Volume 7 Issue 2
The S2C Study – Securing Cannulas in Combat: a simulation-based mixed-method study
Benjamin R Mackie, Christopher Williams, Benjamin Tribe, Jessica Muscat, Bradley Clarke, Jessica Schults,
Gillian Ray‑Barruel, Nicole Marsh, Aldon Delport and Darwin Alvarenga
Keywords catheterisation, Ranger IV, tactical combat casualty care, occlusive dressings, securement, peripheral intravenous
For referencing Mackie BR et al. The S2C Study – Securing Cannulas in Combat: a simulation-based mixed-method study. Vascular Access 2021; 7(2):12-18.
DOI https://doi.org/10.33235/va.7.2.12-18
Abstract
Introduction Haemorrhage is the leading cause of preventable death in combat settings. Pre-hospital practice guidelines advocate for the rapid delivery of blood products and intravenous access to deliver adjunct treatments. However, peripheral intravenous catheter (PIVC) insertion is challenging in the austere setting, and catheter failure is common. This study aims to investigate dressing and securement methods to prevent catheter dislodgement and explore participants’ experiences with these methods within an Australian Defence Force Tactical Combat Casualty Care (TCCC) training course.
Methods and analysis A two-phase mixed-method design will be used to compare the Ruggedised Field (Ranger) method with the S‑Wrap technique to reduce PIVC dislodgement rates during TCCC simulations. The study will be undertaken at the Australian Army School of Health in Victoria, Australia. In Phase 1, we will examine the pull-out force of each dressing securement method in vitro. Phase 2 will involve (i) an observational audit (n=30 participants) of TCCC training to assess the quality and safety of PIVC securement practices and (ii) focus groups with participating healthcare professionals (HCPs) (n<15 participants) to understand their lived experiences of PIVC insertion and securement practices. Associations between pull-out forces will be assessed using analysis of variance, and descriptive statistics will be used to summarise audit data. Focus group data will be analysed using content analysis.
Strengths and limitations of this study
- PIVC insertion is challenging in the austere or combat setting, and catheter failure is common. Data from this study will reveal securement techniques that optimise practice in high threat/disaster settings and enhance patient safety and quality.
- This study will not be conducted in a real-life disaster or combat settings; however, high-fidelity simulations present an alternative option.
Background
The insertion of a peripheral intravenous catheter (PIVC) is one of the most common clinical procedures performed. About 30 million are used in Australia each year, with up to 70% of hospitalised patients requiring a PIVC at some point during their hospital stay.1 However, it is well documented that PIVCs often fail before the completion of intravenous (IV) treatment,2,3 with an incidence as high as 69%.3 Researchers have shown that this failure is, in part, a result of inadequate stabilisation or securement of the catheter to the skin.2,4 Poor securement technique increases the risk of phlebitis and infection and leads to patient pain, dislodgement, occlusion, or blockage of the catheter.1
Optimal dressing and securement of PIVCs in the pre-hospital setting is a crucial practice strategy to reduce complications and failure; however, variations in practice have been reported.5 PIVC dressing and securement are two inter-related interventions.3 A PIVC dressing should cover the insertion site, keeping it dry and clean, be comfortable for the patient, and offer protection from external contamination or trauma.6 Further, PIVCs should be secured to the skin and stabilise the PIVC hub to minimise catheter movement. PIVC dressings should be cost-effective, comfortable for the patient, and easy to remove.7 Recently, secondary analysis of over 40,000 PIVCs revealed that dressing and two-step securement combinations (tape dressing combined with tape or a tubular bandage) – termed ‘methods’ in this study – were significantly associated with decreased catheter site complications compared with a reference combination (simple polyurethane plus non-sterile tape over the dressing).8 Specifically, these two-step securement approaches were associated with fewer phlebitis symptoms, less bruising, and reduced micro-movement of the device within the vein.6,8
During pre-hospital resuscitation, a PIVC is essential for the administration of IV fluids, blood products, and medications, and PIVC failure before treatment completion can negatively impact patient outcomes.9,10 Disaster or high threat conditions, such as treating a trauma patient during a domestic terrorist event11 or combat, pose an even greater risk of accidental PIVC dislodgement (e.g., vehicle extrication, rapid night-time extraction via helicopter, or movement over rough terrain). Further, loss of vascular access in these austere settings may delay crucial fluid resuscitation or lead to missed treatment (e.g., pain relief), as limited opportunity or medical resources may prevent repeated PIVC insertion attempts.12 One way the American military has addressed the dislodgement risk in combat is to develop a securement intervention known as Ruggedised Field (Ranger) IV method.13 Army medics in the Australian Defence Force (ADF) have also developed a novel two-part securement method termed the S‑Wrap to reduce the chance of PIVCs being caught on snag hazards, such as combat webbing, vehicles, or weapons. However, the effectiveness of combat PIVC securement methods is unclear, and no studies were found that evaluated cannula securement in the austere, combat, or disaster context.
