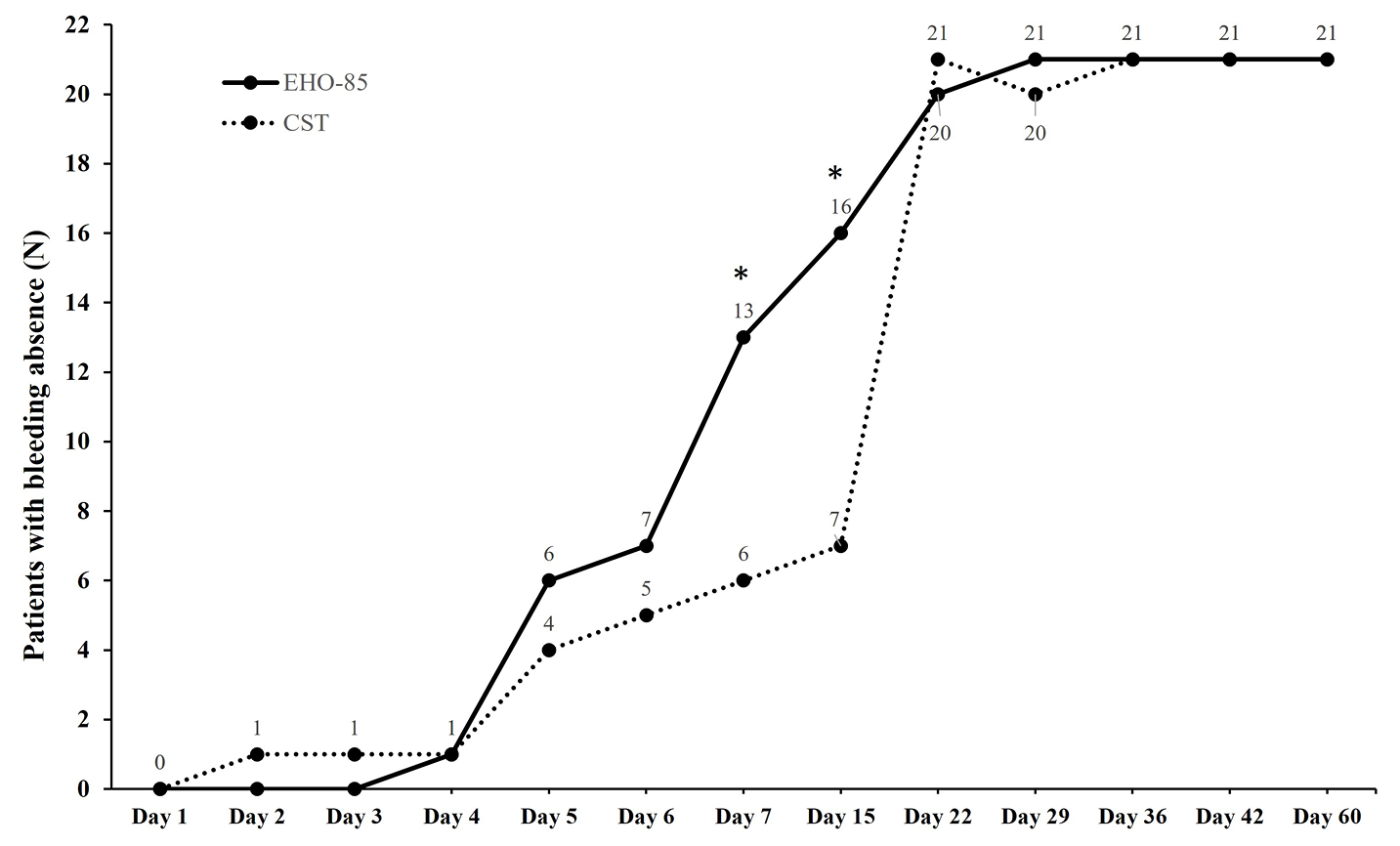
Figure 1. Number of patients with bleeding absence from day 1 to day 60. *p≤0.032. CST, customary supportive therapy.
The study was approved by the National Committee of Bioethics for Medicine and Medical Devices from Romania and by the Ethics Committee for Clinical Trials from the Republic of Bulgaria and performed according to the revised Declaration of Helsinki for biomedical research involving human subjects, the rules of Good Clinical Practice of the European Community CPMP (CPMP/ICH/135/195; ICH Topic E6) and of UNI EN ISO 14155:2012. Trail registration number: ISRCTN40732544, accessible at https://www.isrctn.com/ISRCTN40732544.
Intervention
Dosage schedule for the study products was implemented according to the practitioner’s prescription and based on the approved leaflet. In both EHO-85- and CST-treated groups, each subject received the prescribed treatment by topical route every day, three times per day (morning, noon, and evening) for 42 consecutive days. The patients were carefully instructed on how to correctly administrate the drug. Before application of the product, subjects had to clean the affected area with warm water and mild soap and dry. They were instructed that afterward, EHO-85 and CST should be applied to the anal region with one finger, using the tip of the finger to overcome the sphincter resistance. Patients had to wash their hands thoroughly before and after the application and, in case of need for application inside the rectum, they were told to use a cannula. As for dosage, the amount of product used in each application had to be sufficient to cover the affected area.
