Volume 4 Issue 1
Extended dwell peripheral catheters in patients with difficult venous access: Comparison of a peripheral intravenous catheter and midline catheter
Evan Alexandrou, Nicholas Mifflin, Craig McManus, Vanno Sou, Steven A Frost
Abstract
Introduction: Extending the traditional dwell of peripheral venous catheters from routine replacement has been shown to be successful without increasing risk to patient safety. One group of patients that can benefit from extending catheter dwell are those with difficult venous access, who may endure multiple, painful cannulation attempts. We report our experience on the use of two peripheral devices for extended dwell in patients with difficult venous access.
Methods: The devices chosen and available to the Australian market during the study period were the 100 mm PowerGlide® midline catheter and the 48 mm Nexiva® catheter in both 18 and 20 gauges. Catheter selection was based on ultrasound assessment of vessel depth from skin and diameter. Patient information and catheter characteristics were collected and presented as numbers, ranges and proportions. Non-parametric tests were used to assess differences in catheter groups where appropriate.
Results: Between November 2015 and August 2016, 192 patients had extended dwell peripheral catheters inserted. PowerGlide® catheters were the primary devices placed (n=161, 84%) compared to Nexiva® catheters and the basilic vein was the vessel of choice (n=175, 90%). Primary reasons for device placement were difficult venous access requiring infusion therapy as well as antibiotic therapy. Median dwell of all devices was six days (IQR 3–11 days) and 68% of devices (n=131) were removed for cessation of intravenous therapy.
Conclusion: The majority of devices were placed on first attempt and a number were successfully managed for weeks until cessation of therapy. Rates of phlebitis were minimal, and no infections were reported.
Keywords: Intravenous catheter, peripheral venous catheter, ultrasound.
Conflict of interest
Evan Alexandrou is a member of the Alliance for Vascular Access Teaching and Research (AVATAR) group, which has previously received unrestricted, investigator-initiated research or educational grants from product manufacturers (3M; Adhezion; AngioDynamics;
Baxter; BBraun; Becton Dickinson; CareFusion; Centurion Medical Products; Cook Medical; Entrotech; Medtronic; Smiths Medical).
Funding source
The authors wish to declare no funding was sought or received for this study.
Authors’ contributions
All authors have made substantial contributions to the study conception and design, acquisition of data and analysis and interpretation of data. Each author has contributed to drafting and editing the manuscript and approves the final version for publishing as per the International Committee of Medical Journal Editors (ICMJE) convention.
BACKGROUND
Peripheral intravenous catheters (PIVCs) are the most prevalent medical device used in health care settings across the world. It is estimated that over half of all patients admitted to hospital have a PIVC inserted to receive infusion therapy during an episode of hospitalisation1,2. Midline catheters (MCs) are not as common; however, new generation MCs are now increasingly being used in clinical settings as an alternative to a standard PIVC, or in lieu of a peripherally inserted central catheter (PICC) for peripherally compatible medication3.
PIVCs are known to have a high prevalence of complications that impact patient safety. Up to one in 10 hospitalised patients can suffer from phlebitis (that can include significant pain)1. Other complications include thrombophlebitis, infiltration and dislodgement4-6. Catheter-associated blood stream infection (CABSI) related to PIVCs is a serious adverse event and known to increase morbidity and mortality4,7. Although published rates of complications with MCs are lower than standard PIVCs, adverse outcomes from these devices have included upper extremity thrombosis, leaking at the insertion site and infection, the latter having similar rates to PIVCs8-10.
The mainstay strategy to reduce the incidence of PIVC infection and other complications such as phlebitis has been to routinely replace these devices11-13. A number of high-quality studies have found that routine replacement does not reduce the incidence of PIVC-related complications but, rather, sound insertion technique using a bundle approach, appropriate anatomical placement and catheter size, as well as good securement and dressing practices with ongoing surveillance, has the better outcomes2,5,6,14-17.
Previous investigations have found MCs to be advantageous in some clinical settings as extended dwell peripheral devices (EDPDs); however, they are more expensive than PIVCs and require clinicians with expertise to insert them — often with the use of ultrasound guidance3,8,9,18. Traditional MCs have historically been used as EDPDs; they are made of soft polyurethane, up to 20 cm in length and are trimmable; and they are inserted into the veins of the upper arm such as the cephalic, basilic and brachial veins8. New generation MCs are made of a more rigid material that is non-trimmable, they are 8–10 cm in length, with the shorter devices having the option of being placed below the elbow crease19,20.
The use of standard PIVCs as EDPDs has been adopted successfully by healthcare facilities without increasing the risk of CABSI or phlebitis when insertion and maintenance bundles are implemented with good educational support and surveillance17,21. Evidence-based guidelines also support the practice of PIVCs beyond 72–96 hours and removal when clinically indicated when appropriate processes have been set in place, particularly routine surveillance22-24.
