Volume 1 Issue 1
Percutaneous transluminal angioplasty for central venous disease in dialysis patients: influence on cardiac function
Masataka Banshodani, Hideki Kawanishi, Sadanori Shintaku, Misaki Moriishi, Tetsumasa Yamashita, Rika Ago, Tomoyasu Sato, Shinichiro Tsuchiya
Abstract
Purpose: Increased vascular access flow after percutaneous transluminal angioplasty (PTA) for central venous stenosis and occlusion (central venous disease, CVD) can affect cardiac function in hemodialysis (HD) patients. We evaluated the cardiac function, etiology, and treatment in HD patients with CVD.
Methods: HD patients with CVD treated by PTA between June 2006 and February 2013 were studied.
Results: Of the 26 patients, 22 had left arteriovenous fistulas (AVFs), 1 left arteriovenous graft (AVG), 2 right AVFs, and 1 right AVG. CVD sites were the left brachiocephalic vein (LBCV; n=13), left subclavian vein (LSCV; n=7), both LBCV and LSCV (n=3), right BCV (n=2), and right SCV (n=1). Computed tomography findings indicated a high extrinsic compression rate for the LBCV (91%) and LSCV (50%). The success rate of PTA was 96%. The primary patency rates at 3, 6, 9, and 12 months were 81%, 73%, 65%, and 57%, respectively. The post-PTA brachial artery flow volume was significantly increased compared with the pre-PTA volume (1306 vs. 957 ml/min; p=0.005). The post-PTA left ventricular ejection fraction and expiration inferior vena cava diameter were the same as the pre-PTA values (57% versus 60%, p=0.2 and 17 versus 17 mm, p=0.9, respectively).
Conclusions: Our findings suggest that increased vascular access flow after PTA for CVD has no relation to cardiac function.
Reprinted from J Vasc Access; 2014 (6); 492-497 with permission of the Publisher.
INTRODUCTION
Central venous stenosis and occlusion (central venous disease, CVD) are common and serious complications of vascular access in hemodialysis (HD) patients and caused by the previous placement of central venous catheters (CVCs) (1, 2) or pacemaker wires (3, 4), extrinsic compression (5-7), or high flow and turbulent status resulting from the creation of an arteriovenous fistula (AVF) (8-10). CVD can result in swelling, pain, or the development of collateral veins and disrupts the HD access circuit by causing increased venous counter pressure at dialysis and access flow dysfunction (11-13). In these cases, percutaneous transluminal angioplasty (PTA) must be the primary treatment.
Cardiac mortality and morbidity rates have been demonstrated to be highly elevated in HD patients compared with those in healthy individuals (14). Several reports indicate that the HD procedure is associated with the development of regional left ventricular systolic dysfunction (15, 16) and sudden cardiac death (17). The flow of AVFs affects cardiac function (18). Moreover, high-flow AVFs are associated with an increased risk of cardiac failure (19). However, the associations between cardiac function and increased vascular access flow after PTA for CVD remain unclear.
In this study, we evaluated the influence of PTA on cardiac function as well as the etiology, treatment, and outcome of CVD in HD patients.