Study endpoints
The primary endpoint was the efficacy of EHO-85 vs CST which was evaluated by measuring the time to complete healing (defined as bleeding disappearance). This was a subjective evaluation carried out by means of a patient journal where the participants had to write down if bleeding was present or absent.
Regarding the secondary endpoints, those included
- the proportion of patients that experienced bleeding disappearance was again evaluated by means of a patient journal
- the time to pain reduction was evaluated by a visual analogue scale (VAS). VAS is a well-known validated subjective pain rating scale, scoring between 1 (no pain) and 10 (severe pain)21, 22
- the time to discomfort reduction evaluated by a daily 7-point Likert scale (1=totally unacceptable, 2= unacceptable, 3=slightly unacceptable, 4=neutral, 5=slightly acceptable, 6=acceptable, 7=perfectly acceptable)
- patient quality of life (QoL) by means of the General Health Survey Short Form (SF)-36 questionnaire.23
- treatment safety by evaluating the proportion of patients who experienced adverse events during the study period and the number of patients who withdraw due to adverse events occurring.
Timing
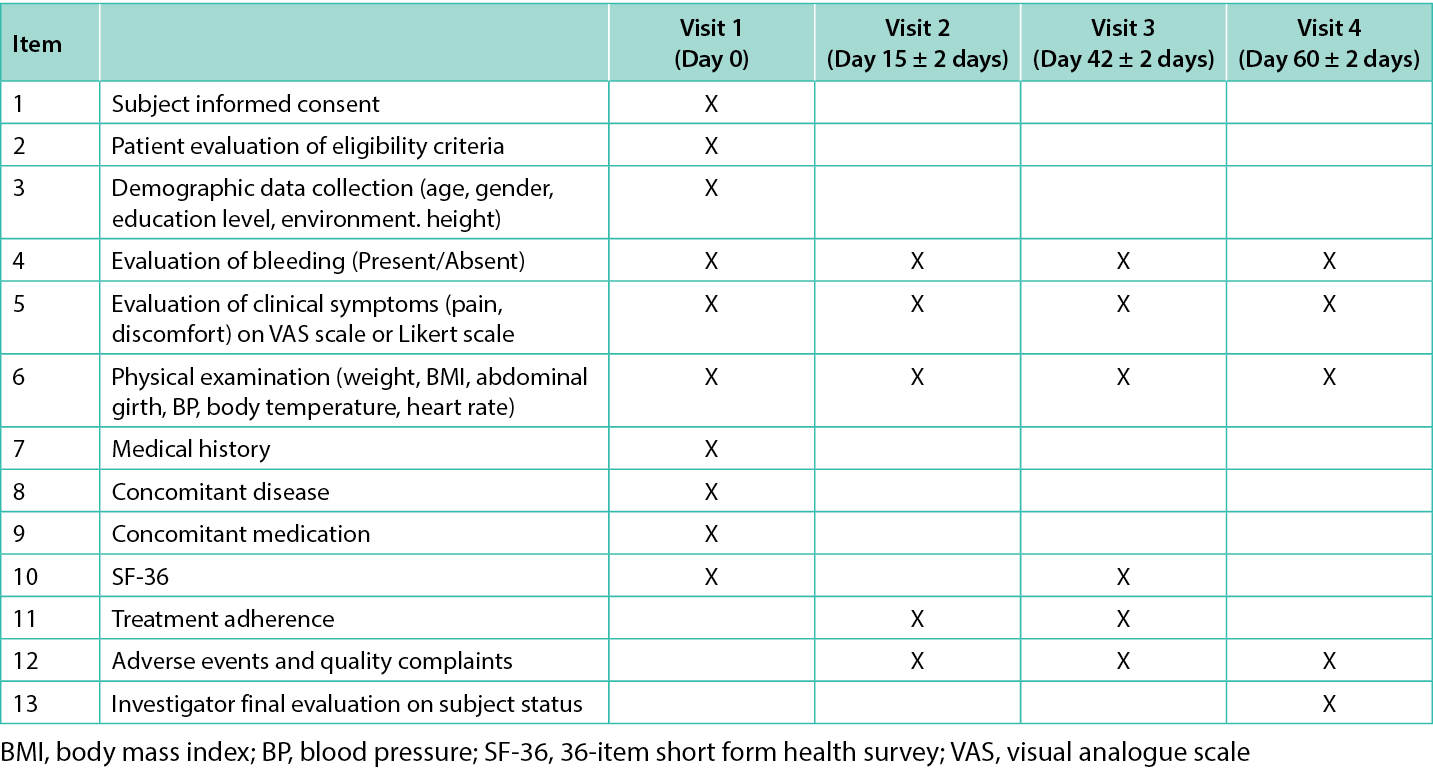
In total, four visits were carried out by each participant: at baseline (visit 1, day 0), visit 2 (day 15), visit 3 (day 42, end of treatment [EOT]), and visit 4 (day 60, end of study [EOS]). In visit 1, after signing the informed consent form, patient eligibility criteria were evaluated and demographic data (age, gender, education level, environment, and height) was collected. Data on previous medical history, concomitant diseases and medications were also obtained at baseline. Subjects reported daily through a patient journal if bleeding was present for the first seven days of treatment and then weekly until EOT (day 42) and at EOS (day 60).