There are a number of PIVCs and MCs suitable as EDPDs. These include shorter length devices (PIVCs) with stabilisation platforms and integrated extension sets (e.g. BD Nexiva® up to 45 mm / Delta Med Delta Ven® up to 48 mm) and new generation, longer length devices such as accelerated Seldinger technique (AST) MCs (for example, Bard PowerGlide® up to 100 mm / Access Scientific POWERWAND® up to 100 mm). All of the above devices have been found to extend dwell times without increasing complications19,20,25-27.
One cohort that can benefit from EDPDs are patients who experience difficult venous access (DiVa). One in three hospitalised patients are reported to have non-visible and non-palpable veins and typically experience numerous painful attempts at peripheral cannulation28,29. Ultrasound-guided cannulation with an EDPD can significantly improve first attempt success, reduce pain associated with the procedure and reduce the need for further cannulation attempts, or the need for a more invasive procedure such as the insertion of a central venous access device (CVAD)28,30.
An increasing presentation of patients with multi-morbidity, suffering from DiVa requiring infusion therapy was the catalyst for this initiative. Treating medical teams and nursing staff were reporting difficulty with cannulating patients, who consequently were referred to the hospital’s central venous access service (CVAS) for the placement of an ultrasound-guided vascular device. Not all referred patients required an invasive CVAD such as a PICC or centrally inserted central catheter, due to the nature of the infusate or expected period of infusion therapy.
The CVAS decided to implement an EDPD program for patients with DiVa with a combination of shorter length integrated PIVCs and new-generation MCs to reduce the need and risk of CVAD placement, and reduce delays for therapy, as well as reduce the number of cannulation attempts. The aim of this study is to report patient and device characteristics, and compare outcomes from the insertion of two EDPDs, based on an ultrasound assessment insertion algorithm, for patients experiencing DiVa.
METHODS
Study design and setting
The setting for this observational study is an 877-bed tertiary referral hospital in South West Sydney, Australia, that services a large geographic area; the emergency department has approximately 80,000 presentations annually. The CVAS reviewed products available to the Australian market that would be suitable as extended dwell devices. The devices’ appearance needed to look unlike PICCs to reduce confusion with treating teams and inadvertent infusion of peripherally incompatible medication (irritant antibiotics, vasopressors, or vesicant chemotherapy). The devices were required to be compatible with power injection of computerised tomography contrast.
Two products were selected after investigation of availability: the 100 mm PowerGlide® midline (Bard Access, Salt Lake City, UT) and the 45 mm Nexiva® closed catheter (Becton and Dickinson, Salt Lake City, UT). Sizes available and chosen as suitable were 18 and 20 gauges in both brands.
The choice of product was based on patient and vessel assessment. The CVAS adheres to evidence-based insertion practices that include appropriate sized veins to accommodate intravascular devices to reduce incidence of venous thrombosis31, as well as ensuring the maximal amount of catheter is placed inside the vein to reduce infiltration risk32.33. Therefore, vessel depth from skin, and vessel diameter were primary indicators for device choice. All devices were placed using ultrasound guidance.
An insertion algorithm was used to guide device choice. If veins were 10 mm (1 cm) or less from the skin (with ultrasound assessment) the BD 45 mm Nexiva® catheter was inserted as the first choice; if, however, the vessels were deeper, the 100 mm Bard PowerGlide® was placed. Catheter gauge was determined after ultrasound assessment of vessel diameter to ensure no more than one-third of the blood vessel was occupied by catheter to ensure adequate blood flow around the device to reduce thrombosis risk, as well as to increase dilution effect of infusate22. For vein diameter 4 mm or greater, an 18 G device was used, and for vein diameter 4 mm or less, a 20 G device was used31,34. All catheters were placed using a sterile technique that incorporated maximal barrier precautions, sterile gown and gloves, as well as mask and cap.
Patient demographics collected included gender, age, clinical category of patient and anatomical placement of device. Catheter characteristics included type of device inserted and gauge. Primary outcome of interest was length of device dwell and secondary outcomes were reasons for device removal to assess rates of failure in the catheter groups. Catheter-associated bloodstream infection (CABSI) was defined as sets of positive blood cultures from separate peripheral veins where no other sources of infection could be found35. Phlebitis was defined as the presence of pain, redness or swelling surrounding the device site13,36,37.
Patient data was collected through routine surveillance by the CVAS and entered into an operational database (Microsoft Access database — Microsoft Office Professional Plus 2010, Version 14.0.7128.5000), situated on a password-protected network drive of the hospital.
Hospital Human Research Ethics Committee approval was granted (reference number: LNR/15LPOOL/518) to review the characteristics and procedural outcomes of patients with difficult access requiring ultrasound-guided cannulation. The Standards for Quality Improvement Reporting Excellence version 2.0 (SQUIRE 2.0) guidelines for reporting improvements in health care were used to present the data38.