PATIENTS AND METHODS
Patient identification
This was a retrospective analysis of data from the Tsuchiya General Hospital undertaken between June 2006 and February 2013. Written informed consent for the procedure was obtained from all patients and patient anonymity was strictly maintained. However, informed consent for inclusion in the study was waived due to the observational nature of the study. We identified HD patients who underwent a first PTA for CVD. CVD was deemed to have occurred if any of the following criteria were met: 1) swelling or development of collateral veins in the affected ipsilateral extremity that sometimes extended to the head, neck, and chest wall on physical examination, and 2) radiological stenosis or occlusion of the subclavian vein, brachiocephalic vein, or superior vena cava. Computed tomography (CT) was indicated on the basis of the clinical signs of CVD in the patients. When clinical signs (e.g., swelling and collateral vein) were present, even if the blood flow was adequate, we performed CT and subsequently PTA for CVD, based on both the clinical signs of stenosis and radiological examination. Clinical manifestations were recorded for each patient after a medical chart review. Data were collected on demographic characteristics, vascular access type and location, HD vintage, history of dialysis CVC or pacemaker placement, and HD status at the diagnosis of CVD. The following data were also collected for 15 of the 26 patients: Kt/V (a urea-based measure of dialysis adequacy), normalized protein catabolic rate (nPCR), venous counter pressure at dialysis, brachial artery (BA) flow volume, and resistance index (RI) of the vascular access site as determined by ultrasonography, and left ventricular ejection fraction (LVEF) and expiration inferior vena cava (IVC) diameters as determined by echocardiography, using a General Electric VIVID 7 system with M4S probe (1.5-4.3 MHz), before and 1-2 weeks after PTA. Both evaluations were implemented before HD. RI is calculated as (peak systolic velocity − peak diastolic velocity)/peak systolic velocity. LVEF is derived from the LV end-diastolic and end-systolic volumes measured using the biplane modified Simpson’s rule and the area-length method, as recommended by the American Society of Echocardiography (20). The diameter of the IVC on expiration was measured approximately 1 cm distal to the right atrium (RA) in a long-axis view.
Pre-procedural assessment
Of the 26 study patients, 19 underwent CVD examination by contrast-enhanced CT angiography. Since January 2012, we performed contrast-enhanced CT angiography before PTA in 8 of 9 patients and determined treatment strategies.
Methods and techniques for percutaneous transluminal angioplasty
Using a transbrachial and/or transfemoral approach, balloon dilation with a semi-compliant balloon was performed. Stent placement was performed for CVD that was resistant or unresponsive to balloon dilation. Balloon and stent sizes were determined according to the diameter of the adjacent normal vein. Since December 2011, we have performed intravascular ultrasound (21, 22) and monitored intra-access pressure during the procedure. Even if good patency is provided by balloon dilation, we performed additional balloon dilation or stent placement when a significant pressure difference (≥5 mmHg) exists between the central and peripheral sides of the CVD lesion. Technical success was defined as less than 30% residual stenosis.
Short-term clinical outcomes
We reviewed post-procedural complications. Primary patency was defined as the length of time from angioplasty until reintervention, or the time of patency measurement.
Statistical analysis
Data were analyzed using the Statistical Package for the Social Sciences 14 software (SPSS Inc., Chicago, Illinois, USA) and expressed as either the number of participants or the percentage (%) of the study population. The remaining data were expressed as means and ranges. Kt/V, nPCR, venous counter pressure at dialysis, BA blood flow, RI, LVEF, expiration IVC diameter, and the intra-access pressure difference were analyzed by paired t-tests with statistical significance determined at p value less than 0.05.
RESULTS
Patient characteristics
Twenty-six HD patients with CVD were treated by PTA between June 2006 and February 2013. Patient characteristics are summarized in Table I. Seventeen men and nine women with a mean age of 69 (range, 40-96) years comprised the study population. The mean HD vintage was 75 (range, 1-188) months. The vascular access site was on the left forearm in 23 patients and on the right forearm in 3 patients. Of the 23 patients with a left forearm access, 22 had an AVF and 1 had an arteriovenous graft (AVG). Of the 3 patients with a right forearm access, 2 had an AVF and 1 had an AVG. The mean duration between vascular access creation and the first PTA for CVD was 56 (range, 1-177) months. Of the 26 patients, 13 initiated HD therapy with a CVC placed in the right (n=9) or left (n=4) internal jugular vein (IJV) before vascular access creation. Ipsilateral CVC placement was performed in only 6 patients (23%). The remaining patient initiated HD therapy with a CVC placed in the right femoral vein. No patient had a history of pacemaker wire placement. The sites of CVD were as follows: left brachiocephalic vein (LBCV; n=13), left subclavian vein (LSCV; n=7), both LBCV and LSCV (n=3), right BCV (RBCV; n=2), and right SCV (RSCV; n=1) (Fig. 1). Of the 26 patients, 9 had extremity swelling, and 9 had both extremity swelling and collateral veins in the ipsilateral extremity. The remaining 8 patients had mild extremity swelling, and in these patients, the blood flow was reduced before the PTA due to stenosis in the anastomosis lesions.
