Volume 6 Issue 1
Difficult vascular access in hospitalised patients: delays to treatment, cannulation attempts, and use of ultrasound
Michael Nye, Amy Sweeny, Stuart Watkins, Jay Ingold and Paul Sharwood
Keywords ultrasound, cannulation, attempts, treatment delays
For referencing Nye M et al. Difficult vascular access in hospitalised patients: delays to treatment, cannulation attempts, and use of ultrasound. Vascular Access 2020; 6(1):5-9.
DOI https://doi.org/10.33235/va.6.1.5-9
Abstract
Background and objectives Placement of peripheral intravenous catheters (PIVCs) is often necessary for hospital-based patients. Some patients are difficult to cannulate using traditional landmark techniques, due to poor vessel visualisation. Multiple PIVC attempts decrease satisfaction and cause treatment delays. At the Gold Coast Hospital & Health Services (GCHHS), the Clinical Team Coordinator (CTC) team members have been trained to place PIVCs with ultrasound guidance. This study aims to describe the practice characteristics (time of call-out, referral ward) and PIVC experience (number of attempts, treatment delays) for a nurse-led PIVC team trained in ultrasound-guided PIVC insertion and the difficult-to-cannulate hospital ward patients they attended.
Method Over the period 1 January 2017 to 31 July 2018, data were collected prospectively by the CTC team using a portable tablet and a custom-made database. Clinical Team Coordinator nurses categorised the activity and data as the time of callout, reason for PIVC, patient characteristics, number of PIVC attempts prior to call-out, CTC attempts, and delay in treatment. All entries marked as ultrasound cannulation assists were extracted and summarised in Excel.
Results During the period, 208 callouts were made for ultrasound cannulation assistance. Nearly half (n=95) of patients had undergone three or more previous cannulation attempts prior to the call-out. In 72% of cases (n=151), the CTC team members required just one attempt at cannulation and 93% (n=195) of all cases were completed with one or two CTC attempts. Treatment delay (the time between PIVC access being required and the eventual establishment of access by the CTC team member) was kept under 1 hour in 40% (n=84) of cases. However, 11% of the cases (n=22) had a 6-hour or greater delay in treatment. The most common reason for PIVC access was for administration of intravenous (IV) antibiotics.
Conclusion Many patients require multiple attempts at cannulation, which are associated with patient discomfort and treatment delays. The availability of a nurse-led ultrasound-guided PIVC team, and increasing ward staff’s knowledge and skills on predicting cannulation difficulty, may improve patients’ cannulation experiences.
Introduction
Placement of a peripheral intravenous catheter (PIVC) is one of the most common procedures completed within the hospital setting, and is often required for administration of antibiotics, other medications, and intravenous (IV) fluids. Peripheral intravenous catheters are estimated to be required in 65% of patients presenting to the Emergency Department (ED).1 As many as 26% of insertions require multiple attempts.2 Interestingly, prior research has shown that up to 50% of PIVCs inserted in Australian EDs are unused.3 The procedure is often straightforward on a healthy patient; however, a range of patient characteristics (such as no palpable or visible vein, a history of IV injections, chemotherapy, or chronic kidney disease) may result in difficulty placing the PIVC and lead to failures.
Portable ultrasound machines have been available for over 20 years,4 yet are not employed routinely to facilitate first-time success with PIVC insertion. However, growing evidence shows that point-of-care ultrasound (POCUS) improves success rates for cannulating difficult intravenous access (DIVA) adult patients.5-8 Some evidence suggests that the use of ultrasound with this group also decreases the number of punctures required,6 the time to successful cannulation,6,8 and increases patient satisfaction.6,8 While the uptake of POCUS-guided PIVC insertion is increasing and research around the area is gaining momentum,6,9,10 the use of POCUS is still not mentioned in the Queensland Government (Australia) Department of Health guidelines on PIVC placements, indicating a slow uptake.11
Prior research has identified that most patients describe PIVC insertion as moderately painful or worse.12,13 It is important to consider patient satisfaction throughout their entire hospital visit, and great insight may be gained from patient feedback. Patients describe experiences as painful, stressful, and concerning, sometimes with significant pain.12 This shows that communication with the patient is critical,13 along with more efficient PIVC procedures to reduce patient distress.
Objective
This study aims to describe the practice characteristics (time of call-out, referral ward, indication for cannulation) and PIVC experience (number of attempts, treatment delays) for a nurse-led PIVC team trained in POCUS-guided PIVC insertion, and the hospitalised DIVA ward patients they attended. This will lead to a better understanding of what influences the PIVC experience and areas to focus on in the future to improve patient care.