Statistical analysis
Data was analysed using the R language for statistical analysis (R Core Team Vienna, Italy). Descriptive statistics are presented as median (interquartile range, IQR) numbers, proportions, and per 1000 catheter days where appropriate. Non-parametric tests such as the Kruskal–Wallis test were used to assess differences in age and dwell time between catheter groups. Differences with categorical variables such as catheter outcomes and clinical specialty were tested using the chi-square test or Fishers exact test, and the time to event of catheter removal was calculated and presented using Kaplan-Meier analysis.
RESULTS
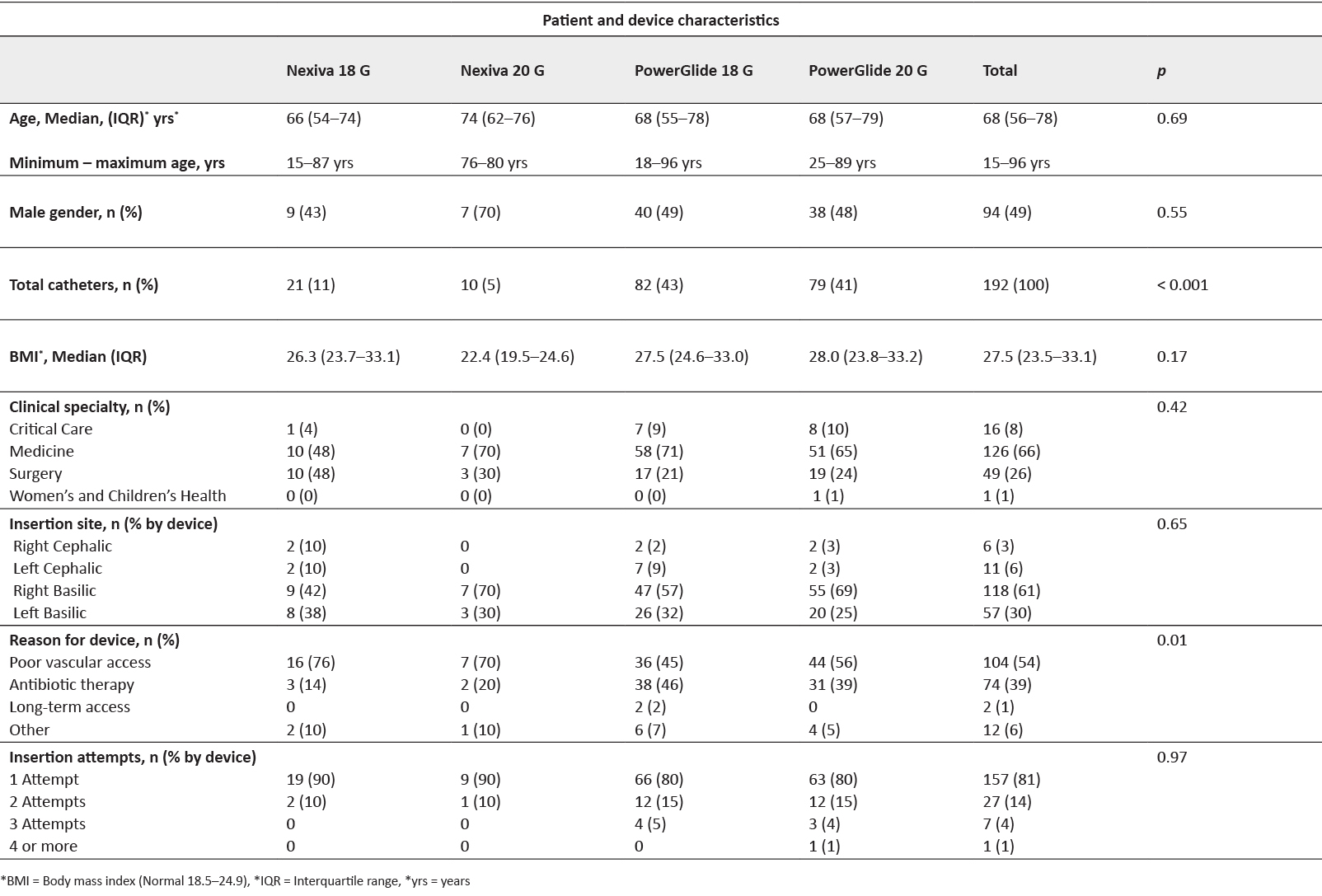
We present the characteristics of 192 patients with a median age of 68 years (IQR 56–78yrs), requiring an extended dwell peripheral catheter between November 2015 and August 2016 (Table 1). Results are presented and stratified in tables by catheter type and catheter gauge (4 groups: 2 x catheter gauge and 2 x catheter type). We found no difference in gender distribution amongst the population (n=94 males, 49%, p=0.55) or in body mass index (Median BMI 27.5, IQR 23.5–33.1,p=0.17), even though an elevated BMI was observed. Amongst this predominantly general medical and surgical population group, we found a difference in catheter choice with a higher number of PowerGlide® catheters inserted according to the insertion algorithm compared to Nexiva® devices. In fact, 4 in every 5 devices inserted were PowerGlide® catheters (PowerGlide® 18G=82, 43%, PowerGlide® 20G=79, 41%, p < 0.001).