Pain perception was reported daily for the first seven days of treatment and then weekly until EOT, discomfort was reported daily, and QoL was assessed in visits 1 and 3. Physical parameters (weight, body mass index, abdominal girth, blood pressure, body temperature, heart rate) were examined every visit. Treatment adherence was assessed in visit 2 and 3 whereas adverse events (AEs) and quality complaints were monitored from visit 2 to visit 4. Each visit’s checklist is presented in Table 1.
Table 1. Checklist of the items assessed at each visit.
Statistical analysis
Continuous data were expressed as the mean and standard deviation (SD) whereas categorical ones as the number of patients providing data at the relevant time point (n), frequency counts, and percentages. Percentages were calculated using N (number per treatment group or overall) as the denominator. Demographics and baseline disease characteristics were summarised by descriptive statistics. Comparisons between treatment groups were carried out with Mann-Whitney test. Statistical significance threshold was p<0.05. All statistical procedures were performed with SPSS® Statistics 27.0 software (IBM Corp, Armonk, NY).
Results
Study population
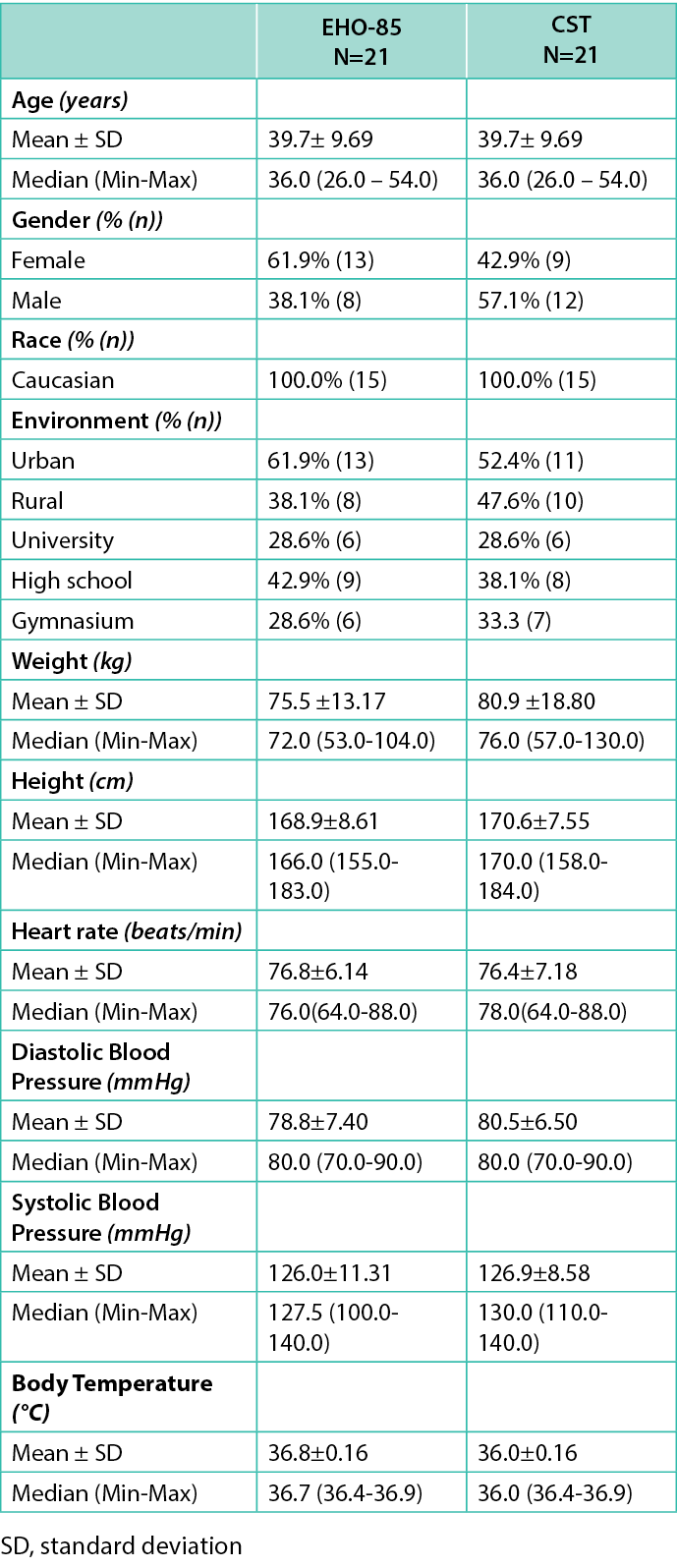
The study was performed between May 2022, and October 2022, with an enrolment period that lasted three months (study period of 60 days per subject). A total of 42 Caucasian patients (100% acceptability rate) were screened and enrolled (10 patients at the Romanian center and 32 at the Bulgarian ones), including 52.4% (n=22) female subjects (13 in the EHO-85 group). The mean age of the participants included in both groups was 39.7±9.7 years (range: 26.0–54.0). Both groups were similar in terms of weight, height, heart rate, and diastolic and systolic blood pressure. The patients’ demographic data are represented in Table 2.
