Volume 4 Issue 1
Registered nurses’ experiences of using four methods of peripheral intravenous catheter dressings and securement during a randomised controlled trial. A survey.
Christine Woods, Gillian Ray-Barruel, Nicole Marsh, Julie Flynn, Emily Larsen, Claire M Rickard
Abstract
Background: Peripheral intravenous catheters (PIVC) have undesirably high failure rates (20−69%), despite billions used around the world annually. An assortment of dressing and securement methods has been designed to anchor these catheters and prevent PIVC complications or failure. However, there is inadequate evidence about optimal dressing and securement methods and how decisions for their use are made.
Objective: The study aim was to understand staff perceptions of four catheter dressing and securement methods tested in a randomised controlled trial (RCT), so as to provide context for the RCT findings.
Method: A printed survey was distributed among nurses involved with the trial at a large tertiary hospital in southeast Queensland, Australia.
Results: Of 150 surveys distributed, 109 (72.6%) surveys were completed and analysed. The majority of respondents came from the surgical department and had fewer than 5 years’ clinical nursing experience (44%). Covering the insertion site was the most important factor identified by staff when choosing a PIVC dressing securement approach (61%), followed by stabilising the cannula (34%). Nurses reported that all four dressing and securement arms in the trial had satisfactory perceived performance and acceptability.
Conclusion: Available supplies of PIVC dressings and securements change regularly due to new products or suppliers, which affects clinical practice. The use of a staff survey was a useful way to drill down and gather feedback from the nurses about the performance and acceptability of all four dressing and securement arms in the trial. Active inclusion and involvement of the nursing staff is an important element in the desired endpoint of translation of research evidence into practice.
Keywords: Peripheral intravenous catheters, dressings and securement devices, surveys, research translation.
Key points already known
• Peripheral intravenous catheter dressing and securement remains problematic in the clinical environment, with many devices failing before treatment completion.
• The use of audit tools, evaluations and surveys has been chosen to gain valuable knowledge on the performance of various dressing and securement devices for peripheral intravenous catheters.
• Inadequate evidence exists to inform decisions about optimal peripheral intravenous catheter dressing and securement.
What this paper adds
• Personal preferences and insights of staff nurses can illuminate factors that may affect the implementation of research findings into practice.
• Adequate site coverage and catheter stabilisation are recognised by staff nurses as integral to catheter dressing and securement performance, regardless of specific dressing choice.
INTRODUCTION
Peripheral intravenous catheters (PIVCs) are common in global health care, with billions used annually1. In Australia alone, approximately 14 million intravenous devices are used each year2. Dressing and securement products (either one product or multiple products used in combination) to secure PIVCs are essential; without a secure PIVC, subsequent device failure is likely. There is currently a plethora of PIVC dressing and securement products on the market and numerous polices pertaining to the care and management of these important devices. However, a recent Cochrane systematic review highlighted the inadequate evidence base that currently exists to inform decisions about optimal PIVC dressing and securement3.
In 2013−2015, a multicentre randomised controlled trial (RCT), Securing All intraVenous devices Effectively in hospitalised patients — the SAVE trial4, was undertaken to identify the most effective dressing and securement for PIVCs. Over a three-year period, 1709 adult patients were recruited from two large teaching hospitals in south-east Queensland. The aim of the RCT was to test the clinical efficacy and cost-effectiveness of four different PIV dressing and securement methods to prevent catheter failure. The four methods investigated were: 1. simple polyurethane dressing (SPU); 2. bordered polyurethane dressing (BPU); 3. sutureless securement device + simple polyurethane dressing (SSD); and 4. tissue adhesive + simple polyurethane dressing (TA).
Decisions about which dressing or securement devices to use can be ad hoc, with little structured decision making or clinical research base to explain these actions. Other studies have investigated catheter dressings and securement devices5, with a few studies also looking at staff satisfaction6. Trial results about efficacy may be limited in usefulness without understanding what drives the preferences and choices of nurses who apply and maintain PIVC dressings. Patients may inform performance also, and as known advocates for patients, nurses are the first point of call to receive this valuable feedback.