Researching in a disaster or combat setting has numerous inherent risks, including physical harm to researchers, breach of confidentiality, legal action, or psychological discomfort.14 When real-life situations preclude research opportunities, high-fidelity simulations present an alternative option. High fidelity simulations are defined as a healthcare education methodology that involves sophisticated life-like manikins. The ADF conducts high fidelity simulated combat casualty care training, termed Tactical Combat Casualty Care (TCCC). The TCCC training is mandated in the ADF for all healthcare professionals (HCPs), such as medics, paramedics, nurses, and physicians; thus, simulated TCCC training in the ADF affords a unique opportunity to evaluate the effectiveness of dressing and securement practices in the austere setting. This study will address this knowledge gap and assess combat PIVC securement to reduce catheter failure and any resultant patient harm.
Aims and objectives
The main aim of this study is to determine whether the Ranger IV or S‑Wrap securement method is associated with lower rates of PIVC dislodgement. A secondary aim is to evaluate PIVC dressing and securement practices and HCPs’ experiences during TCCC training.
Methods
Study design
A pragmatic mixed-method design with two phases will be used to compare the Ranger IV method to the S‑Wrap technique to reduce PIVC dislodgement rates during TCCC simulations.15 In Phase 1, the effectiveness of each method to prevent cannula dislodgement in vitro will be evaluated. In Phase 2, an observational audit of TCCC training will be conducted to understand the quality and safety of each method. At the completion of this training, focus groups with HCPs will occur to explore their experiences of PIVC insertion and securement practices.
Setting
The study will be undertaken at the Australian Army School of Health (ASH) in Victoria, Australia. The ASH has world-class facilities, particularly simulation that affords realistic medical training for members of the ADF who will provide patient care during military operations.
Intervention (PIVC securement methods)
Ruggedised Field (Ranger) IV method
This method involves a peripheral 18 gauge (G) IV catheter and saline lock that is established and covered with a bordered polyurethane dressing; the next step uses an 18G steel needle to connect to the IV administration tubing. The hard needle is inserted through the transparent dressing into the saline lock and IV administration tubing secured with a Line-backer IV securing device to the skin/limb (e.g., Morrison Medical Line-backer Tapeless, USA). The Line-backer IV Securing Device is a tapeless, secondary securement device that holds IV tubing in place with a secure hook and loop closure (Figure 1).13
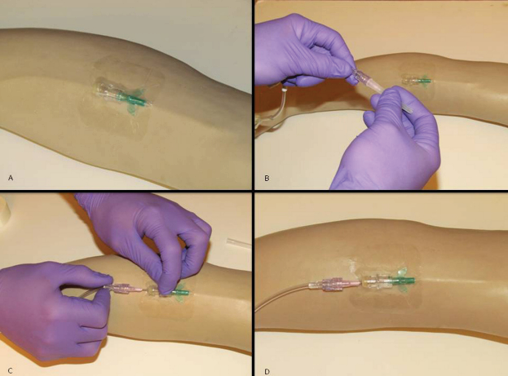
Figure 1. Ranger lock IV
S‑Wrap method
Following successful PIVC insertion, the S‑Wrap technique combines a standardised securement technique using an occlusive 15x20cm film dressing (e.g., Tegaderm™ IV Advanced Securement Dressing, 3M Healthcare, USA) and tape (i.e., 3M™ Micropore, 1 inch). The IV administration set is connected to the PIVC and then looped in an S‑shape between several layers of crepe bandage (10cm) (Figure 2).