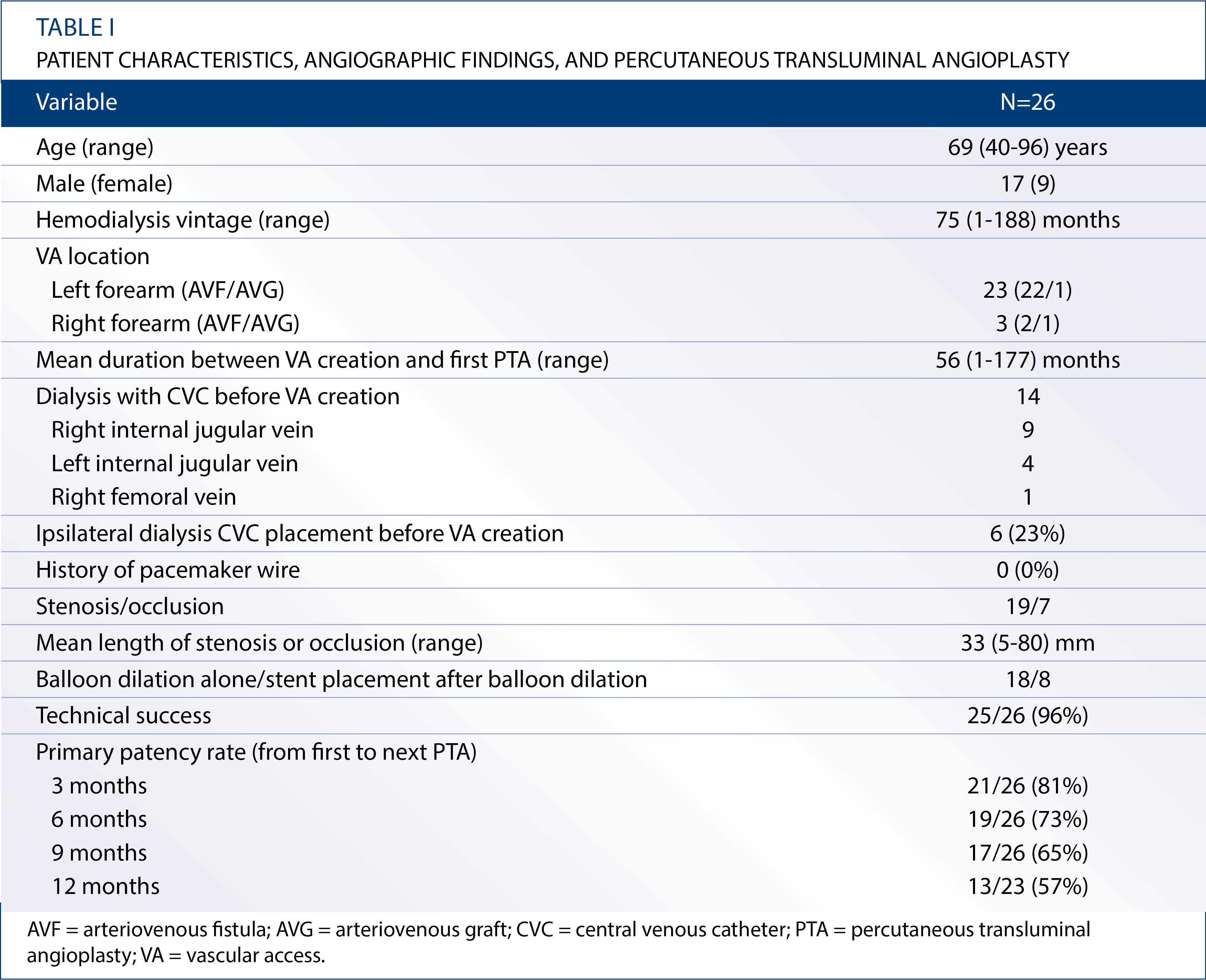
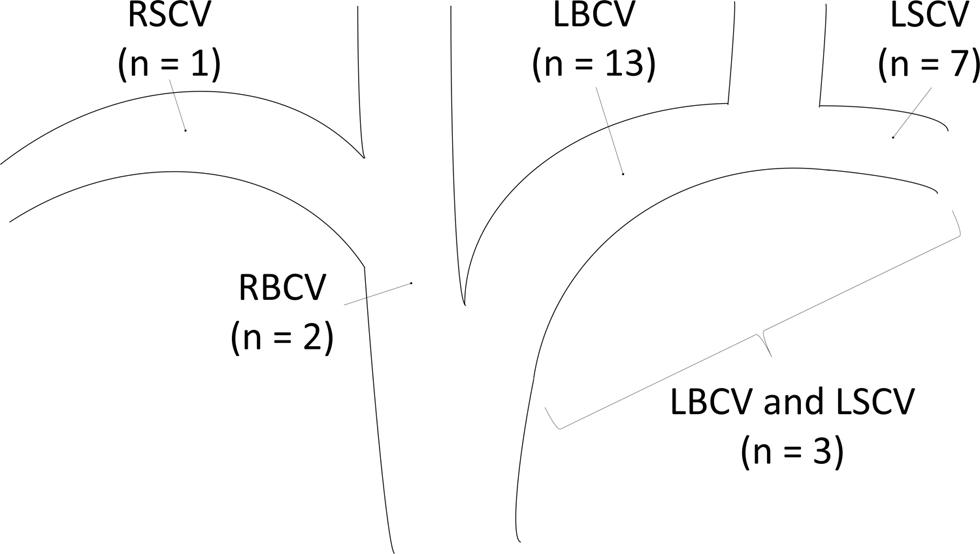
Figure 1. Distribution of central venous stenosis or occlusion. Values are expressed as the number of patients. LBCV = left brachiocephalic vein; LSCV = left subclavian vein; RBCV = right brachiocephalic vein; RSCV = right subclavian vein.
Hemodialysis status at the diagnosis of CVD
During HD, heparin was used as an anticoagulant, and high-flux membrane dialyzers were used in all cases. The mean blood flow during HD and the maximum interdialytic weight gain rate were 225 (range, 150-300) ml/min and 5.4% (range, 0%-12%), respectively. In all patients, systolic blood pressure stabilized at 90-160 mmHg during HD without the use of a vasopressor.
Contrast-enhanced computed tomography findings
Of the 11 patients with LBCV disease, compression of the LBCV by the brachiocephalic artery or ascending aorta was apparent in 10 (91%). Of the 6 patients with LSCV disease, compression of the LSCV between the clavicle and first rib was apparent in 3 (50%). Of the 2 patients with right CVD, compression of the central vein by the surrounding tissue was not apparent in either of them.
Angiographic findings and percutaneous transluminal angioplasty
PTA data are summarized in Table I. Of the 26 patients, 19 had stenosis and 7 had occlusion. The mean length of CVD was 34 (5-80) mm. Balloon size was 5 mm (n=3), 8 mm (n=5), 9 mm (n=3), 10 mm (n=7), 12 mm (n=5), 14 mm (n=1), or unknown (n=2), and the mean maximum balloon inflation pressure was 9.1 (2-16) atm. Of the 26 patients, 8 patients underwent stent placement after balloon dilation (LBCV, n=4; LSCV, n=1; LBCV and LSCV, n=1; RBCV, n=1; RSCV, n=1). Six self-expandable and two balloon-expandable stents were delivered. The stent size was 8 mm (n=1), 10 mm (n=5), 12 mm (n=1), or 14 mm (n=1). The mean intra-access pressure differences were significantly decreased from 32 (range, 20-62) to 1.4 (range, 0-4) mmHg by PTA (n=5; p=0.02).