Previous research shows that staff preference is an integral component of decision making about PIVC dressings and securement7. Audit tools, evaluations and staff satisfaction surveys have been used to gain valuable knowledge on the performance of various dressing and securement devices for both peripheral and central catheters6,8-10. The overall findings of these studies illustrate the high value of staff input when introducing a new dressing and securement device for PIVCs. Giving staff the opportunity to rate their acceptability enables them to take part-ownership. When implementing a new method of PIVC dressings, nursing staff who are engaged and informed will be more motivated and invested in clinical practice compliance, leading to positive outcomes for both the organisation and the patient11.
Taking a planned, coordinated approach to the introduction of new clinical practice measures is essential. By exploring staff acceptability and performance of the dressing and securement methods tested in the RCT, it allows the researcher to use that knowledge to promote the research and translate it into practice while retaining responsibility for health care dollars. Preventing PIVC failure is an important patient outcome12.
In this paper, we report the findings of a survey undertaken to investigate staff acceptability of the different dressing and securement devices used in the four-arm RCT. The data gained from the survey can be used as translational research, that is, to assist with implementation and decision-making processes to improve hospital standards and quality within health care systems. The use of a survey allowed us to examine the discernments and insights of key stakeholders as factors that may affect decision making and implementation of evidence. The research knowledge gained can form the basis of an implementation plan, and therefore reach the patients for whom it was intended to assist.
METHODS
This sub-study concept was created within the original SAVE trial protocol. The aim of this sub-study was to explore nursing staff acceptability and performance of the different dressing and securement devices tested in the RCT, with the objective of providing evidence for decision making and implementation of research findings into clinical practice.
Design
The design of this sub-study was a questionnaire survey, in print format. The survey was designed to answer the following research questions:
- What do nurses consider is the most important characteristic of an IV dressing?
- What are nurses’ perceptions of the performance and acceptability of each of the dressing securement methods used in the trial?
- What is the ease and acceptability to nurses of trial paperwork and process?
Sampling
During nursing shift handover periods, the research nurse distributed the survey to a purposive sample of 150 staff working in the trial wards, encompassing medical, surgical, cancer care, coronary care, and infectious diseases. The research nurse provided information about the survey and allowed time for staff completion and questions. The surveys were designed for all levels of nursing staff, with the inclusion criteria being that staff had participated in the trial by caring for patients enrolled in the study. The surveys were voluntary and implied consent was given when completed.
Survey tool
The survey was constructed to assess nurses’ perceptions of the factors affecting their clinical practice regarding the acceptability and performance of the four PIVC dressing and securement methods used in the RCT. The survey was reviewed for clarity, readability and validity by experienced researchers and research nurses familiar with care of PIVC dressings, with some changes made to the original survey prior to distribution. The survey contained 12 questions in total. There were two questions about the respondents’ demographics and professional characteristics; one question about preferred characteristics of PIVC dressing; two questions about each of the four trial dressing and securement methods, asking respondents to rate separately the “performance” and “acceptability” on an 11-point scale of 0 (poor) to 10 (excellent), as well as provide free text comments about each product; one question on “ease and acceptability” of the trial paperwork and processes on an 11 point (0–10) scale as well as provide free text comments; and a final question inviting any further free text comments on either the trial or on PIVC dressing and securement. All responses were anonymous and confidential. The full survey is included in Appendix 1.
Ethical and other approvals
As part of the overall project, the trial (including survey) was registered with the ANZCTR (ACTRN12611000769987). Hospital and University ethics committee approvals were obtained prior to commencement (HREC/11/QRCH/152; NRS/46/11/HREC). Due to logistical reasons, the survey was distributed at only one of the two study hospitals involved in the RCT (Royal Brisbane and Women’s Hospital).
Analysis
Quantitative responses were tabulated and summarised as counts and proportions for categorical variables. Ordinal data was summarised as means/medians and standard deviations/interquartile ranges if normally/abnormally distributed. Responses were compared for the four study groups using ANOVA, with approximate value of < 0.05 considered significant. A simple descriptive review of the text comments was conducted by two researchers, CW and GRB.