Table 2. The patients’ demographic data
Primary endpoint
All 21 subjects in both arms were available for the intention-to-treat analysis. At day 1, all subjects presented bleeding at defecation (42/42 patients; p=0.317; Figure 1). At days 7 and 15, there were more subjects reporting bleeding absence at defecation in the EHO-85 group than in the CST group (13/21 vs 6/21 [p=0.032] and 16/21 vs 7/21 [p=0.006], respectively). From day 22 onwards, there were no statistically significant differences between groups regarding bleeding absence. Moreover, all the patients (21/21) reported bleeding absence from day 29 onwards in the EHO-85 group and from day 36 onwards in the CST group (21/21). Finally, during the follow-up visit on day 60, all subjects in both treatment groups reported bleeding absence at defecation (42/42 patients; p=1.000).
Secondary endpoints
As for the proportion of patients that experienced bleeding disappearance, the results are shown in Table 3. All 21 subjects in both arms were available for the intention-to-treat analysis. At day 7, there was a statistically significant difference between arms (p=0.030), when 61.9% (13/21) of subjects in the EHO-85 group reported bleeding absence at defecation while that figure was 28.6% (6/21) in the CST group.
Table 3. Proportion (%) of subjects that experienced bleeding disappearance from day 1 to day 60
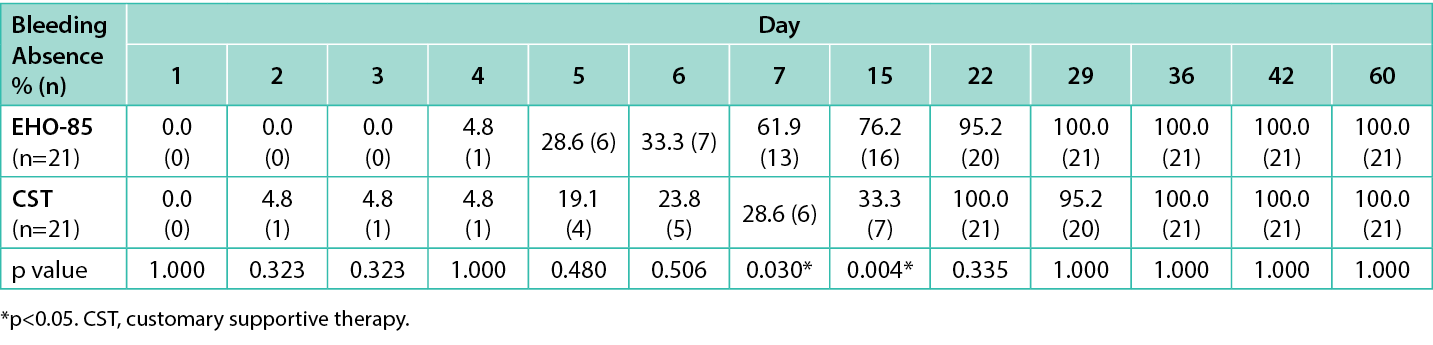
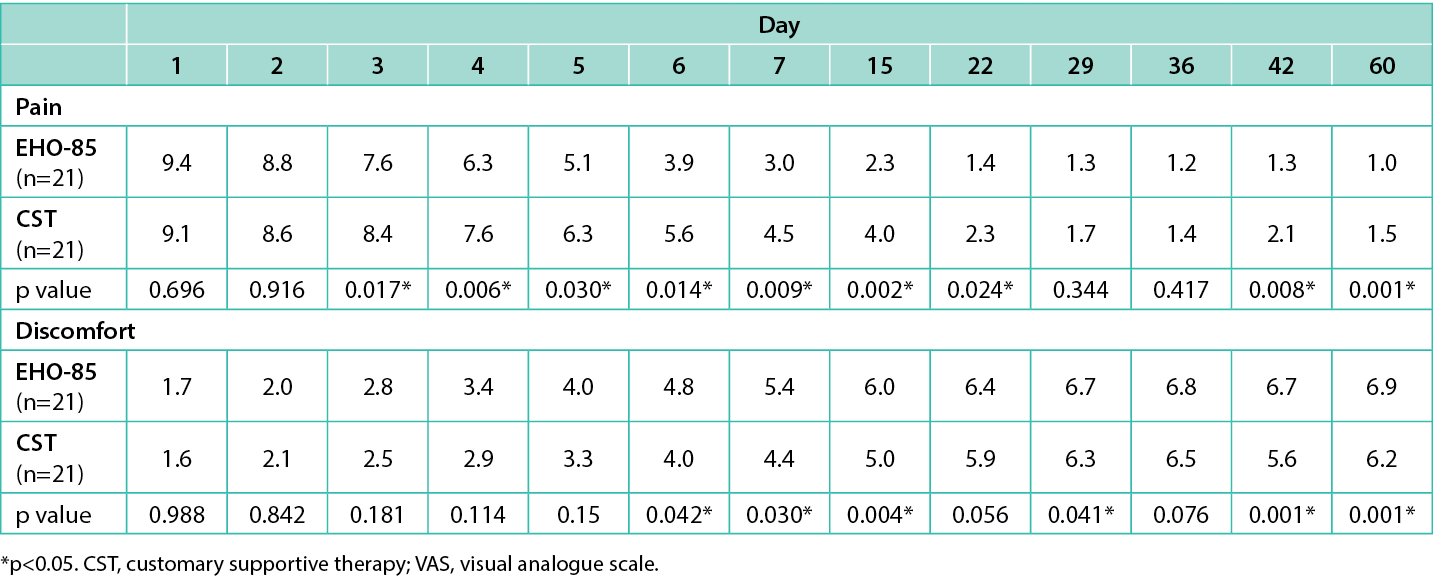
A significant difference was also found between groups at day 15 [76.2% (16/21) vs 33.3% (7/21), respectively; p=0.004]. Regarding pain reduction, at day 1, similar mean pain scores evaluated by VAS scale were observed in the EHO-85 and CST groups [9.38 vs 9.05, respectively (severe pain)]. Also, time to discomfort level reduction, evaluated by the 7-point Likert scale, was considered unacceptable for both groups (1.71 vs 1.62, respectively). However, there were statistically significant differences in pain reduction between patients treated with EHO-85 and with CST at days 3, 4, 5, 6, 7, 15, 22, 42, and 60 (p≤0.030; Table 4), and in discomfort level reduction at days 6, 7, 15, 29, 42, and 60 (p≤0.042; Table 4).
