Volume 39 Number 1
Chemotherapy-induced pyoderma gangrenosum
Michelle Wai Kuen Lee, Steven Kar Kay Chan, Amy Choi Ching Fong and Kristie Wai Sze Ho
Keywords Pyoderma gangrenosum, chemotherapy, azacitidine
For referencing Lee MWK et al. Chemotherapy-induced pyoderma gangrenosum. WCET® Journal 2019; 39(1):9-17
DOI https://doi.org/10.33235/wcet.39.1.9-17
Abstract
Pyoderma gangrenosum (PG) is a refractory, painful, non-infectious, ulcerative and inflammatory skin condition. Approximately 50% of patients with PG showed an existing systemic disease, such as inflammatory bowel conditions, haematological disorders, rheumatoid diseases or hepatopathies. Some patients developed PG following acute trauma or injury in a process known as pathergy. In the other cases, PG is characterised by isolated skin lesions with unknown causes and classified as idiopathic. However, in recent decades, PG has been reported in patients treated with certain medications. In this manuscript, we report two cases of PG, which were triggered by chemotherapy in patients with myelodysplastic syndrome (MDS) and chronic myelomonocytic leukaemia (CMML).
Introduction
Pyoderma gangrenosum (PG) is a refractory, painful, non-infectious, ulcerative and inflammatory skin condition, which was first described by Brocq in 19161. In 1930, Brunstring et al. named it pyoderma gangrenosum2. It is commonly associated with underlying systemic diseases and occurs most frequently between 40 and 60 years old1,3-6. Typical PG can occur on any skin surface, but is most commonly seen over lower limbs and often leaves cribriform scars after the wounds have healed2,7.
Approximately 50% of patients with PG showed an existing systemic disease, such as inflammatory bowel conditions, haematological disorders, rheumatoid diseases or hepatopathies1,8. Some patients developed PG following acute trauma or injury in a process known as pathergy2,9-11. In the other cases, PG is characterised by isolated skin lesions with unknown causes and classified as idiopathic12. However, in recent decades, PG has been reported in patients treated with certain medications. In the review by Wu et al.13, 43 cases of drug-induced PG were identified. To follow is a report of two cases of PG, which were triggered by chemotherapy in patients with myelodysplastic syndrome (MDS) and chronic myelomonocytic leukaemia (CMML).
Case 1
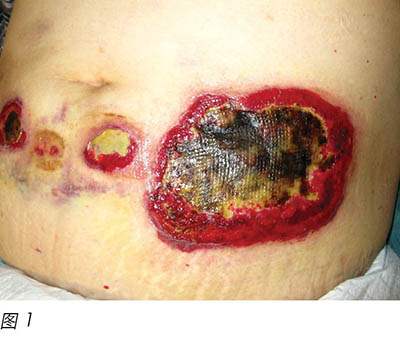
A 58-year-old female was diagnosed with MDS. MDS is a collection of pathologically and cytogenetically distinct bone marrow disorders characterised by peripheral blood cytopenias and will result in an increased risk of bleeding and infectious complications14. In addition, these patients have a tendency to develop acute myeloid leukaemia (AML)14,15. Azacitidine, a chemical analogue of cytosine, is a chemotherapy drug used to treat conditions that affect the blood and the bone marrow. This was given via subcutaneous injection for the patient for one week. On day 8, the patient developed non-neutropenic septic shock and multiple skin lesions were noted over her abdomen (injection site), which required admission to the intensive care unit. Initially the lesions were erythematous, which rapidly progressed into blisters and finally skin necrosis occurred. The wounds were well circumscribed, with a ring-shaped large ulceration and elevated oedematous borders (Figure 1).