RESULTS
Respondents
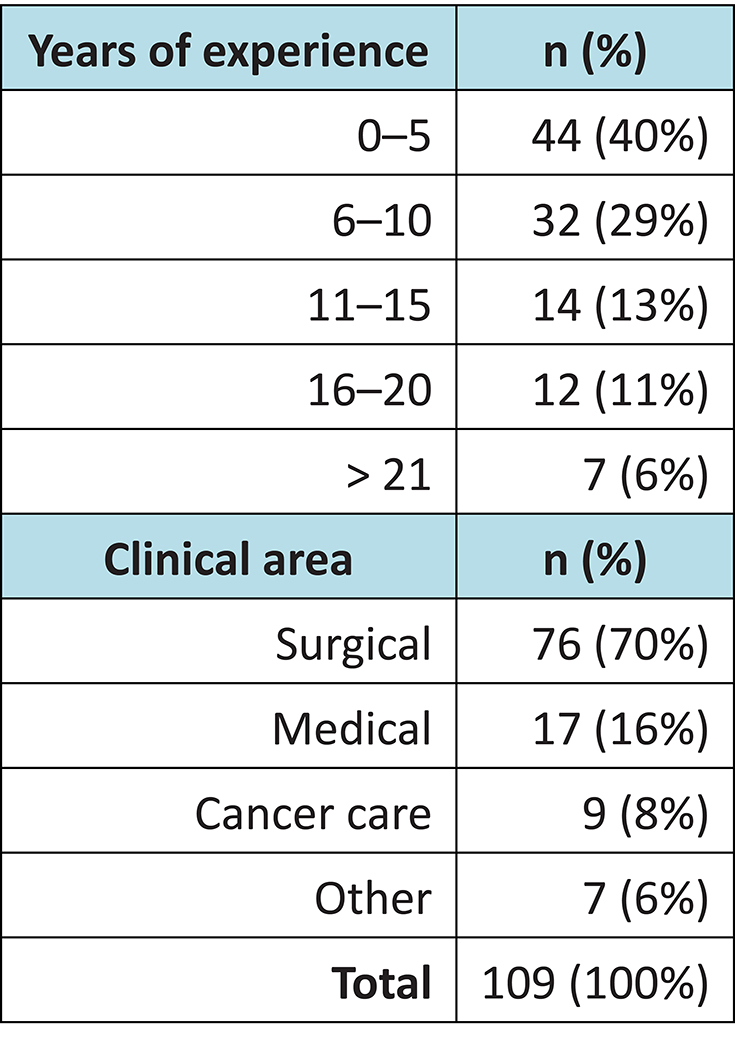
Of the 150 surveys distributed, 109 were returned: a response rate of 72.6%. The majority of staff came from the surgical department and had fewer than five years’ clinical nursing experience (Table 1).
Table 1: Demographic overview of survey respondents (n=109)
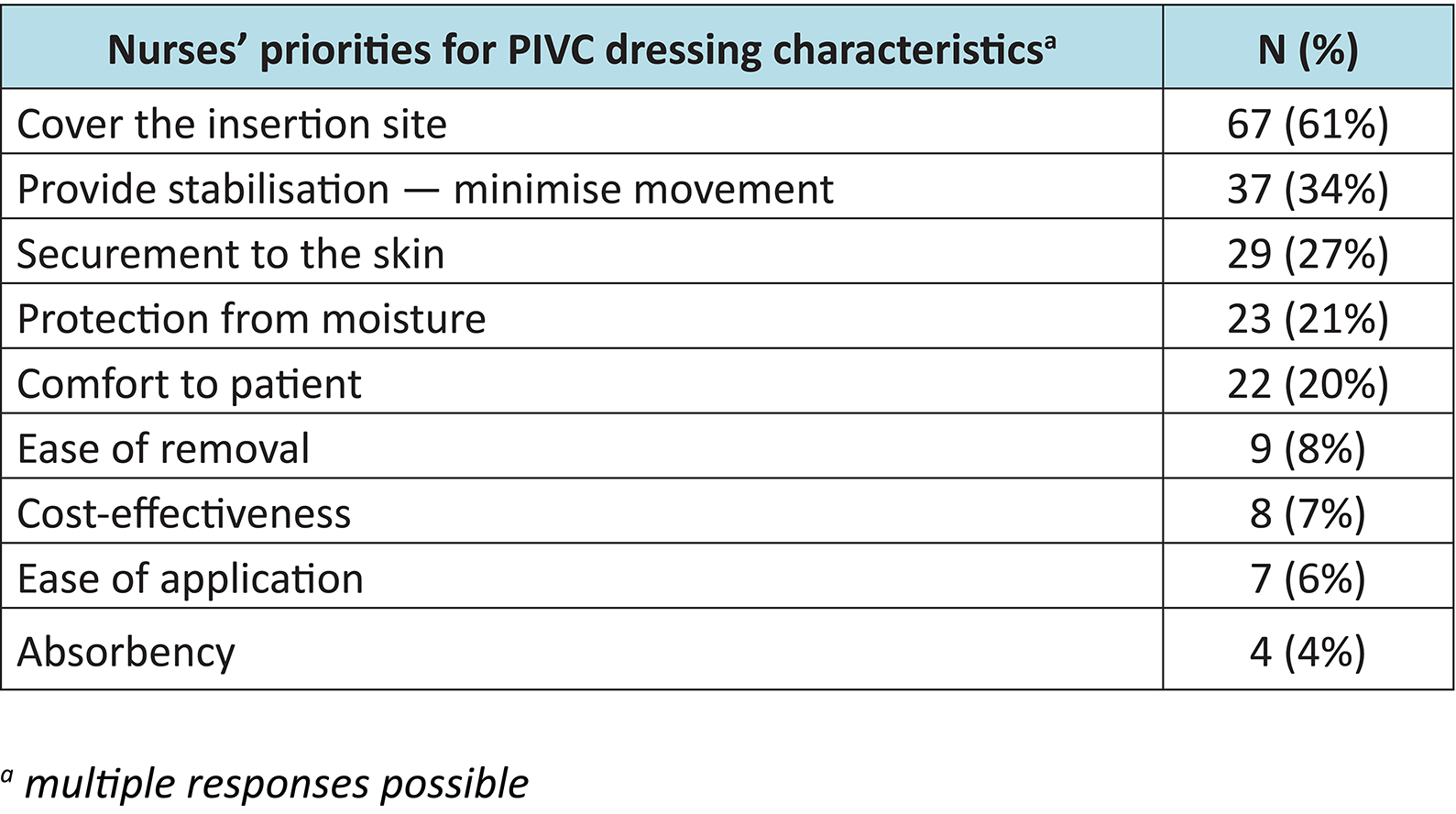
Nurses’ priorities for PIV dressing characteristics
Nurses’ priorities for PIVC dressings are shown in Table 2. More than one answer could be selected. The data revealed that the majority of nurses (61%) acknowledged that covering the insertion site was most important.
Table 2: Nurses priorities for PIVC dressing characteristics (n=109)a
Performance and acceptability of dressing and securement devices
Nurses reported the clinical performance and acceptability of each of the four dressing and securement devices used in the RCT as follows (Figures 1 and 2).
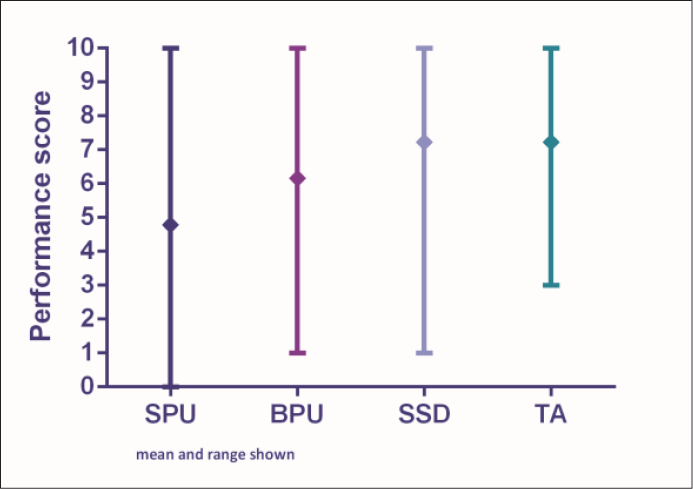
Figure 1: Performance scores
SPU = simple polyurethane dressing; BPU = bordered polyurethane dressing; SSD = sutureless securement device + simple polyurethane dressing; TA = tissue adhesive + simple polyurethane dressing
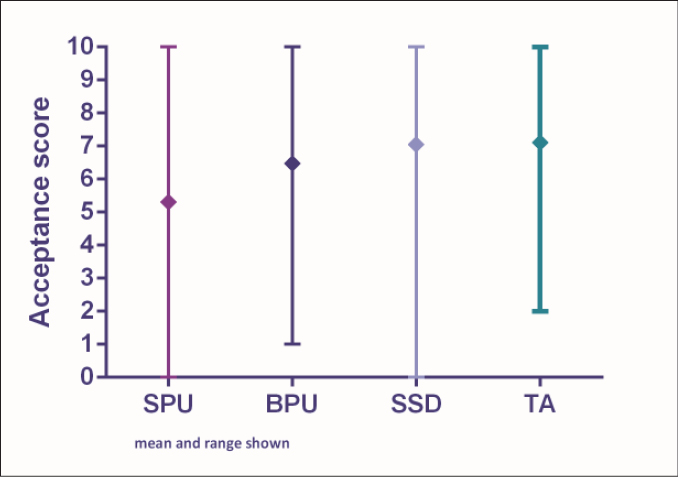
Figure 2: Acceptability scores
SPU = simple polyurethane dressing; BPU = bordered polyurethane dressing; SSD = sutureless securement device + simple polyurethane dressing; TA = tissue adhesive + simple polyurethane dressing
Performance and acceptability of the simple polyurethane dressing (SPU)
The median score was 5.69 for performance and 5.98 for acceptability (interquartile range shown in Figures 1 and 2). Five respondents (5%) provided free comments regarding the overall performance and acceptability of the SPU dressing. Positive comments described the dressing to be “secure with good visibility of the PIVC site”. Negative comments included that “the dressing edges rolled and lifted too easily”.