Methods
Study design
This was a prospective observational cohort study of all referrals of ward patients for POCUS-guided PIVC.
Setting
Gold Coast University Hospital (GCUH) (Gold Coast, Australia) is a tertiary-level facility that opened in 2013.14 It comprises 750 beds, has the busiest ED in Australia (nearly 110,000 presentations in the 2017–18 fiscal year), and over 5,000 births in the same period. The hospital provides a wide range of services, including trauma, cancer services, diagnostic and emergency medicine, mental health, speciality procedures, and women’s and newborn services.
At GCUH, the Clinical Team Coordinator (CTC) team members have been trained and are considered experts at POCUS-guided PIVC placement. The CTC team member can be called from any medical or surgical ward in the hospital whenever a practitioner deems PIVC placement is difficult. At the time of this study, there were 5.1 full-time equivalent (FTE) positions in the CTC team, covered by six individuals.
This study used all call-outs for POCUS-guided cannulation assistance occurring during the period 1 January 2017 to 31 July 2018. Ethics approval for this audit was obtained (HREC/17/QGC/189).
Participants
Participants were all adult ward patients who required a PIVC and were referred to the CTC team member for POCUS-guided cannulation. A clinician (nurse or doctor) caring for the patient made referrals by phone. Any patient on whom a POCUS-guided PIVC was to be used was categorised by the CTC team member on-duty as a POCUS-guided cannulation call-out. No patients were excluded for this study.
Outcome and practice variables
The CTC team member collected the following data: date, time and ward of call-out; the type of clinician who requested the assistance; the number of attempts that occurred prior to call-out; the number of POCUS-guided attempts taken by the CTC team member; the reason PIVC was required; the delay in treatment due to failure to cannulate earlier; and patient risk factors for difficult cannulation. The number of attempts prior to CTC call-out was determined by asking the patient.
Treatment delay was calculated as the difference between the time successful cannulation occurred and the time the IV treatment had been scheduled in the patient’s chart.
Data sources
Data were collected prospectively by the CTC team members using a portable tablet and a custom-made database. The database was designed to capture all call-out activity, not just POCUS-guided PIVC call-outs. A CTC team member is available 24/7 and are called to investigate deteriorating patients, for all medical emergencies (code blues), and to any call regarding aggressive patients (code blacks). In some cases, and at the CTC team members’ discretion, the CTC team member supervised another clinician performing the POCUS-guided cannulation, rather than performing it themselves, to teach more clinicians how to use the ultrasound machine. However, they did not attend a difficult case just to train another clinician.
Bias
The CTC team were the clinicians performing the POCUS-guided PIVC placement and the data collectors. Although the CTC team members entered their own performance data, at the time of collection they were not aware that it would be summarised apart from their other practices.
Data analysis
Data were exported into Microsoft Excel and summarised using simple counts and proportions. Data were missing on the number of ultrasound attempts in nine records. For these cases, the number of attempts was inferred based on the time taken for the POCUS-guided attempt. If the time taken was under 30 minutes, one attempt was recorded, otherwise two attempts were recorded.
Results
Practice and patient characteristics
Between January 2017 and July 2018, 208 call-outs for PIVC assistance from a CTC team member occurred. The average age and interquartile range (IQR) of DIVA patients was 62 years (46–76 years) with 60% of patients being female. In more than half of the cases (58%, n=121), the reason the patient was identified as DIVA (the reason for call-out) was a history of difficult access. The other common reasons included having no vein palpable (11%, n=24), no vein visible (8%, n=18), or a history of IV injections (7%, n=16).
Most call-outs (67%, n=140) occurred after-hours (outside of 8am-4pm). The night session (4pm–midnight) was the busiest time of day, accounting for 50% (n=105) of cases, compared to the morning (midnight–8am) having only 17% (n=35) of cases. The gastroenterology inpatient unit accounted for the most referrals (19.2%, n=40), followed by the short stay surgical unit (9.1%, n=19). Other wards with more than 10 call-outs were orthopaedics inpatient unit, cancer inpatient unit, immunology/sleep studies/rehab, neurology, surgical inpatient unit, general medicine inpatient unit, and the renal inpatient unit.
In over 40% (n=85) of cases, access was required for the administration of IV antibiotics, with a further 15% (n=33) being for other medications. Intravenous fluids (14%, n=30) and blood samples (13%, n=28) were other common reasons for access (Figure 1).