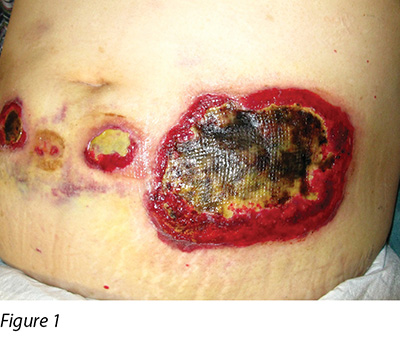
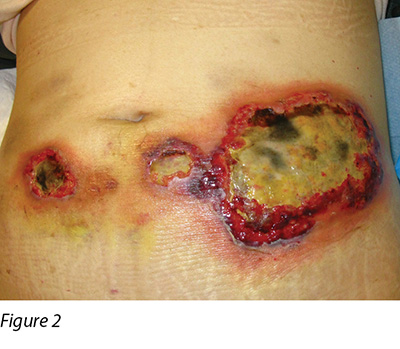
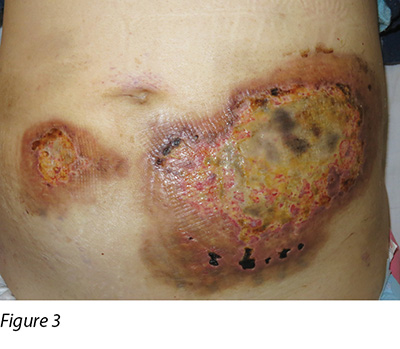
Wound culture indicated there was no particular bacterial, fungal or mycobacterial organisms. The wound biopsy demonstrated inflammatory neutrophilic dermatosis. A dermatologist was consulted and PG was finally diagnosed. Methylprednisolone 50 mg daily was commenced orally. One month following oral steroid therapy, the edge of the wound remained violaceous and it was evident that the PG was still active (Figure 2). Cyclosporine, an immunosuppressant medication, and doxycycline, a broad-spectrum antibiotic of the tetracycline class, were added to the treatment regimen. Subsequently, the wounds were less violaceous in appearance and epithelialisation was noted from the edge (Figure 3). In addition, less pain was experienced by the patient. Methylprednisolone was then decreased gradually to 5 mg with cyclosporine 70 mg and doxycycline 100 mg daily as a maintenance dose. The patient was discharged from the hospital afterwards and wound care was continued by the community nurse every alternate day.
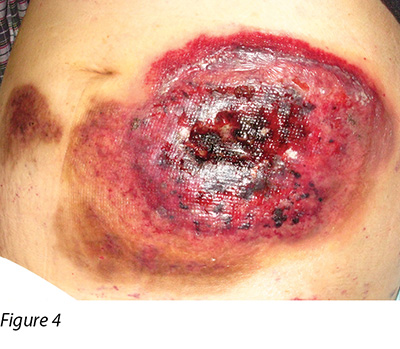
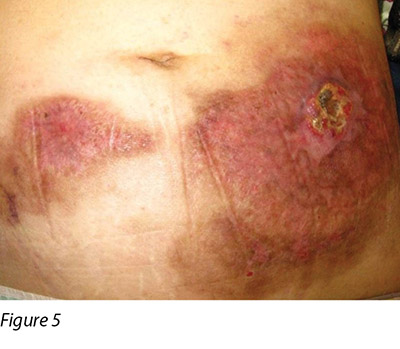
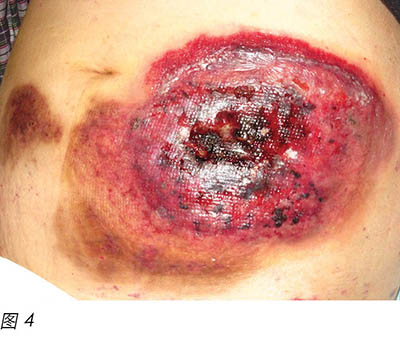
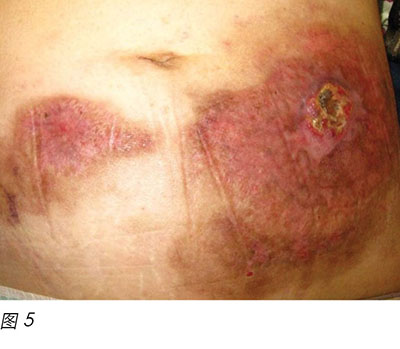
Two months later, the patient's general condition deteriorated and her white blood cells were found to be in a rising trend during follow-up in the haematology clinic. After discussion with the patient and her family members, the patient was admitted to the hospital again and decitabine cycle 1 was given intravenously. Decitabine is another DNA methyltransferase depleting drug for the treatment of MDS16. Unfortunately, two weeks following the introduction of this medication, the patient reacted with neutropenic fever again and a flare-up of PG eventuated (Figure 4). Methylprednisolone 30 mg daily and cyclosporine 40 mg twice a day were recommenced, with recognised improvement in the wound (Figure 5). Dosage of both drugs was gradually decreased as the improvement continued. Conversely, another two months later, PG flared up again after decitabine cycle 2 was given. A high dose of methylprednisolone and cyclosporine were recommenced. However, the patient's prolonged neutropenic state complicated her deteriorating health and she passed away two months following active treatment.