Performance and acceptability of the bordered polyurethane dressing (BPU)
The median score was 6.94 for performance and 7.19 for acceptability (interquartile range shown in Figures 1 and 2). Four respondents (4%) provided free comments about the performance and acceptability of the BPU dressing, with all comments detailing negative outcomes such as: the dressing had too many “bits and pieces”; “secure placement of the dressing can be difficult”; and “the edges of the dressing lift easily”. One respondent commented that the dressing was “not secure for patients with excessive body hair and reduced skin elastin”.
Performance and acceptability of the sutureless securement device + simple polyurethane dressing (SSD)
The median score was 7.42 for performance and 7.13 for acceptability (interquartile range, Figures 1 and 2). Twelve respondents (11%) provided free comments about the acceptability and performance of the SSD method. Negative comments discussed the size of the dressing saying that it was “cumbersome and bulky on patient skin” and “impractical for patients”. A positive remark stated that the dressing was “very secure”.
Performance and acceptability of the tissue adhesive + simple polyurethane dressing (TA)
The median score was 7.34 for performance and 7.20 for acceptability (interquartile range shown in Figures 1 and 2). Nine respondents (8%) provided free comments about the performance and acceptability of this dressing method. Positive comments reported that “the dressing was secure”, with “good visibility allowing for assessment of the PIVC site”. Other comments associated with the TA was that the dressings were “easy to remove”, but “for some patients the TA failed after day 2”, and the “sticking capacity was greatly reduced for diaphoretic patients”.
Ease and acceptability of trial paperwork and process