Table 4. Time to pain reduction evaluated by VAS scale and time to discomfort level reduction evaluated by 7-point Likert scale from day 1 to day 60.
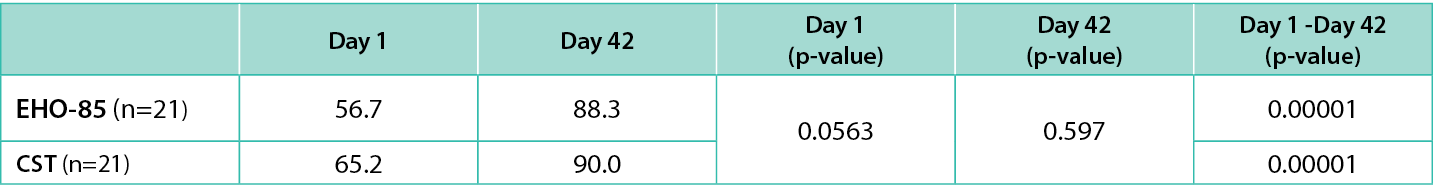
As for the QoL evaluation, no differences were found at day 1 or day 42 between groups, but the difference was significant between the day 1 and day 42 SF-36 values within the two treatment groups (56.7 vs 88.3 for the EHO-85 group and 65.1 vs 90.0 for the CST group; p<0.001 in both cases; Table 5).
Table 5. QoL (SF-36) evaluation Day 1 to Day 42.
Safety
No AEs were recorded during the study period and no quality complaints were reported either. No subject withdrew from the study or discontinued treatment due to AEs. All subjects were adherent to the therapy and did not miss any dose. Moreover, no allergic reactions to the product administration were reported.
Discussion
While conservative treatment intends to avoid constipation and pharmacological treatments mainly seek to decrease IAS pressure transiently,10 EHO-85 approaches AF by primarily targeting oxidative damage to improve healing. In this sense, some antioxidant compounds, such as curcumin, quercetin, or chitosan have shown efficacy in the wound healing process.24 Furthermore, OELE is an important source of non-enzymatic antioxidants with effective antioxidant activity against reactive species that has been demonstrated to have protective properties against oxidative damage to human erythrocytes.25
In preclinical studies, EHO-85 was shown to have important ROS scavenger capacity in vitro due to the high phenolic content of OELE. This makes it able to protect and promote the viability of the cells involved in the structure and regeneration of the skin.26 Also, the hydrogel proved to be a remarkable moisturiser with significant antioxidant and pH-decreasing effects. In addition, it presented superior wound healing rates compared to hydrocolloid dressing. In animals, EHO-85 has the ability to speed up hard-to-heal wound healing.18 Moreover, when compared with Indian/Asiatic pennywort, hyaluronic acid, or dexpanthenol-containing treatments, EHO-85 demonstrated equal or superior effect on tissue regeneration in a rat model of excisional wounds.27
Not only in preclinical studies, but in a randomised clinical trial (RCT), it was observed that the OELE-based hydrogel accelerated wound healing and was superior to the widely used VariHesive® amorphous hydrogel.28 Later, in 2023, from the same group of patients, the superiority of EHO-85 over the standard hydrogel VariHesive® was again tested in an observer-blinded RCT (MACAON), using only patients with difficult-to-heal diabetic foot-ulcers. Again, EHO-85 effect was superior to VariHesive®. A 51.6% of wound-area reduction was observed using the EHO-85 when compared to the 18.9% reduction with VariHesive® (p<0.001).29 In accordance, recent data demonstrated that EHO-85 allows an easier application and improved performance due to its particular shear thinning properties, when compared to VariHesive®, Intrasite, and Nu-gel hydrogels. The same study corroborated EHO-85 superiority versus VariHesive®, in the healing process of venous leg ulcers, pressure ulcers and diabetic foot ulcers. Indeed, during the RCT the average ulcer closure was 26% higher in patients treated with EHO-85 within two weeks of treatment (p=0.002), and the closure rate was three times higher for the same group (p<0.01) [30].