The basilic veins of the upper arms were the primary choice for cannulation, these vessels comprised just over 90% of all catheter insertions (right basilic = 118, 61%, left basilic = 57, 30%, p=0.65). The primary reason for catheter insertion was difficult venous access requiring infusion therapy (n=104, 54%) followed by antibiotic therapy (n=74, 39%. p=0.01). Four in every five catheter insertions were successful at first attempt (n=157, 81%, p=0.97) with BD Nexiva® devices having a higher first attempt success rate (Table 1).
Table 1: Patient characteristics
We found the median dwell of all devices to be 6 days (IQR 3–11 days) with a total of 1645 catheter days (Table 2). Median dwell differed between the four catheter groups (p=0.01). PowerGlide®catheters were observed to have twice the dwell compared to Nexiva® catheters; this also included maximum days in dwell with the longest being a PowerGlide® 20 G catheter in dwell for 69 days. The longest a Nexiva® catheter (18 G) was in situ was 28 days (Table 2).
Table 2: Device outcomes
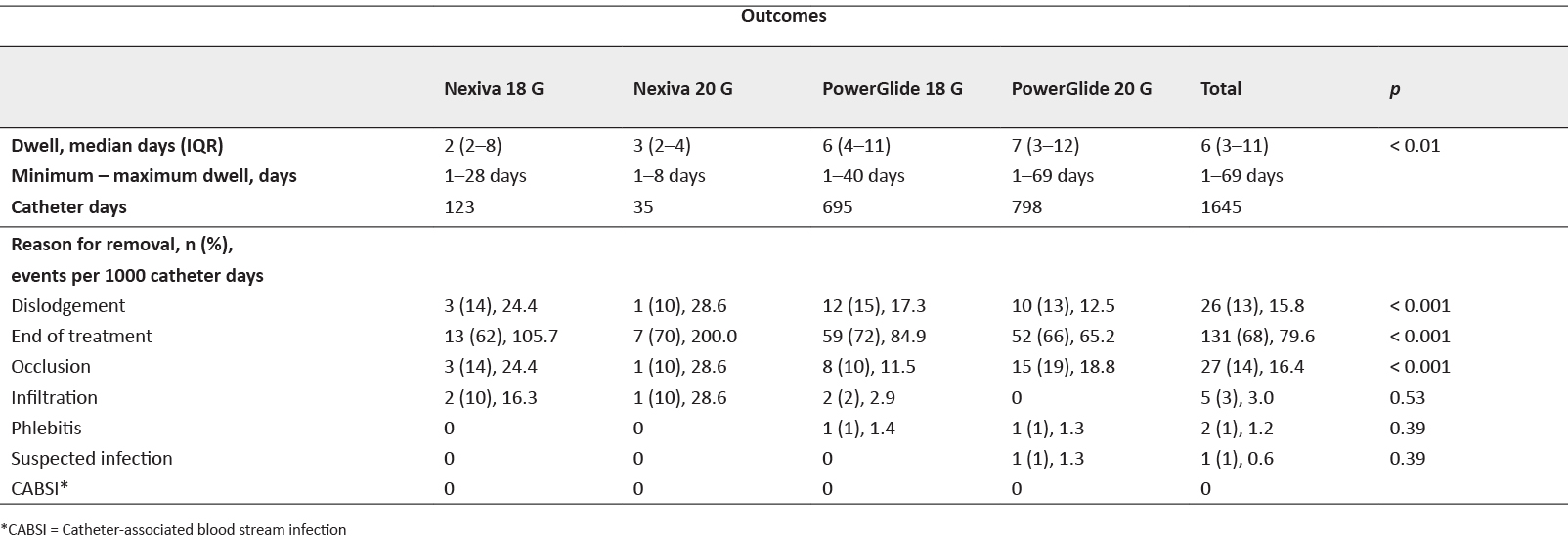
Overall, 131 catheters (68%) were removed for cessation of intravenous therapy (79.6 per 1000 catheter days); however, this differed amongst the observed groups. Nexiva® catheters had shorter median dwell (18 G = 2 days, 20 G = 3 days) compared to PowerGlide® catheters (18 G = 6 days, 20 G = 7 days, p < 0.01), but had greater observed survival per 1000 catheter days, although Nexiva®devices showed a higher probability of failure in the initial days of placement (Figure 1). The Kaplan-Meier analysis found no difference in survival rates between the four catheter groups (p=0.4).