The median score was 7.18 (IQR 6, 8) relating to the ease and acceptability of the trial paperwork and processes. Qualitative feedback included both negative and positive comments. Nineteen respondents (17%) provided comments in response to this question. The positive comments stated that the paperwork was “simple and straightforward to understand” and “easy to fill out”. Some negative comments suggested that it was “not always feasible to complete the paperwork” and that the “forms sometimes got missed or forgotten”.
Further comments or suggestions about the trial
Overall, 45 respondents (41%) provided free comments. Of these, 19 comments related to the RCT, and 19 to PIVC dressing/securement, with 7 unrelated comments (such as “bring the IV service back”). Trial comments largely related to praising the research nurses for their skills and professionalism, for example, “excellent in their job”, “extremely helpful”. Other comments discussed the benefits associated with the trial relating to the communication and education of staff and patients about the importance of dressing and securement devices in reducing poor PIVC outcomes.
DISCUSSION
PIVC securement remains problematic in the clinical environment, with many devices failing before treatment completion5. The SAVE RCT sought to identify the best PIVC dressing and securement method to prevent PIVC failure, testing four different products. Before implementing a new process, it was important to identify the factors that affect nurses’ clinical practice with PIVC dressing and securement. We conducted a staff survey to investigate the performance and acceptability of each dressing/securement method, as well the overall ease and acceptance of the trial paperwork and processes. This was done to provide a platform to understand the essential steps when introducing practice change for PIVC dressing and securement options. However, there is a paucity of research investigating different dressing and securement methods with the objective of using the data for knowledge translation.