Case 2
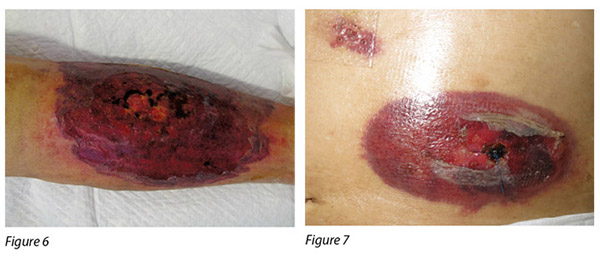
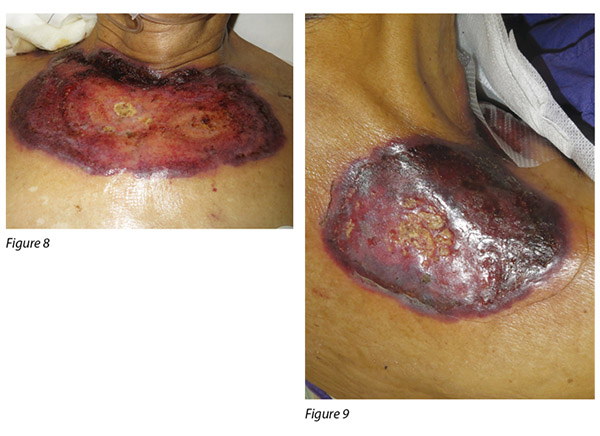
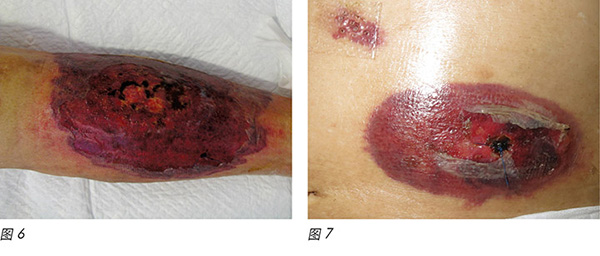
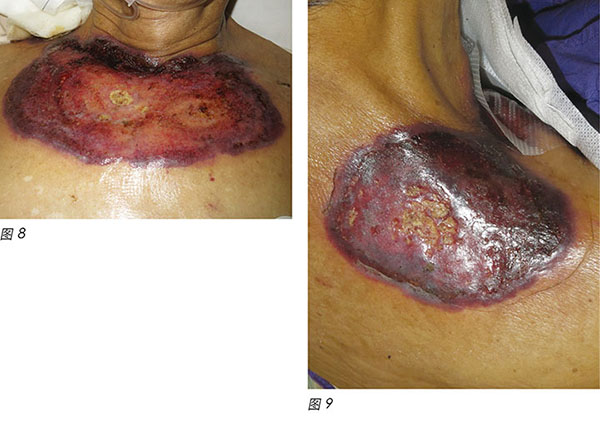
A 72-year-old male was diagnosed with CMML. CMML is a pathologically heterogeneous disease with overlapping morphologic features of both myelodysplastic syndromes and myeloproliferative neoplasms17. It is accompanied by bone marrow dysplasia, cytopenias and hepatosplenomegaly18. As a result of the patient having progressive anaemia and thrombocytopenia, azacitidine was commenced. The first cycle of azacitidine was well tolerated by the patient. Four days into his second cycle, multiple erythematous, painful pustular plaques with violaceous borders appeared initially on the left lower limb, then became generalised over his abdomen, chest wall and shoulder (Figure 6-9).