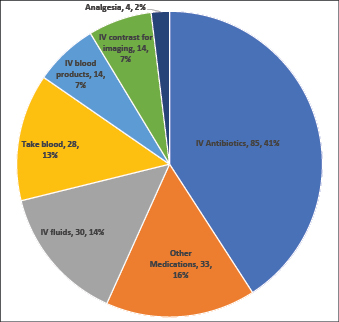
Figure 1. Reason IV access was required for 208 DIVA patients
Patient experience and CTC team member outcomes
Number of attempts
Between zero and nine attempts to cannulate a patient were reported prior to a call-out for assistance (Figure 2), with nearly half (n=95) of the patients undergoing three or more attempts prior to call-out. The CTC team members achieved successful POCUS-guided PIVC placement in more than two-thirds of cases (72%, n=151). Of all CTC team member cannulations, 93% (n=195) were completed with one or two attempts (Figure 3).
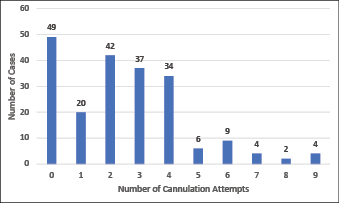
Figure 2. Number of peripheral intravenous cannulation attempts prior to call-out for assistance
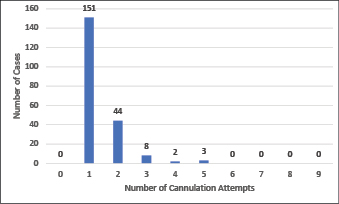
Figure 3. Number of POCUS-guided peripheral intravenous cannulation attempts by the CTC team member after call for assistance
Delay in treatment
The treatment delay is recorded as the time between when the PIVC access was required (for medications or other treatment reasons), and when access was obtained by the CTC team member. This delay was kept to under 1 hour in 40% (n=84) of cases. However, treatments were delayed by 3 or more hours in nearly half the cases (Figure 4). The average delay was 2.25 hours (IQR 0.5–2.5). Twenty-two cases (11%) had a 6-hour or greater treatment delay.
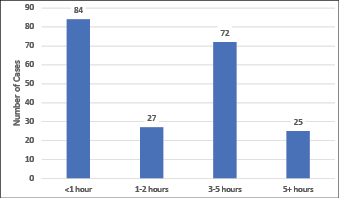
Figure 4. Length of delay for IV treatment due to difficulty in placing PIVC for 208 DIVA patients
Discussion
This study finds that dedicated staff who have been trained in using POCUS-guided cannulation can gain DIVA patients’ PIVC access with fewer attempts. The expert service described in this paper was used mostly after-hours, and usually not until three or more attempts to cannulate had occurred. Some patients underwent nine attempts at cannulation prior to an attending clinician calling out for help from a team member trained in POCUS-guided PIVC.
Difficulty cannulating these patients resulted in a delay to treatment of 3 or more hours for more than half of these cases. It is important to recognise the important role the CTC team member and POCUS-guided PIVC play in reducing this delay to treatment. Earlier identification of DIVA patients and referral to a CTC team member can further reduce these delays.
In terms of success rates using POCUS-guided PIVC placement techniques, the results found in this study are comparable with published literature,9,10,15 indicating that our result of 72% first attempt success with POCUS guidance is achievable in other settings.
Point-of-care ultrasound-guided PIVC placement can reduce the number of attempts required to cannulate patients in general, with past research indicating a reduction of both number of attempts and procedure time.16,17 Other trials that categorised patients as difficult or not difficult to cannulate also found higher success rates when POCUS guidance was used, with more improvement in difficult access patients.18 However, successful execution of this procedure requires training in the identification of difficult-to-cannulate patients, training in the use of POCUS-guided PIVC insertion, and access to ultrasound machines.
Using POCUS guidance is of most benefit when used on patients with poor vessel visualisation, as this is the best predictor of DIVA.19 Patients with poor vessel visualisation alone qualified as 20% of difficult-to-cannulate patients in this study, and up to 17% in other studies.20 Quality assessment of the patient and identification of potential DIVA patients should both be a mandatory component of any cannulation training program.