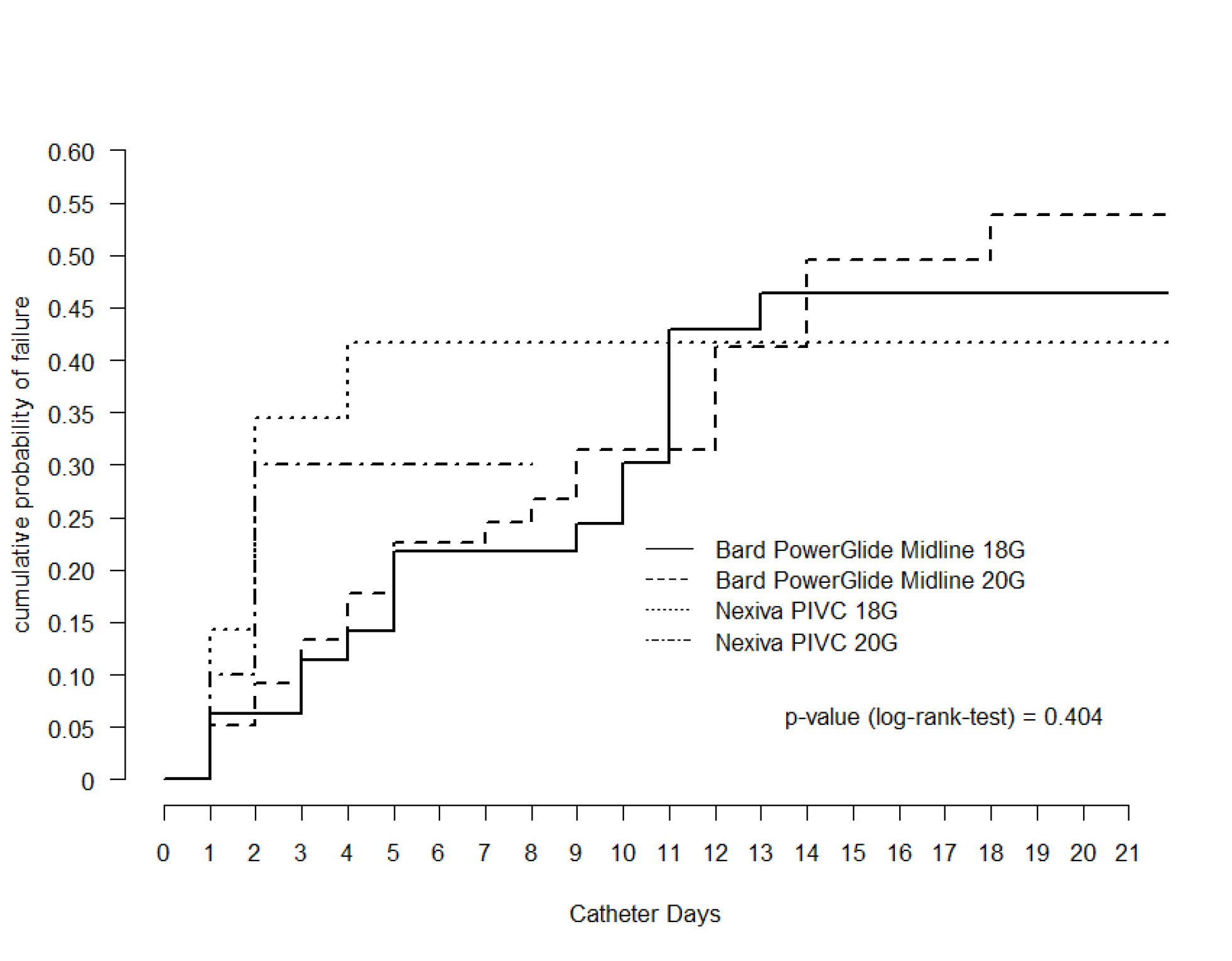
Figure 1: Kaplan-Meier analysis of time to event of catheter removal
We also observed one in every seven catheters inserted failed due to occlusion (n=26, 13%). This rate was highest with Nexiva® 20 G devices (n=15, 28.6/1000 catheter days, p < 0.001). Infiltration rate was higher in Nexiva® devices compared to PowerGlide® catheters and although one device was removed for suspected infection, no CABSI was observed and no symptomatic thrombosis was recorded. A low incidence of phlebitis was also recorded (n=1, 1%, p=0.39)
DISCUSSION
The insertion of EDPDs that include new-generation MCs have been used in practice in both adult and paediatric populations3,19,25. These devices, when appropriately inserted, secured and managed well can have a clinical and cost benefit by reducing the number of unnecessary cannulations per patient14,15,17,39. They are also a successful alternative to more invasive CVADs for peripherally compatible medication for intermediate term therapy20,40.
Choosing an appropriately sized peripheral device for extended dwell based on ultrasound-guided assessment has not been explored to date, to our knowledge. Both devices we placed had previously been used for extended dwell purposes (or remained in place until clinical indication for removal)19,25. We therefore wanted to assess the effectiveness of these devices in the DiVa cohort based on our insertion algorithm.