Figure 2. S-Wrap
Study procedures
Phase 1
The study will investigate the pull-out force in vitro of two PIVC securement methods. Pull-out force is defined in this study as the tensile force (N) required to produce PIVC dislodgement. Pigskin (i.e., in vitro method) secured to a mannekin/simulated IV phlebotomy training arm will be used as a surrogate for human skin because it shares similar epidermal and dermal thickness ratios to human skin.16
Force measurement
The force during continuously more vigorous pulling until PIVC dislodgement occurs will be recorded. Each PIVC will be secured to a pork belly cutlet. Six PIVCs will be secured using the Ranger IV method, and six using the S‑Wrap method. Administration sets with a Luer lock adaptor (Baxter Healthcare, Old Toongabbie, NSW) will be attached to the PIVCs, and the IV administration line injection port will be attached to a cord at two alternating angles. An angle of 30° and 0° (to the positive x-axis) have been selected because they attempt to replicate how a PIVC administration set may become caught on a fixed object from any angle (e.g., tree branch, stretcher, body armour) in an austere combat setting. The cord will pass through a pulley and attach to a Digital Force Gauge (DFG). A DFG is a measuring instrument used across industries to measure the force during a push or pull test and aligns with previous studies examining PIVC dressing securement.10,17
A study team member experienced in cannulation [CW] will perform all the securement methods, while another member of the research team [BT] observes their practice to ensure consistency and accuracy. The third study member [BM] will perform the pull-out force tests by continuously increasing tension on the catheter securement (Figure 3). The peak force occurring before dislodgement or equipment failure will be recorded from continuous force recordings.

Figure 3. Experimental setup – force measurement
(a) IV line injection port; (b) Pulley at 30° to the horizontal; pulley will be rotated to achieve lateral 0°; (c) Digital Force Gauge and direction of pull.
Phase 2
Sample
Given the focussed nature of this study, purposeful sampling will be used. Thus, this study will be conducted in conjunction with existing training courses, and students completing the TCCC course will be invited to participate. The ASH conducts TCCC training for approximately 200 medics, paramedics, nurses, and medical doctors each year. Due to the TCCC course requirements, all students receive familiarisation training and will practice standardised PIVC securement approaches, the S‑Wrap securement method, and will cannulate simulated casualties via a Laerdal™ training arm. During the field phase of the TCCC course, students who choose to participate in the study will be allocated using the randomised block approach, to use either the Ranger IV method or the S‑Wrap method. All students >18 years attending the course will be eligible for participation.