Training courses in POCUS-guided PIVC placement have been shown to decrease the total number of required attempts for clinicians in general.21 One randomised controlled trial performed with first- and second-year medical students demonstrated a significant decrease in the number of attempts required when using POCUS guidance instead of standard techniques.22 The delivery and effectiveness of PIVC cannulation training has also been studied recently. One study of a group of medical students concluded that students with face-to-face teaching performed significantly better than those receiving no education in POCUS-guided PIVC placement.23 A similar study of students in PIVC insertion training compared peer-assisted groups versus instructor-led groups and found both methods effective teaching techniques.24 These studies highlight the importance of training, and also offer an insight into different training methods that may be utilised.
As with most procedures, successful cannulation requires adequate training, demonstrated competency, ongoing practice, as well as regular monitoring and assessment of outcomes.
Limitations
A number of limitations exist in this study. First, this is a single-site study and, as such, may not be generalisable to other settings. This study describes the experience with one site’s model of deploying POCUS-guided assistance. The deployment team’s success is described, but other clinicians’ POCUS-guided attempts (if any), were not available for analysis, and are not described. Because the CTC team members were frequently called out to assist with POCUS-guided cannulation, it is likely that they are the most adept at this technique. Therefore, the results found may represent a ‘best-achievable’ practice. Second, data entry was not mandatory; some call-outs may have been missed. However, this is unlikely given that the CTC team members are motivated to show their productivity.
Strengths
This study offers information on a practice that is difficult to track prospectively. Data were collected using bedside portable devices immediately after PIVC insertion in a large tertiary care setting. Data collection in these types of settings is generally difficult. In this study, the nature of the CTC team’s operation made this data collection possible: the team is available 24/7, their services are available to the entire hospital, and the team makes active use of portable devices allowing data capture in real-time. The data collected by the CTC team members provide a unique insight into the effectiveness of POCUS-guided cannulations. It is rare to get prospective data on cannulations collected, so this dataset provides a good insight into cannulation attempts and the effects they have on patient care.
Conclusion
Hospitalised DIVA patients frequently endure multiple attempts at cannulation and hours of delays in treatment. Point-of-care ultrasound-guided cannulation, performed by members of a team trained in this procedure, can achieve cannulation with one or two attempts in the vast majority of cases. Evidence-based assessment of the quality of a patient’s vascular access and the initial attending clinician’s confidence in securing the PIVC access should be assessed. When DIVA patients are identified, POCUS-guidance should be employed to ensure quick PIVC access with minimal patient discomfort.
Future studies on this topic should assess barriers to POCUS-guided PIVC and compare models of deployment. It is important to determine whether broad-based training of clinicians or training of a team, as in this study, confers the greatest benefit to patients and best reduces delays. The best model may vary by size and/or type of hospital. The impact of training in, and use of, POCUS-guided PIVC placement procedures on first attempt success, as well as the attributes indicating a DIVA patient, also need to be considered. Last, we encourage those that deliver education on PIVC placement to include universal patient assessment for identification of DIVA patients as a necessary component, and to train prospective cannulators on POCUS-guided cannulation.
Author(s)
*Michael Nye1; Amy Sweeny1,2; Stuart Watkins2,3; Jay Ingold2; Paul Sharwood2
1Griffith University, Brisbane, QLD, Australia
2Gold Coast Hospital and Health Service, Southport, QLD, Australia
3Australian Institute of Ultrasound, Broadbeach Waters, QLD, Australia
*Corresponding author
Michael Nye, Medical Student, Teaching Griffith Health Centre, G40, Gold Coast Campus, Griffith University, Southport, QLD 4222 Australia
Email michael.nye@griffithuni.edu.au
References
- Hawkins T, Greenslade JH, Suna J, Williams J, Rickard CM, Jensen M, et al. Peripheral intravenous cannula insertion and use in the emergency department: an intervention study. Acad Emerg Med. 2018;25(1):26-32.
- Sabri A, Szalas J, Holmes KS, Labib L, Mussivand T. Failed attempts and improvement strategies in peripheral intravenous catheterization. Biomed Mater Eng. 2013;23(1-2):93-108.
- Limm EI, Fang X, Dendle C, Stuart RL, Egerton Warburton D. Half of all peripheral intravenous lines in an Australian tertiary emergency department are unused: pain with no gain? Ann Emerg Med. 2013;62(5):521-5.
- Mandavia, Diku. 20 Years Of SonoSite 2019 [Internet] Video: 1:43 min. Available from: https://www.sonosite.com/about/sonosites-history
- Egan G, Healy D, O’Neill H, Clarke-Moloney M, Grace PA, Walsh SR. Ultrasound guidance for difficult peripheral venous access: systematic review and meta-analysis. Emerg Med J. 2013;30(7):521-6.