EDPDs placed according to vessel depth and diameter can be advantageous for DiVa patients who may incur multiple attempts by multiple personnel to gain peripheral venous access. The cost of this (sometimes futile) exercise is not only economic for the facility but also entails psychological and physical cost for the patient where nearly one in 10 PIVCs fail within eight hours of placement and nearly half within 24 hours, even with ultrasound for this cohort41-45.
The use of ultrasound allows access to veins situated deeper in the muscle tissue (particularly in the upper arms) that are not usually visible or palpable; however, the disadvantage is that catheters are more prone to movement and more likely to dislodge or occlude, as we observed with our PowerGlide® devices placed in deeper vessels44,45. Vessel depth from the skin is a known risk factor for catheter failure; therefore, longer length devices such as PowerGlide® can be beneficial for deeper veins3,32,46.
We chose the 10 mm depth from skin rule for catheter length selection to maximise catheter material within the vein. The higher number of PowerGlide® catheters used compared to the shorter Nexiva®catheters reflects the depth of vessels from the skin and above average BMI of the study group. These characteristics may also explain the slightly higher infiltration rate with the shorter 45 mm length Nexiva® catheters.
Just over 80% of devices were placed on the first attempt. We observed increased attempts with the longer PowerGlide® devices, and this was most likely due to the technical complexity associated with accessing deeper basilic veins where most devices were placed43,46,47. Preference for these upper arm veins was related to their larger diameter, the unlikelihood they had been accessed previously, and their anatomical position away from areas of high flexion44,46,48.
Dwell times differed between the catheter groups. We found PowerGlide® devices were in situlonger than Nexiva® catheters. One possible explanation could be related to patient complexity and the requirement for extended infusion therapy. Regardless of this difference, the majority of devices were in place until the cessation of intravenous therapy, and our observed failure rates were lower than have previously been described, particularly for shorter PIVCs1,4,6,37. This finding suggests that the use of integrated devices such as the Nexiva® may be appropriate for extended dwell (the longest we observed was 28 days) when placed in suitable patients (shallower vessels), at a significantly reduced cost compared to new-generation accelerated seldinger devices such as the PowerGlide®.
The leading concerns of extending the length of dwell of peripheral catheters is the risk of failure related to phlebitis and infection11-13. Most bacteraemia related to PIVCs occur shortly after insertion or removal, suggesting sub-optimal insertion and maintenance practices as the primary reason11,49. All our devices were inserted using sterile technique and cared for by clinical staff using evidence-based hospital policies. Some of the devices we placed remained in situ for a number of weeks with the incidence of phlebitis being minimal and no infections observed.
The results of this observational study may be subject to bias or confounding, and interpretation of superiority of product should not be considered as an outcome. The insertion algorithm we used assisted clinically in device choice; this may have influenced catheter survival rates but, without appropriate testing and adjustment, causation should not be assumed. Another important potential weakness of our data is that the catheters were not assigned randomly to patients. In particular, the outcomes of the catheters may have potentially been subject to indication bias where outcomes of specific catheters could have been influenced by other factors that may have been strong predictors of catheter choice by the inserting clinician.
CONCLUSION
The insertion of EDPDs has been successful at our institution. Most devices were placed on the first attempt and some were in place for many weeks. We observed minimal phlebitis and no infections over the study period. We found our approach to extend the dwell of integrated PIVCs until clinical indication for removal alongside MCs for DiVa patients to be a logical and sensible one. Future studies aimed at defining best practice approaches for managing DiVa patients are required.
Author(s)
Evan Alexandrou RN, BHealth, ICU Cert, MPH, PhD Liverpool Hospital, NSW, Australia Centre for Applied Nursing Research, Western Sydney University & South Western Sydney Local Health District, NSW, Australia Alliance for Vascular Access Teaching and Research Group, Menzies Health Institute Queensland, Griffith University, Brisbane, Qld, Australia South Western Sydney Clinical School & University of New South Wales, NSW, Australia Nicholas Mifflin RN, ICU Cert Liverpool Hospital, NSW, Australia Craig McManus RN, ICU Cert Liverpool Hospital, NSW, Australia Vanno Sou RN, ICU Cert Liverpool Hospital, NSW, Australia Steven A Frost RN, ICU Cert, MPH, PhD Liverpool Hospital, NSW, Australia Centre for Applied Nursing Research, Western Sydney University & South Western Sydney Local Health District, NSW, Australia South Western Sydney Clinical School & University of New South Wales, NSW, Australia Simpson Centre for Health Services Research and Ingham Institute of Applied Medical Research, Liverpool, NSW, Australia Corresponding author Evan Alexandrou, Western Sydney University Building EB, Ground Level Room 44, Parramatta South Campus Locked Bag 1797, Penrith South, DC 1797, NSW 2751, Australia Email: E.Alexandrou@westernsydney.edu.au Pages 20-24
References
- Alexandrou E, Ray-Barruel G, Carr PJ et al. International prevalence of the use of peripheral intravenous catheters. J Hosp Med 2015; 10(8):530–533.