Sample size
The recruitment target is 30 participants. Authors recommend that for clinical observational audit projects, a ‘snapshot’ sample of between 20–50 cases for process-based audits is adequate.18
Data collection
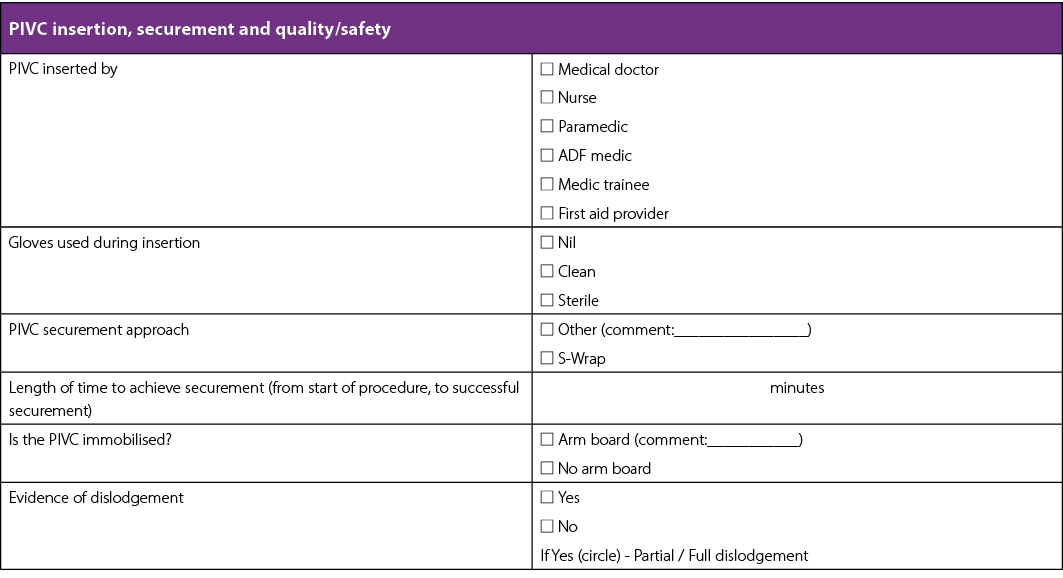
Audit evaluation: The quality and safety of PIVC securement will be assessed within existing TCCC courses using an observational audit checklist. Observational data collection embedded in ADF training has previously been reported with minimal impact on student teaching.19 As part of the TCCC course, students are routinely observed by their instructors, and two study team members [CW, BT], both experts in TCCC training, will attend the combat field simulations to observe the safety and quality of securement practices. A research team member experienced in observational data collection [BM] will also observe for evidence of PIVC insertion and securement complications using an observational field guide (Appendix 1 – observational data collection tool). Maintaining situational awareness is a fundamental component of TCCC; thus, the time taken to complete the securement method will also be measured. Also, the use of an arm board with the S‑Wrap method may occur in the field if the patient is deemed agitated and immobilisation of a limb is necessary; thus, the use of an arm board to aid in PIVC securement will be recorded.
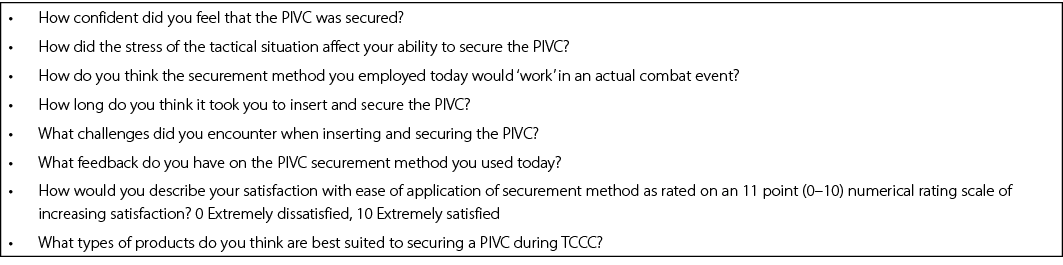
Qualitative evaluation: At the completion of TCCC training in which PIVC securement has occurred, a purposive sample of HCPs will be invited to provide feedback on their experiences. The sample size will be determined by achievement of data saturation; however, previous studies20 suggest 10–15 participants will be recruited. Focus groups will be conducted using an interview guide and take no more than 30 minutes to complete. Semi-structured questions will be posed to elicit participants’ experiences of inserting and securing PIVCs using both methods (Appendix 2). Data will be audio-recorded and professionally transcribed verbatim. Demographic and background data will be collected from all participants at the beginning of the focus groups/interviews, and include: rank, Corps, previous experience in cannulation, method approach used during TCCC, and current healthcare qualifications.