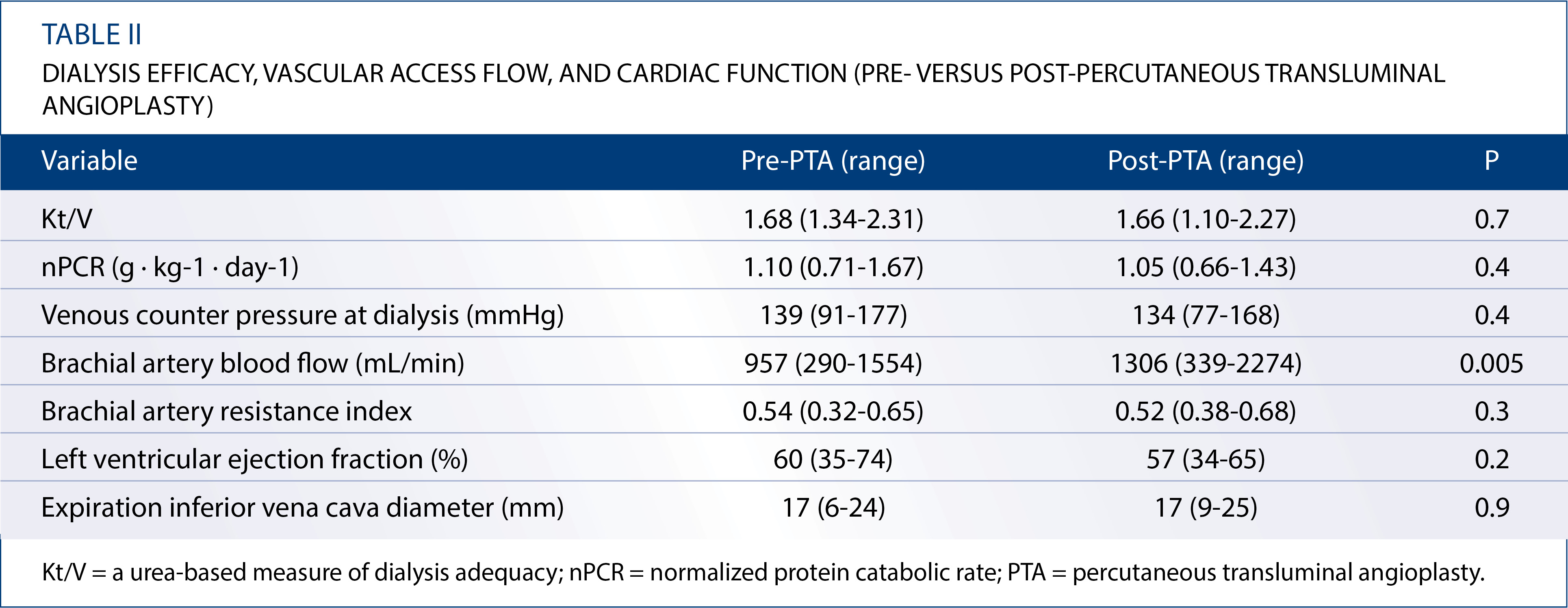
Dialysis efficacy, vascular access flow, and cardiac function (pre versus post-percutaneous transluminal angioplasty)
The pre and post-PTA dialysis efficacy, vascular access flow, and cardiac function of 15 of the 26 patients are summarized in Table II. Means of the following parameters were not significantly different before and after PTA: Kt/V (1.68 [range, 1.34-2.31] versus 1.66 [1.10-2.27], P=0.7), nPCR (1.10 [0.71-1.67] versus 1.05 [0.66-1.43] g/kg/day; p=0.4), and venous counter pressure at dialysis (mean for consecutive three times) (139 [91-177] versus 134 [77-168] mmHg; P=0.4). Compared with the pre-PTA value, the mean BA flow volume was significantly increased after PTA (957 [290-1554] versus 1306 [339-2274] ml/min; p=0.005). However, the mean BA RI (0.54 [0.32-0.65] versus 0.52 [0.38-0.68]; p=0.3), LVEF (60% [35%-74%] versus 57% [34%-65%]; p=0.2), and expiration IVC diameter (17 [6-24] versus 17 [9-25] mm; p=0.9) were the same before and after PTA.