- Ansel B, Boyce M & Embree JL. Extending short peripheral catheter dwell time: a best practice discussion. J Infus Nurs 2017; 40(3):143–146.
- Moureau N & Chopra V. Indications for peripheral, midline and central catheters: summary of the MAGIC recommendations. Br J Nurs 2016; 25(8):S15–S24.
- Zingg W & Pittet D. Peripheral venous catheters: an under-evaluated problem. Int J Antimicrob Agents 2009; 34:S38–S42.
- Marsh N, Webster J, Larson E, Cooke M, Mihala G & Rickard C. Observational study of peripheral intravenous catheter outcomes in adult hospitalized patients: a multivariable analysis of peripheral intravenous catheter failure. J Hosp Med 2017; E1–E7.
- Wallis MC, McGrail M, Webster J et al. Risk factors for peripheral intravenous catheter failure: a multivariate analysis of data from a randomized controlled trial. Infect Control Hosp Epidemiol 2014; 35(1):63–68.
- Pujol M, Hornero A, Saballs M et al. Clinical epidemiology and outcomes of peripheral venous catheter-related bloodstream infections at a university-affiliated hospital. J Hosp Infect 2007; 67(1):22–29.
- Alexandrou E, Ramjan LM, Spencer T et al. The use of midline catheters in the adult acute care setting–clinical implications and recommendations for practice. J Assoc Vasc Access 2011; 16(1):35–38.
- Adams DZ, Little A, Vinsant C & Khandelwal S. The midline catheter: A clinical review. J Emerg Med 2016; 51(3):252–258.
- Beard R. Complications associated with midline catheters. J Assoc Vasc Access 2015; 20(4):252–253.
- Mermel LA. Short-term peripheral venous catheter–related bloodstream infections: a systematic review. Clin Infect Dis 2017; 65(10):1757–1762.
- Powell J, Tarnow KG & Perucca R. The relationship between peripheral intravenous catheter indwell time and the incidence of phlebitis. J Infus Nurs 2008; 31(1):39–45.
- Cicolini G, Manzoli L, Simonetti V, et al. Phlebitis risk varies by peripheral venous catheter site and increases after 96 hours: a large multi-centre prospective study. J Adv Nurs 2014; 70(11):2539–2549.
- Rickard CM, Webster J, Wallis MC et al. Routine versus clinically indicated replacement of peripheral intravenous catheters: a randomised controlled equivalence trial. Lancet 2012; 380(9847):1066–1074.
- Webster J, Osborne S, Rickard CM & New K. Clinically-indicated replacement versus routine replacement of peripheral venous catheters. Cochrane Libr 2015.
- Carr PJ, Higgins NS, Cooke ML, Rippey J & Rickard CM. Tools, clinical prediction rules, and algorithms for the insertion of peripheral intravenous catheters in adult hospitalized patients: a systematic scoping review of literature. J Hosp Med 2017; 12(10).
- DeVries M, Valentine M, Mancos P. Protected clinical indication of peripheral intravenous lines: Successful implementation. J Assoc Vasc Access 2016; 21(2):89–92.
- Scoppettuolo G, Pittiruti M, Pitoni S et al. Ultrasound-guided “short” midline catheters for difficult venous access in the emergency department: a retrospective analysis. Int. J Emerg Med 2016; 9(1):3.
- Moureau N, Sigl G & Hill M. How to establish an effective midline program: a case study of 2 hospitals. J Assoc Vasc Access 2015; 20(3):179–188.
- Caparas JV, Hu J-P. Safe administration of vancomycin through a novel midline catheter: a randomized, prospective clinical trial. J Vasc Access 2014; 15(4):251–256.
- Crowell J, O’Neil K & Drager L. Project HANDS: a bundled approach to increase short peripheral catheter dwell time. J Infus Nurs 2017; 40(5):274–280.
- Gorski L, Hadaway L, Hagle M, McGoldrick M, Orr M & Doellman D. Infusion therapy standards of practice. J Infus Nurs 2016; 39(suppl 1):S1–S159.
- Loveday H, Wilson J, Pratt R et al. epic3: national evidence-based guidelines for preventing healthcare-associated infections in NHS hospitals in England. J Hosp Infect 2014; 86:S1–S70.
- O’Grady NP, Alexander M, Burns LA et al. Guidelines for the prevention of intravascular catheter-related infections. Clin Infect Dis 2011; 52(9):e162–e193.
- Gonzalez López J, Vilela AA, del Palacio EF, Corral JO, Martí CB & Portal PH. Indwell times, complications and costs of open vs closed safety peripheral intravenous catheters: a randomized study. J Hosp Infect 2014; 86(2):117–126.
- Pathak R, Patel A, Enuh H, Adekunle O, Shrisgantharajah V & Diaz K. The Incidence of central line–associated bacteremia after the Introduction of midline catheters in a ventilator unit population. Infect Dis Clin Prac 2015; 23(3):131.