The majority of respondents in this study were surgical nurses (70%) with 0–5 years’ clinical experience (40%). Most reported that covering the insertion site, minimising movement, and securement to the skin were the main characteristics required for the optimal PIVC dressing. Currently the United Kingdom’s epic3 guidelines recommend the use of a sterile, transparent polyurethane dressing to cover the insertion site13. Interestingly, the performance and acceptability of the SPU dressing was ranked as the least preferred dressing option. Respondents caring for patients with this dressing method stated that the SPU dressing’s tendency to lift and/or roll off easily from the skin outweighed the fact that the dressing provides good visibility of the PIVC site.
No dressing in the study was a clear favourite for nurses, although stabilising the PIVC was identified as an important characteristic. This is a similar finding to Bolton who reported that 94% of staff audited believed that there was benefit to the use of a stabilisation device8. In our survey, respondents commented that both the TA and the SSD achieved stabilisation by reducing movement and preventing potential catheter dislodgement. A recent review of the use of TA for VAD securement found a likely benefit of TA preventing VAD complications and failure14. Other studies have reported that PIVC stabilisation is a key factor in reducing the risks of complications8,15,16, particularly for PIVCs dwelling over 24 hours17. From the comments gathered in this study we identified that nursing staff believed that TA may have limited use for patients who are diaphoretic or excessively hirsute.
PIVC securements and dressings change constantly, as new products and suppliers enter the market, evidence-based guidelines are updated, and hospital policies and standards change, often driven by economic considerations18. Changes in all of these factors affect clinical practice. Therefore, deciding the best PIVC dressing/securements should not be about choosing a specific dressing, rather about understanding the key dressing characteristics most effective in reducing catheter complications and failure. Implementation strategies involving educating clinical staff and patients about dressings are a strategic but often neglected step in reducing PIVC complications and costs.
The limitations of this study were that only one hospital participated in the survey, which may not be generalisable to others. Also, not all nurses may have used all four products and possibly would have given different feedback. Direct patient feedback on their preferred PIVC dressing is also absent in this sub-study. Nevertheless, the quantitative and accompanying textual data gained from this survey has generated useful information about the perceived performance and acceptability of four different dressing and securement methods, as well as identifying characteristics that nurses deem important when selecting a PIVC dressing. PIVC policies and education programs are predominately created and managed by nurses19, and this feedback should be considered in their design.
Maximising the culture of safety and quality in health care is a collaborative effort. For improvements to occur in any industry requires measurements and analysis20. Research of this type is imperative so that the correct use and management of these devices occurs. The value of research in this area is significant, both clinically and economically.
A crucial element in the larger RCT was to take measures to communicate with staff and make them part of the process evaluation, thereby giving them shared ownership of the change. Organisational culture in hospitals is often rigid, relying heavily on treatment plans and policies written by management rather than bedside staff. Gathering user data on the ease and acceptability of the trial paperwork and processes was useful, as this knowledge now provides a platform for understanding the context of the final RCT results, when available, and in the design of future research studies.
The use of a staff survey that engages key stakeholders whenever there is institutional change of PIVC dressings due to emerging products, research or new suppliers, calls for consideration. We have learnt that deciding the best PIVC dressing/securement should not be about choosing a specific dressing, rather instead about understanding the key characteristics of PIVC dressings, such as stabilisation of the catheter, to provide a more effective tool in reducing catheter complications. Theemphasis is not only on the production of new knowledge but also the usefulness of knowledge. Active inclusion and involvement of the nursing staff is an important element in the goal of study translation of research evidence into practice.
Appendix 1

PARTICIPANT INFORMATION
Chief Investigators: Prof Claire Rickard & Prof Joan Webster on behalf of the SAVE trial team.
Description of Research Study
IV drip insertion is the most common invasive procedure performed in hospitals with approximately 14 million used in Australia each year. IV drips often fail by getting blocked, inflamed, infected or moving out of the vein. This affects up to 40% of patients. Such failure probably happens because the IV drips are not well enough secured to the skin and we think this should be mostly preventable. Currently hospitals spend millions of dollars spent on dressings to secure IV drips to the skin. Nurses and doctors spend countless hours applying them. Current failure rates indicate current dressings are inadequate. The SAVE trial was a large multisite randomised controlled trial evaluating a range of IV dressing and securement mechanisms. The aim of this study is to survey nursing acceptability and utility of the various dressing and securement products used in the SAVE trial.