Here, we assessed the efficacy of the hydrogel in AF healing, a particular type of wound. In accordance with the previous studies, EHO-85 was easily administered by topical route. Moreover, the number and proportion of patients treated with EHO-85 who achieved bleeding absence was significantly higher than the ones treated with CST on days 7 and 15. All patients were free from bleeding from day 29 in the EHO-85 group and at day 36 in the CST group. Therefore, in this study, the time to complete healing (i.e., absence of bleeding) was shorter in the hydrogel group. Moreover, the pain and discomfort levels were lower in the EHO-85 compared to the CST arm at several time points, including EOT and EOS. These effects of the hydrogel on bleeding, pain, and discomfort were in line with the QoL assessment that yielded a significant improvement of 31.9 points between baseline and EOT; the difference in the CST group between those visits was of 24.9. The apparent superiority of the OELE-based hydrogel could be explained by its multiple wound-healing properties (i.e., moisturiser, antioxidant, and pH reduction in the wound bed),26,29 whereas CST acts on AF healing by means of a single Cicatridina® moisturising effect.31 Regarding safety, no AEs were reported in the study, which is in accordance with the safety results of the RCT where the hydrogel’s safety profile was considered excellent.28
There are a few limitations to the study’s findings that should be considered. Although this analysis yielded significant data, evidencing the strength of the product, with a good acceptance rate, a follow-up period of 60 days can be considered short to assess long-term efficacy and potential recurrences, as some reports describe high recurrent rates nine months after the initial healing.32,33 Moreover, patients with acute and also chronic AF with an acute episode, were included. Nonetheless, no distinction or sub-analysis was performed. Finally, the statistical significance threshold used for this pilot study might increase the risk of type I error, so further studies’ thresholds will be corrected in the protocol.
Even so, this 42-patient sample pilot study demonstrated significant effects in terms of pain and bleeding absence at defecation with no withdrawals observed. Hence, it supports the feasibility and acceptability of conducting a large and definitive double-blinded randomised study. A longer follow-up period and a broader sample of AF patients from medical registries including the elderly patients should be considered to assess potential recurrences and enhance generalisability. Finally, bleeding presence/absence is a widely used indicator of treatment efficacy as is one of the most common symptoms in AF disease.20,34,35 However, to increase validity of the results and assess complete healing, it will be interesting to couple these subjective parameters with some objective ones such as the assessment of complete epithelialisation, functional rectal capacity, inhibitory rectoanal reflex, and anal pressure.34–36
Conclusions
The OELE-based hydrogel studied here showed efficacy and safety for the treatment of acute episodes of AFs and might therefore be considered as an alternative to current CST. However, as open-label pilot studies are not sufficient to change clinical practice, randomised clinical trials are needed to confirm these results.
Implications for clinical practice
Clinicians need to appreciate that AFs are the most common causes of severe anorectal pain in adults. AFs typically take around six weeks to properly heal or, if not healed, can evolve into CAF. Also, it is important to acknowledge that standard therapies available can alleviate the symptoms by treating constipation, not being specifically focused on wound healing and modulation. Moreover, it also needs to be considered that more aggressive treatments are available, including surgical approaches, particularly in CAF cases. However, they are commonly associated with side effects and post-surgery complications.
Here, the importance of addressing wound-repair locally and accelerating AF healing is highlighted, and the beneficial effects of EHO-85 as an efficient, cost-effective, and easy-to-apply alternative therapy for AF wound repair are demonstrated. Although the results presented are only reflective of one pilot study, it is expected that these methods may serve as a template for bigger and more complex study designs addressing the effects of EHO-85 in AF-wound healing.
Acknowledgments
We thank Meisys for providing medical writing support.
Declarations
Competing interests: F.B. is an employee of Noventure, S.L. (Barcelona, Spain).
Conflict of Interest
Competing interests: FB is an employee of Noventure, SL. (Barcelona, Spain).
Funding
This research was funded by Noventure, S.L. (Barcelona, Spain). It was allocated to CRO and research committee fees and of authors’ honoraria.
Ethics approval
The study was performed according to the revised Declaration of Helsinki for biomedical research involving human subjects, the rules of Good Clinical Practice of the European Community CPMP (CPMP/ICH/135/195; ICH Topic E6) and of UNI EN ISO 14155:2012. The IRB numbers were 0859/11.07.2022 and DM/30.05.2022 for the three centers in Bulgaria and for the center in Romania, respectively.
Author contributions
FB participated in the interpretation of the results and oversaw the process as well as critically reviewed it. All authors have read and agreed to the published version of the manuscript.