- Bausone-Gazda D, Lefaiver CA & Walters S-A. A randomized controlled trial to compare the complications of 2 peripheral intravenous catheter-stabilization systems. J Infus Nurs 2010; 33(6):371–384.
- Sou V, McManus C, Mifflin N, Frost SA, Ale J & Alexandrou E. A clinical pathway for the management of difficult venous access. BMC Nurs 2017; 16(1):64.
- van Loon FH, Puijn LA, Houterman S & Bouwman AR. Development of the A-DIVA scale: a clinical predictive scale to identify difficult intravenous access in adult patients based on clinical observations. Medicine 2016; 95(16).
- Stolz L, Stolz U, Howe C, Farrell I & Adhikari S. Ultrasound-guided peripheral venous access: a meta-analysis and systematic review. J Vasc Access 2015; 16(4):321–326.
- Sharp R, Cummings M, Childs J et al. Measurement of vein diameter for peripherally inserted central catheter (PICC) insertion: an observational study. J Infus Nurs 2015; 38(5):351–357.
- Elia F, Ferrari G, Molino P et al. Standard-length catheters vs long catheters in ultrasound-guided peripheral vein cannulation. Am J Emerg Med 2012; 30(5):712–716.
- Reeves T, Morrison D &Altmiller G. A nurse-led ultrasound-enhanced vascular access preservation program. Am J Nurs 2017; 117(12):56–64.
- Sharp R, Cummings M, Fielder A, Mikocka-Walus A, Grech C & Esterman A. The catheter to vein ratio and rates of symptomatic venous thromboembolism in patients with a peripherally inserted central catheter (PICC): a prospective cohort study. Int J Nurs Stud 2015; 52(3):677–685.
- O’Grady NP, Alexander M, Burns LA et al. Guidelines for the prevention of intravascular catheter-related infections. Am J Infect Control 2011; 39(4):S1–S34.
- Ray-Barruel G, Polit DF, Murfield JE & Rickard CM. Infusion phlebitis assessment measures: a systematic review. J Eval Clin Pract 2014; 20(2):191–202.
- Helm RE, Klausner JD, Klemperer JD, Flint LM & Huang E. Accepted but unacceptable: peripheral IV catheter failure. J Infus Nurs 2015; 38(3):189–203.
- Ogrinc G, Davies L, Goodman D, Batalden P, Davidoff F & Stevens D. SQUIRE 2.0 (Standards for QUality Improvement Reporting Excellence): revised publication guidelines from a detailed consensus process. J Cont Edu Nurs 2015; 46(11):501–507.
- Tuffaha HW, Rickard CM, Webster J et al. Cost-effectiveness analysis of clinically indicated versus routine replacement of peripheral intravenous catheters. Appl Health Econ Health Policy 2014; 12(1):51–58.
- Anderson J, Greenwell A, Louderback J, Polivka BJ & Behr JH. Comparison of outcomes of extended dwell/midline peripheral intravenous catheters and peripherally inserted central catheters in children. J Assoc Vasc Access 2016; 21(3):158–164.
- Bauman M, Braude D & Crandall C. Ultrasound-guidance vs. standard technique in difficult vascular access patients by ED technicians. Am J Emerg Med 2009; 27(2):135–140.
- Robinson-Reilly M, Paliadelis P & Cruickshank M. Venous access: the patient experience. Support Care Cancer 2016; 24(3):1181–1187.
- İsmailoğlu EG, Zaybak A, Akarca FK & Kıyan S. The effect of the use of ultrasound in the success of peripheral venous catheterisation. Int Emerg Nurs 2015; 23(2):89–93.
- Fields JM, Dean AJ, Todman RW et al. The effect of vessel depth, diameter, and location on ultrasound-guided peripheral intravenous catheter longevity. Am J Emerg Med 2012; 30(7):1134–1140.
- Dargin JM, Rebholz CM, Lowenstein RA, Mitchell PM & Feldman JA. Ultrasonography-guided peripheral intravenous catheter survival in ED patients with difficult access. Am J Emerg Med 2010; 28(1):1–7.
- Mills CN, Liebmann O, Stone MB & Frazee BW. Ultrasonographically guided insertion of a 15-cm catheter into the deep brachial or basilic vein in patients with difficult intravenous access. Ann Emerg Med 2007; 50(1):68–72.
- Witting MD, Schenkel SM, Lawner BJ & Euerle BD. Effects of vein width and depth on ultrasound-guided peripheral intravenous success rates. J Emerg Med 2010; 39(1):70–75.
- Keyes LE, Frazee BW, Snoey ER, Simon BC & Christy D. Ultrasound-guided brachial and basilic vein cannulation in emergency department patients with difficult intravenous access. Ann Emerg Med 1999; 34(6):711–714.
- Sato A, Nakamura I, Fujita H et al. Peripheral venous catheter-related bloodstream infection is associated with severe complications and potential death: a retrospective observational study. BMC Infect Dis 2017; 17(1):434.