Why you have been chosen You are invited to participate in this important study because you are a registered nurse on a ward included in the SAVE trial.
What we will ask you to do You are invited to complete the survey listed. There are a total of six questions including a couple of basic demographic questions as well as other asking you to rate the performance and acceptability of products used in the SAVE trial and trial paperwork/processes
Risks to you There are no foreseeable risks through participation in the study. Your participation is entirely voluntary. No individual’s practice is being examined. Only aggregate data will be published.
Benefits to you We do not expect you to get any direct benefit through participation in the study. However, we value your responses and the results of this study may contribute to improvements in health care practice and patient outcomes in the future. A summary of the results of the study will be available upon request at the completion of the study.
Confidentiality Data collected during this study will be treated confidentially. All survey responses will be anonymous and there is no way to link answers to any individual. Data generated will be stored securely at the Hospital and University. Only aggregate data will be published. No individual person or unit will be identifiable in the final report or related publications. Research records will be destroyed 15 years after the study completion.
Choosing to participate or not Your participation is entirely voluntary and if you decide not to participate this will not affect your relationship with research staff. Your consent to participate in the survey will be assumed by completion of the survey.
If you have any questions If you have any questions now, or at a later time, please don’t hesitate to contact Prof Rickard or Julie Flynn - SAVE Survey coordinator 3636 4121 or julie.flynn@griffith.edu.au, and they will be happy to answer your questions.
This study has been reviewed and approved by the Queensland Children’s Health Services (RCH) Human Research Ethics Committee which was designated by the Queensland Health Office of Health and Medical Research for this multi-site study. Should you wish to discuss the study with someone not directly involved, in particular in relation to matters concerning policies, information about the conduct of the study, or should you wish to make an independent complaint, please contact the Chairperson of the Queensland Children’s Health Services (RCH) Human Research Ethics Committee, Professor John Pearn, 3365 5323.



Author(s)
* Christine Woods RN, MOccupEnviroHlth Nurse Researcher, Alliance for Vascular Access Teaching and Research (AVATAR) group, Menzies Health Institute Queensland, Griffith University, Health Sciences Building N48_0.06, 170 Kessels Road, Nathan, Qld 4111, Australia Email c.woods@griffith.edu.au Gillian Ray-Barruel RN, PhD Research Fellow, Alliance for Vascular Access Teaching and Research (AVATAR) group, Menzies Health Institute Queensland, Griffith University, Brisbane, Qld, Australia Nicole Marsh RN, BN, MAdvPrac (HlthRes) Vascular Access Research Fellow, Royal Brisbane and Women’s Hospital, Brisbane, Qld, Australia School of Nursing and Midwifery, Griffith University, Brisbane, Qld, Australia Alliance for Vascular Access Teaching and Research (AVATAR) group, Menzies Health Institute Queensland, Griffith University, Brisbane, Qld, Australia Julie Flynn RN, BN, MAdvPrac (HlthRes) Senior Research Assistant & Registered Nurse, Alliance for Vascular Access Teaching and Research (AVATAR) group, Menzies Health Institute Queensland, Griffith University, Brisbane, Qld, Australia Royal Brisbane and Women’s Hospital, Brisbane, Qld, Australia Emily Larsen RN, BN, GDip(HlthRes) Senior Research Assistant, Alliance for Vascular Access Teaching and Research (AVATAR) group, Menzies Health Institute Queensland, Griffith University, Brisbane, Qld, Australia Royal Brisbane and Women’s Hospital, Brisbane, Qld, Australia Claire M Rickard RN, PhD Professor of Nursing, Alliance for Vascular Access Teaching and Research (AVATAR) group, Menzies Health Institute Queensland, Griffith University, Brisbane, Qld, Australia School of Nursing and Midwifery, Griffith University, Brisbane, Qld, Australia Royal Brisbane and Women’s & Princess Alexandra Hospitals, Brisbane, Qld, Australia Division of Nursing, Midwifery and Social Work, University of Manchester, UK * Corresponding author Pages 3-10
References
- Alexandrou E, Ray-Barruel G, Carr PJ, Frost S, Inwood S, Higgins N et al. International prevalence of the use of peripheral intravenous catheters. J Hosp Med 2015; 10(8):530–3.