Data analysis
Descriptive statistics will be used to summarise the data collected (Phases 1 and 2), including the use of mean/median, range, and standard deviation values (as appropriate) for continuous data, and frequencies and percentages for categorical data. In Phase 1, associations between pull-out force and method characteristics will be assessed using one-way analysis of variance, followed by a posthoc Bonferroni test. The Bartlett test will be used to verify assumptions. Statistical significance will be determined at p≤0.05. Data will be analysed using PASW 22.0 (SPSS Inc., Chicago, IL). In Phase 2, focus group data will be analysed using content analysis, where data are grouped around central, recurrent ideas and themes developed through research team consensus.21 Focus group member-checking will occur as needed to ensure the trustworthiness and validity of the findings.
Ethics
This study was reviewed by the Departments of Defence and Veterans’ Affairs Human Research Ethics Committee (DDVA HREC) and deemed to be an evaluation activity, and the proposal upholds the principles of the National Statement on Ethical Conduct in Human Research and the Ethical Consideration in Quality Assurance and Evaluation Activities.22 Participants will be provided an information flyer on the first day of the TCCC course explaining the nature and purpose of the study in simple language. Participation is voluntary; that will be made explicit to HCPs attending the TCCC course. There is no obligation to take part in the study. If they choose not to participate, there will be no detriment to their learning or course outcomes.
On completion of the field training component of TCCC, HCPs will be invited to participate in a brief focus group, and informed consent procedures will be followed. Anonymity cannot be guaranteed due to the nature of the focus groups, however, participant information will be treated in the strictest confidence and they will not be individually identifiable in the resulting report or other publications. At the commencement of data collection, each participant will be given a pseudonym and any identifying information (such as rank) will be removed. No names will be used in any reports or papers. Data will be stored securely in a password-protected database and paper copies in a locked filing cabinet, as per the Australian National Health and Medical Research Council guidelines.23
Discussion
Haemorrhage from the injured extremity is the primary cause of preventable death in military settings,24,25 and TCCC guidelines advocate for the rapid delivery of IV blood products and adjunct treatments such as tranexamic acid for the hypovolaemic trauma patient in the tactical environment.13 However, PIVC insertion is challenging in the austere setting, and failure is common. This study will compare two techniques to optimise PIVC dressing and securement in a simulated setting. As prior research in this area is limited, the study findings will inform defence personnel training in high threat and disaster settings to improve patient safety and quality. Further, publication of the results will ensure the best PIVC dressing and securement practices are embedded into future ADF or equivalent pre-hospital training to reduce catheter failure and any resultant patient harm. This study may also inform the civilian pre-hospital management of PIVCs and those involved in inter-hospital patient retrieval and transport.
Strengths and limitations
This single-centre simulation-based mixed-method study inevitably will not be blinded; however, it will be the first to evaluate PIVC securement in the austere, combat context. In Phase 1, the dressings and securements will only be attached for a short period of time before the pulling force will be applied. Therefore, cannulation site (e.g., cubital fossa, hand), and the effects of soiling, sweating, blood, or moisture will not be taken into account. The impact of each securement method on IV flow rates will not be examined. Further, only longitudinal and lateral traction forces will be examined in phase one. However, in Phase 2, observations of practice, and participants’ experiences of inserting and securing PIVC during TCCC training will occur. Additionally, member-checking will occur following each focus group to ensure the trustworthiness and validity of findings. The S‑Wrap method could not be taught and observed during TCCC because of limitations in accessing the required clinical consumables within the ADF. This study is not being conducted in a real-life disaster or combat setting; however, high-fidelity simulations present a pragmatic option.
Conclusion
PIVC insertion is challenging in the austere or combat setting and catheter failure is common. This study will compare two securement techniques that may optimise cannulation practice in high threat and disaster settings to enhance patient safety and quality. Findings from this study may also inform the civilian pre-hospital management of PIVCs, as well as those involved in inter-hospital patient retrieval and transport.
Ethics
This study was reviewed by the Departments of Defence and Veterans’ Affairs Human Research Ethics Committee (DDVA HREC) and deemed to be an evaluation activity.
Trial registration
ACTRN12621000314820
Conflict of interest
The authors declare no conflicts of interest.
Funding
The authors received no funding for this study.