- van Loon FHJ, Buise MP, Claassen JJF, Dierick-van Daele ATM, Bouwman ARA. Comparison of ultrasound guidance with palpation and direct visualisation for peripheral vein cannulation in adult patients: a systematic review and meta-analysis. Br J Anaesth. 2018;121(2):358-66.
- Stolz LA, Stolz U, Howe C, Farrell IJ, Adhikari S. Ultrasound-guided peripheral venous access: a meta-analysis and systematic review. J Vasc Access. 2015;16(4):321-6.
- Bahl A, Pandurangadu AV, Tucker J, Bagan M. A randomized controlled trial assessing the use of ultrasound for nurse-performed IV placement in difficult access ED patients. Am J Emerg Med. 2016;34(10):1950-4.
- Gregg SC, Murthi SB, Sisley AC, Stein DM, Scalea TM. Ultrasound-guided peripheral intravenous access in the intensive care unit. J Crit Care. 2010;25(3):514-9.
- Pare JR, Pollock SE, Liu JH, Leo MM, Nelson KP. Central venous catheter placement after ultrasound guided peripheral IV placement for difficult vascular access patients. Am J Emerg Med. 2019;37(2):317-20.
- Guideline - Peripheral interavenous catheter (PIVC). In: Health Do, editor. 2015.
- Cooke M, Ullman AJ, Ray-Barruel G, Wallis M, Corley A, Rickard CM. Not “just” an intravenous line: consumer perspectives on peripheral intravenous cannulation (PIVC). An international cross-sectional survey of 25 countries. PloS one. 2018;13(2):e0193436.
- Larsen E, Keogh S, Marsh N, Rickard C. Experiences of peripheral IV insertion in hospital: a qualitative study. Br J Nurs (Mark Allen Publishing). 2017;26(19):S18-s25.
- Gold Coast Hospital and Health Service. Annual Report 2017–18. In: Health Do, editor. 2018.
- Smith C. Should nurses be trained to use ultrasound for intravenous access to patients with difficult veins? Emerg Nurs. 2018 Jul 6;26(2):18-24.
- Costantino TG, Parikh AK, Satz WA, Fojtik JP. Ultrasonography-guided peripheral intravenous access versus traditional approaches in patients with difficult intravenous access. Ann Emerg Med. 2005;46(5):456-61.
- Heinrichs J, Fritze Z, Vandermeer B, Klassen T, Curtis S. Ultrasonographically guided peripheral intravenous cannulation of children and adults: a systematic review and meta-analysis. Ann Emerg Med. 2013;61(4):444-54.e1.
- McCarthy ML, Shokoohi H, Boniface KS, Eggelton R, Lowey A, Lim K, et al. Ultrasonography versus landmark for peripheral intravenous cannulation: a randomized controlled trial. Ann Emerg Med. 2016;68(1):10-8.
- Shokoohi H, Boniface K, Vahali S, Eggleton R, Ashraf R, Ding R, et al. 72 Predicting difficult peripheral intravenous access in adult emergency department patients. Ann Emerg Med. 2014;64(4):S26-S7.
- Carr PJ, Rippey JCR, Budgeon CA, Cooke ML, Higgins N, Rickard CM. Insertion of peripheral intravenous cannulae in the emergency department: factors associated with first-time insertion success. J Vasc Access. 2016;17(2):182-90.
- Feinsmith S, Huebinger R, Pitts M, Baran E, Haas S. Outcomes of a simplified ultrasound-guided intravenous training course for emergency nurses. J Emerg Nurs. 2018;44(2):169-75.e2.
- Vitto MJ, Myers M, Vitto CM, Evans DP. Perceived difficulty and success rate of standard versus ultrasound-guided peripheral intravenous cannulation in a novice study group: a randomized crossover trial. J Ultrasound Med. 2016;35(5):895-8.
- Lian A, Rippey JCR, Carr PJ. Teaching medical students ultrasound-guided vascular access - which learning method is best? J Vasc Access. 2017;18(3):255-8.
- Pelloux S, Gregoire A, Kirmizigul P, Maillot S, Bui-Xuan B, Llorca G, et al. Peripheral venous catheter insertion simulation training: A randomized controlled trial comparing performance after instructor-led teaching versus peer-assisted learning. Anaesth Crit Care Pain Med. 2017;36(6):397-402.