- Rickard CM, Marsh NM, Webster J, Gavin NC, McGrail MR, Larsen E et al. Intravascular device administration sets: replacement after standard versus prolonged use in hospitalised patients — a study protocol for a randomised controlled trial (The RSVP Trial). BMJ Open 2015; 5(2):e007257.
- Marsh N, Webster J, Mihala G & Rickard CM. Devices and dressings to secure peripheral venous catheters to prevent complications. Cochrane Database Syst Rev 2015; Issue 6, Art No.: CD011070.
- Rickard CM, Marsh N, Webster J, Playford EG, McGrail MR, Larsen E et al. Securing All intraVenous devices Effectively in hospitalised patients — the SAVE trial: study protocol for a multicentre randomised controlled trial. BMJ Open 2015; 5(9):e008689.
- Marsh N, Webster J, Mihala G & Rickard CM. Devices and dressings to secure peripheral venous catheters: a Cochrane systematic review and meta-analysis. Int J Nurs Stud 2017; 67:12–9.
- Allain J. Quality as standard: the process of evaluating cannulae for trust-wide use. Prof Nurse 2004; 19(10):48–9.
- Flippo PL & Lee J. Clinical evaluation of the Sorbaview Shield securement device used on peripheral intravenous catheters in the acute care setting. J Vasc Access 2011; 16(2):95,100–98,2.
- Bolton D. Improving peripheral cannulation practice at an NHS Trust. Br J Nurs 2010; 19(21):1346–50.
- Jeanes A & Bitmead J. Reducing bloodstream infection with a chlorhexidine gel IV dressing. Br J Nurs 2015; 24(Sup19):S14–S9.
- Maryniak K. Clinical performance and nursing satisfaction of a transparent chlorhexidine gluconate IV securement dressing with peripherally inserted central catheters. J Vasc Access 2009; 14(4):200–3.
- Stark P. 6 Reasons to involve employees in decision making. San Diego, CA: PeterBarronStark; 2010. Accessed 8 March, 2018. Available from: https://peterstark.com/key-to-engagement/#
- Bausone-Gazada D, Lefaiver CA & Walters SA. A randomized controlled trial to compare the complications of 2 peripheral intravenous catheter-stabilization systems. J Infus Nurs 2010; 33(6):371–84.
- Loveday HP, Wilson JA, Pratt RJ, Golsorkhi M, Tingle A, Bak A et al. epic3: national evidence-based guidelines for preventing healthcare-associated infections in NHS hospitals in England. J Hosp Infect 2014; 86(Suppl 1):S1–70.
- Corley A, Marsh N, Ullman AJ & Rickard CM. Tissue adhesive for vascular access devices: who, what, where and when? Br J Nurs 2017; 26(19):S4–S17.
- Goossens GAPRN, Hadaway LMRNBCC. Key strategies for improving outcomes of patients with peripheral venous catheters: report of an international panel discussion. J Vasc Access 2014; 19(3):135–7.
- Shears G. Summary of product trials for 10 614 patients: comparing an intravenous stabilizing device to tape. J Infus Nurs 2006; 29(4):225–31.
- Bugden S, Shean K, Scott M, Mihala G, Clark S, Johnstone C et al. Skin glue reduces the failure rate of emergency department-inserted peripheral intravenous catheters: a randomized controlled trial. Ann Emerg Med 2016; 68(2):196–201.
- Chico-Padrón RM, Carrión-García L, Delle-Vedove-Rosales L, González-Vargas CS, Marrero-Perera M, Medina-Chico S et al. Comparative safety and costs of transparent versus gauze wound dressings in intravenous catheterization. J Nurs Care Qual 2011; 26(4):371–6.
- Schuster C, Stahl B, Murray C, Keleekai NL & Glover K. Development and testing of a short peripheral intravenous catheter insertion skills checklist. J Vasc Access 2016; 21(4):196–204.
- Scobie S, Thomson R, McNeil JJ & Phillips PA. Measurement of the safety and quality of health care. Med J Aust 2006; 184(10):S51–S5.