Appendix 1. Observational data collection tool
Appendix 2. Focus group schedule
Author(s)
Benjamin R Mackie*1,2,3,4, Christopher Williams1, Benjamin Tribe1, Jessica Muscat1, Bradley Clarke1, Jessica Schults5,6,
Gillian Ray‑Barruel2,3,4,7, Nicole Marsh3,8,9, Aldon Delport10, Darwin Alvarenga11
1 Army School of Health, VIC, Australia
2 Menzies Health Institute, QLD, Australia
3 Griffith University, School of Nursing and Midwifery, Brisbane, QLD, Australia
4 University of the Sunshine Coast, School of Nursing, Midwifery and Paramedicine, QLD, Australia
5 University of Queensland, School of Nursing Midwifery and Social Work, QLD, Australia
6 Department of Anaesthesia, Queensland Children’s Hospital, Brisbane, QLD, Australia
7 Queen Elizabeth II Jubilee Hospital, Brisbane, QLD, Australia
8 Royal Brisbane and Women’s Hospital, QLD, Australia
9 Queensland University of Technology, School of Nursing, QLD, Australia
10 Central Queensland University, School of Health, Medicine, and Applied Sciences, QLD, Australia
11 2nd General Health Battalion, QLD, Australia
*Corresponding author
Benjamin Mackie, Australian Army, Army School of Health, VIC, Latchford Barracks, Bandiana MILPO, 3694, Australia
Email Benjamin.mackie@defence.gov.au
References
- Keogh S, Alexandrou E, Bulmer A, Cooke M, Coventry L. Peripheral intravenous catheters: a review of guidelines and research; 2019 [cited 2020 Nov 19]. Available from: https://www.safetyandquality.gov.au/sites/default/files/2019-06/literature-review-peripheral-intravenous-catheters-a-review-of-guidelines-and-research_qut.pdf
- Marsh N, Webster J, Mihala G, Rickard CM. Devices and dressings to secure peripheral venous catheters to prevent complications. Cochrane Database Syst Rev. 2015(6).
- Marsh N, Larsen E, Genzel J, Mihala G, Ullman AJ, Kleidon T, et al. A novel integrated dressing to secure peripheral intravenous catheters in an adult acute hospital: a pilot randomised controlled trial. Trials. 2018;19(1):1–10.
- Royer T. Improving short peripheral IV outcomes: a clinical trial of two securement methods. JAVA. 2003;8(4):45–9.
- Mason MF, Wallis M, Lord B, Barr N. Prehospital use of peripheral intravenous catheters and intraosseous devices: an integrative literature review of current practices and issues. Australas Emerg Care. 2020.
- Rickard C, Ullman A, Kleidon T, Marsh N. Ten tips for dressing and securement of IV device wounds. Aust Nurs Midwifery J. 2017;24(10):32–4.
- Campbell C, Bowden T. Peripheral vascular access devices: care and maintenance. Br J Card Nurs. 2011;6(3):132–40.
- Corley A, Ullman AJ, Mihala G, Ray-Barruel G, Alexandrou E, Rickard CM. Peripheral intravenous catheter dressing and securement practice is associated with site complications and suboptimal dressing integrity: a secondary analysis of 40,637 catheters. Int J Nurs Stud. 2019;100:103409.
- Bester B, Sobuwa S. Utilisation of prehospital intravenous access. SAMJ. 2014;104(9):615–8.
- Schmutz A, Menz L, Schumann S, Heinrich S. Dislodgement forces and cost effectiveness of dressings and securement for peripheral intravenous catheters: a randomized controlled trial. J Clin Med. 2020;9(10):3192.
- Quaile A. Manchester stands united after terrorist attack. JPP. 2018;10(7):300.
- Oliver G, Jones M. ECG or x-ray as the ‘gold standard’ for establishing PICC-tip location? Br J Nurs. 2014;23(Sup19):S10-S6.
- Morgan TR. Evaluation of fluid bolus administration rates using ruggedized field intravenous systems. WEM. 2014;25(2):204–9.