Author(s)
Diana Petrisor1 MD, Zlatka Etropolska2, Mariya Armova3 MD, Stefan Dimitrov4, Petar Yanev5, Félix Berrocal* MD6
1Medical Center Endodigest, Oradea, Bihor, Romania
2Ambulatory practice for Primary Outpatient Medical Care SANA OOD, Sofia, Bulgaria
3Outpatient clinic for Individual practice for primary medical care Dr Elenski EOOD, Plovdiv, Bulgaria
4Medical Center Prolet EOOD, Ruse, Bulgaria
5Medical Center Prolet EOOD, Ruse, Bulgaria
6Noventure, S.L., Barcelona, Spain
*Corresponding author email fberrocal@noventure.com
References
- Beaty JS, Shashidharan M. Anal fissure. Clin Colon Rectal Surg. 2016 Mar; 29(1):30–37.
- Boland PA, Kelly ME, Donlon NE, Bolger JC, Larkin JO, Mehigan BJ, et al. Management options for chronic anal fissure: a systematic review of randomised controlled trials. Int J Colorectal Dis. 2020 Oct; 35(10):1807–1815.
- Schlichtemeier S, Engel A. Anal fissure. Aust Prescr. 2016 Feb; 39(1):14–17.
- Hananel N, Gordon PH. Re-examination of clinical manifestations and response to therapy of fissure-in-ano. Dis Colon Rectum. 1997 Feb; 40(2):229–323.
- Mapel DW, Schum M, Von Worley A. The epidemiology and treatment of anal fissures in a population-based cohort. BMC Gastroenterol. 2014 Jul 16; 14129.
- Villanueva Herrero JA, Henning W, Sharma N, Deppen JG. Internal anal sphincterotomy. Treasure Island (FL): StatPearls Publishing; 2023.
- Melguizo-Rodriguez L, de Luna-Bertos E, Ramos-Torrecillas J, Illescas-Montesa R, Costela-Ruiz VJ, Garcia-Martinez O. Potential effects of phenolic compounds that can be found in olive oil on wound healing. Foods. 2021 Jul 15; 10(7):1642.
- Cano Sanchez M, Lancel S, Boulanger E, Neviere R. Targeting oxidative stress and mitochondrial dysfunction in the treatment of impaired wound healing: a systematic review. Antioxidants (Basel). 2018 Jul 24; 7(8).
- Stewart DB, Sr., Gaertner W, Glasgow S, Migaly J, Feingold D, Steele SR. Clinical practice guidelines for the management of anal fissures. Dis Colon Rectum. 2017 Jan; 60(1):7–14.
- Arroyo A, Montes E, Calderon T, Blesa I, Elia M, Salgado G, et al. Treatment algorithm for anal fissure. Consensus document of the Spanish Association of Coloproctology and the Coloproctology Division of the Spanish Association of Surgeons. Cir Esp (Engl Ed). 2018 May; 96(5):260–267.
- Alvandipour M, Ala S, Khalvati M, Yazdanicharati J, Koulaeinejad N. Topical Minoxidil versus topical Diltiazem for chemical sphincterotomy of chronic anal fissure: a prospective, randomized, double-blind, clinical trial. World J Surg. 2018 Jul; 42(7):2252–2258.
- Arslan K, Erenoglu B, Dogru O, Turan E, Eryilmaz MA, Atay A, et al. Lateral internal sphincterotomy versus 0.25 % isosorbide dinitrate ointment for chronic anal fissures: a prospective randomized controlled trial. Surg Today. 2013 May; 43(5):500–505.
- Berkel AE, Rosman C, Koop R, van Duijvendijk P, van der Palen J, Klaase JM. Isosorbide dinitrate ointment vs botulinum toxin A (Dysport) as the primary treatment for chronic anal fissure: a randomized multicentre study. Colorectal Dis. 2014 Oct; 16(10):O360–366.
- Davids JS, Hawkins AT, Bhama AR, Feinberg AE, Grieco MJ, Lightner AL, et al. The American Society of Colon and Rectal Surgeons Clinical Practice Guidelines for the Management of Anal Fissures. Dis Colon Rectum. 2023 Feb 1; 66(2):190–199.
- Nyam DC, Pemberton JH. Long-term results of lateral internal sphincterotomy for chronic anal fissure with particular reference to incidence of fecal incontinence. Dis Colon Rectum. 1999 Oct; 42(10):1306–1310.
- Somova LI, Shode FO, Ramnanan P, Nadar A. Antihypertensive, antiatherosclerotic and antioxidant activity of triterpenoids isolated from Olea europaea, subspecies africana leaves. J Ethnopharmacol. 2003 Feb; 84(2-3):299–305.
- El SN, Karakaya S. Olive tree (Olea europaea) leaves: potential beneficial effects on human health. Nutr Rev. 2009 Nov; 67(11):632–628.
- Casado-Diaz A, La Torre M, Priego-Capote F, Verdu-Soriano J, Lazaro-Martinez JL, Rodriguez-Manas L, et al. EHO-85: A multifunctional amorphous hydrogel for wound healing containing Olea europaea leaf extract: effects on wound microenvironment and preclinical evaluation. J Clin Med. 2022 Feb 24; 11(5).
- Noventure SL. ULKOX® OLE leaflet. Accessed July 18, 2023. https://noventure.com/sites/default/files/2022-10/ulkox_ole_leaflet_rev.05_clean.pdf
- Kenny SE, Irvine T, Driver CP, Nunn AT, Losty PD, Jones MO, et al. Double blind randomised controlled trial of topical glyceryl trinitrate in anal fissure. Arch Dis Child. 2001 Nov; 85(5):404–407.