Short-term clinical outcomes
In 25 out of the 26 patients (96%), the vascular accesses were salvaged by PTA without any complications, indicating technical and clinical success. In the remaining case, balloon dilation was stopped halfway through owing to the occurrence of chest pain. In all patients, the symptoms caused by CVD improved after PTA. The reintervention rate was 62% (16/26). The primary patency rate at 3, 6, 9, and 12 months was 81% (21/26), 73% (19/26), 65% (17/26), and 57% (13/23), respectively (Tab. I). The overall 90-day survival rate was 100%.
DISCUSSION
CVD is a significant complication in HD patients. The incidence of CVD has been reported to be in the range of 14%-40% in the literature (11). Strong association of CVD with previous placement of CVCs and pacemaker wires has been reported (13). However, in the present study, extrinsic compression is considered a fundamental factor for CVD because the rate of extrinsic compression of LBCV and LSCV was higher than that of previous CVC placement.
One of the largest studies on PTA for CVD, comprising 47 patients, showed a technical success rate of 77% and a primary patency rate of 58% at 3 months, 45% at 6 months, and 29% at 12 months (23). The results of PTA for CVD in our study were excellent by comparison.
Cardiac failure is a life-threatening complication in HD patients. Although satisfactory vascular access flow is necessary for dialysis adequacy, high vascular access flow induces high-output cardiac failure (19, 24). Horita et al reported that increased vascular access flow after PTA for CVD resulted in increased RA and right ventricle diameters, but these changes were completely recovered. Moreover, there were no significant pre and post-PTA differences in LVEF (25). However, the flow volume of the vascular access was not evaluated, and their study included only six patients. In the present study, although ipsilateral BA flow, which reflects vascular access flow, significantly increased after PTA compared with its pre-PTA value, the LVEF and expiration IVC diameter, which reflect RA pressure, were the same before and after PTA. These findings suggest that increased vascular access flow after PTA for CVD has no relation to cardiac function. The existence of collateral veins is believed to be the reason for the lack of a significant difference before and after PTA in dialysis efficacy, venous counter pressure at dialysis, and BA RI. The development of collateral veins may prevent the increase of venous counter pressure or BA RI due to CVD. Moreover, there were no significant hemodynamic changes after PTA, with the exception of minor increases in access flow. This relatively small change may explain the lack of observed cardiovascular changes.
This study has several limitations. First, this study was based on a group of patients being treated at a single center and the sample size was small. Second, the association between previous ipsilateral CVC placement and CVD was not examined sufficiently because at our institution, the CVC side was usually on the right and the vascular access side was usually on the left. Finally, the association between increased vascular access flow after PTA and cardiac function in HD patients with low cardiac function was not examined sufficiently. We believe that further studies with a larger sample size are needed to confirm our findings. However, given the small number of published papers that have examined outcomes including vascular access flow and cardiac function in HD patients with CVD, our study is valuable relative to the existing information in the field.
Among HD patients, CVD may be caused by extrinsic compression rather than previous ipsilateral dialysis catheter placement in the IJV. The results of PTA for CVD were comparatively excellent. Our findings suggest that increased vascular access flow after PTA for CVD has no relation to cardiac function.
Financial support: The authors have no financial disclosures to make.
Conflict of interest: The authors have no conflict of interest.
Address for correspondence:
Masataka Banshodani
Tsuchiya General Hospital
Hiroshima, Japan
Author(s)
Masataka Banshodani1, Hideki Kawanishi1,2, Sadanori Shintaku1, Misaki Moriishi1, Tetsumasa Yamashita1, Rika Ago1, Tomoyasu Sato3, Shinichiro Tsuchiya1 1. Department of Artificial Organs, Akane-Foundation, Tsuchiya General Hospital 3-30, Nakajimacho, Naka-ku, Hiroshima - Japan 2. Faculty of Medicine, Hiroshima University, 1-2-3 Kasumi, Minami-ku, Hiroshima - Japan 3. Department of Radiology, Akane-Foundation, Tsuchiya General Hospital 3-30, Nakajimacho, Naka-ku, Hiroshima - Japan