- Collogan LK, Tuma F, Dolan-Sewell R, Borja S, Fleischman AR. Ethical issues pertaining to research in the aftermath of disaster. J Trauma Stress. 2004;17(5):363–72.
- Teddlie C, Tashakkori A. A general typology of research designs featuring mixed methods. Research in the Schools. 2006;13(1):12–28.
- Summerfield A, Meurens F, Ricklin ME. The immunology of the porcine skin and its value as a model for human skin. Mol Immunol. 2015;66(1):14–21.
- Simonova G, Rickard C, Dunster K, Smyth D, McMillan D, Fraser J. Cyanoacrylate tissue adhesives–effective securement technique for intravascular catheters: in vitro testing of safety and feasibility. Anaesth Intensive Care. 2012;40(3):460–6.
- University Hospital of Bristol. How to: set an audit sample & plan your data collection; 2009 [cited 2020 Nov 20]. Available from: http://www.uhbristol.nhs.uk/files/nhs-ubht/5%20How%20To%20Sample%20Data%20Collection%20and%20Form%20v3.pdf
- Whitney SJ, Fidock JJ, Ferguson N. Assessing the effectiveness of simulation-based counter-IED training. J Battlef Technol. 2012;15(1):57–64.
- Hancock ME, Amankwaa L, Revell MA, Mueller D. Focus group data saturation: a new approach to data analysis. Qual Rep. 2016;21(11):2124.
- DeSantis L, Ugarriza DN. The concept of theme as used in qualitative nursing research. West J Nurs Res. 2000;22(3):351–72.
- NHMRC. Ethical considerations in quality assurance and evaluation activities. Australian Government; 2014. Available from: https://www.google.com/url?q=https://www.nhmrc.gov.au/about-us/resources/ ethical-considerations-quality-assurance-and-evaluation-activities&sa =D&source=editors&ust=1628737372899494&usg=AOvVaw3SRR3X- zYOnnmFACXw0oiO]
- NHMRC. Australian National Health and Medical Research Council guidelines; 2020. Available from: https://www.google.com/url?q=https://www.nhmrc.gov.au/ guidelines&sa=D&source=editors&ust= 1628737372898654&usg=AOvVaw0pX ArFbnFmxdGksXxBKvdx]
- Unlu A, Kaya E, Guvenc I, Kaymak S, Cetinkaya R, Lapsekili E, et al. An evaluation of combat application tourniquets on training military personnel: changes in application times and success rates in three successive phases. BMJ Mil Health. 2015;161(4):332–5.
- Kotwal RS, Montgomery HR, Kotwal BM, Champion HR, Butler FK, Mabry RL, Cain JS, Blackbourne LH, Mechler KK, Holcomb JB. Eliminating preventable death on the battlefield. Arch Surg. 2011 Dec 1;146(12):1350–8.
___________________________

Vascular Access – Call for Papers
The next issue deadline for submissions is 24 January 2022
The Australian Vascular Access Society (AVAS) is an association of healthcare professionals founded to promote the vascular access specialty (http://avas.org.au/). Our multidisciplinary membership strives to advance vascular access research, promotes professional and public education to shape practice and enhance patient outcomes, and partners with industry to develop evidence-based innovations in vascular access.
The electronic journal Vascular Access is the official publication of AVAS, and provides a venue for national and international scholars and practitioners to publish high-quality peer-reviewed research and educational reviews relevant to vascular access in Australia and globally. The journal also provides a space for evidence-based discussions and debate on issues of importance to patients requiring vascular access.
Vascular Access is published twice a year (April and October) and manuscripts pertaining to this specialty are invited. The editor welcomes manuscripts in the form of research findings, scoping or systematic protocols, clinical papers, case studies, reports, literature reviews, letters and product appraisals. Video submissions are also welcomed. Submissions will be accepted from any country but must be in English.
For more information or to receive a copy of the Author Guidelines, please contact Linda M. Verde, Editor-in-Chief, at info@avas.org.au.