- Delgado DA, Lambert BS, Boutris N, McCulloch PC, Robbins AB, Moreno MR, et al. Validation of digital visual analog scale pain scoring with a traditional paper-based visual analog scale in adults. J Am Acad Orthop Surg Glob Res Rev. 2018 Mar; 2(3):e088.
- Todd KH, Funk KG, Funk JP, Bonacci R. Clinical significance of reported changes in pain severity. Ann Emerg Med. 1996 Apr; 27(4):485–489.
- Hawker GA, Mian S, Kendzerska T, French M. Measures of adult pain: Visual Analog Scale for Pain (VAS Pain), Numeric Rating Scale for Pain (NRS Pain), McGill Pain Questionnaire (MPQ), Short-Form McGill Pain Questionnaire (SF-MPQ), Chronic Pain Grade Scale (CPGS), Short Form-36 Bodily Pain Scale (SF-36 BPS), and Measure of Intermittent and Constant Osteoarthritis Pain (ICOAP). Arthritis Care Res (Hoboken). 2011 Nov; 63 Suppl 11S240–252.
- Comino-Sanz IM, Lopez-Franco MD, Castro B, Pancorbo-Hidalgo PL. The role of antioxidants on wound healing: a review of the current evidence. J Clin Med. 2021 Aug 13; 10(16).
- Lins PG, Marina Piccoli Pugine S, Scatolini AM, de Melo MP. In vitro antioxidant activity of olive leaf extract (Olea europaea L.) and its protective effect on oxidative damage in human erythrocytes. Heliyon. 2018 Sep; 4(9):e00805.
- Casado-Diaz A, Moreno-Rojas JM, Verdu-Soriano J, Lazaro-Martinez JL, Rodriguez-Manas L, Tunez I, et al. Evaluation of antioxidant and wound-healing properties of eho-85, a novel multifunctional amorphous hydrogel containing Olea europaea leaf extract. Pharmaceutics. 2022 Feb 1; 14(2).
- Torrecillas-Baena B, Camacho-Cardenosa M, Carmona-Luque MD, Dorado G, Berenguer-Perez M, Quesada-Gomez JM, et al. Comparative study of the efficacy of EHO-85, a hydrogel containing olive tree (Olea europaea) leaf extract, in skin wound healing. Int J Mol Sci. 2023 Aug 28; 24(17).
- Verdu-Soriano J, de Cristino-Espinar M, Luna-Morales S, Dios-Guerra C, Caballero-Villarraso J, Moreno-Moreno P, et al. Superiority of a novel multifunctional amorphous hydrogel containing Olea europaea leaf extract (EHO-85) for the treatment of skin ulcers: a randomized, active-controlled clinical trial. J Clin Med. 2022 Feb 25; 11(5).
- Verdu-Soriano J, Casado-Diaz A, de Cristino-Espinar M, Luna-Morales S, Dios-Guerra C, Moreno-Moreno P, et al. Hard-to-heal wound healing: superiority of hydrogel EHO-85 (containing Olea europaea leaf extract) vs. a standard hydrogel. a randomized controlled trial. Gels. 2023 Dec 8; 9(12).
- Verdu-Soriano J, de Cristino-Espinar M, Luna-Morales S, Dios-Guerra C, Casado-Diaz A, Quesada-Gomez JM, et al. EHO-85, Novel amorphous antioxidant hydrogel, containing Olea europaea leaf extract-rheological properties, and superiority over a standard hydrogel in accelerating early wound healing: a randomized controlled trial. Pharmaceutics. 2023 Jul 11; 15(7).
- Farma-Derma srl. Cicatridina® leaflet. Accessed July 18, 2023. https://www.cicatridina.com/products/12-products/13-ointment.html.
- Carapeti EAK, M.A.; McDonald, P.J.; Chadwick, S.J.D.; Melville, D.; Phillips, R.K.S. Randomised controlled trial shows that glyceryl trinitrate heals anal fissures, higher doses are not more effective, and there is a high recurrence rate. Gut. 1999; 44727–44730.
- Graziano ASL, L.; Lencinas, S.; Masciangioli, G.; Gualdrini, U.; Bosisio, O. Long-term results of topical nitroglycerin in the treatment of chronic anal fissures are disappointing. Tech Coloproctol. 2001; 5143–5147.
- Cundall JD, Gardiner A, Laden G, Grout P, Duthie GS. Use of hyperbaric oxygen to treat chronic anal fissure. Br J Surg. 2003 Apr; 90(4):452–453.
- Parellada C. Randomized, prospective trial comparing 0.2 percent isosorbide dinitrate ointment with sphincterotomy in treatment of chronic anal fissure: a two-year follow-up. Dis Colon Rectum. 2004 Apr; 47(4):437–443.
- Tankova L, Yoncheva K, Muhtarov M, Kadyan H, Draganov V. Topical mononitrate treatment in patients with anal fissure. Aliment Pharmacol Ther. 2002 Jan; 16(1):101–103.