An incisional wound biopsy over the abdomen and left lower limb demonstrated diffuse dense infiltration of the dermis and superficial subcutaneous tissue by polymorphs with focal fat necrosis. The overall features were consistent with neutrophilic dermatosis and indicative of PG. Microbiological studies of the wounds were negative for both aerobic and anaerobic bacterial growth. However, the patient developed a neutropenic fever and multiple antibiotics were given. In the presence of a depressed immune system, the sepsis could not be controlled and the patient died two weeks following the commencement of treatment.
Discussion
Azacitidine (AZA) is a DNA methyltransferase inhibitor, which has been shown to improve overall survival in patients with MDS and its sub-types19-21. However, AZA-induced demethylation of DNA may cause epigenetic changes, which lead to increased interferon production and cytoskeletal rearrangements; these changes may support the pathogenesis of AZA-induced PG by upregulating inflammation and neutrophil migration13. This side effect had been demonstrated in individual reports and literature21-23. The report of two patient case studies have also demonstrated AZA-induced PG.
Azacitidine can be administered by using intravenous or subcutaneous routes. However, it was known that skin lesions, ecchymosis, petechiae and skin induration following subcutaneous injection could be developed in up to 97% of patients23-24. Azacitidine-induced injection site PG was rare, but a single case was reported recently by Roy et al.21. In case study 1, the patient suffered from injection site complications following eight days of treatment. Some literature reported that changing the needle with no azacitidine residue before injection could reduce the incidence of injection-site reactions but control studies measuring this were limited24.
Another drug used in case study 1 was decitabine. It is a DNA methyltransferase (DNMT1)-depleting drug approved for treatment of MDS. In 2017, Saleh and Saunthararajah reported successfully treated MDS-induced PG by using decitabine25. However, PG relapsed in case study 1 during the treatment of decitabine. Further studies concerning the relationship between PG and decitabine are warranted.
Wound management
Topical therapy is a significant issue in all the patients with PG but there is no consensus in the management of these wounds1,26. The treatment is largely empirical and depends on the severity and extent of the lesions. The overall goals of local wound management are to reduce lesion inflammation, decrease pain and promote wound healing1-2. Some topical drugs, such as the application of tacrolimus, have been reported, where wounds show no further extension, regression of the inflammatory border and pain relief. However, patients' serum creatinine was increased27-28. Therefore, systemic absorption should be closely monitored and a clinical trial in this area is suggested to measure the risk and benefits of the topical drug.
The literature has indicated moist wound management to be the cornerstone in managing PG wounds as it can improve wound-related pain, facilitate autolytic debridement and promote angiogenesis8. Various dressings, such as polyurethane foam, Hydrofiber and alginate dressings, are documented in individual PG wound management with resolving erythema, flattened epibole edges and pain relief1,29. In the first case, the Hydrofiber dressing had been tried but the patient could not tolerate it because of severe pain. It might be due to the hydrophilic effect of the dressing. In addition, because of less exudate of the wound, the dressing adhered to the wound bed and increased pain on removal. This might also increase the potential to trigger pathergy30. Hydrogel dressings are formulations of water, polymers and other ingredients. They are designed to hydrate the wound tissue, keep nerve endings moist to reduce pain and maintain a moist environment for cell migration31. In light of there being no bacterial growth within the tissues of the PG wounds for our two patients, Hydrogel with a tulle dressing were applied to facilitate autolytic debridement and reduce pain. On the other hand, although the underlying cause of PG is non-infectious, most of the patients are prescribed corticosteroids; therefore, caution should be made to prevent bacterial infection. The wounds were closely monitored for clinical signs of infection, such as erythema, warmth, increased pain, increased exudate and malodour30. Although skin flora was identified from the wound in the later stage of case 1, it was assessed that topic antimicrobial wound dressings were not necessary.
In view of the potential pathergy in the development and acceleration of the condition, both of the reported cases did not receive any surgical intervention nor conservative sharp wound debridement during the treatment period1. The evidence has shown that effective management of the systemic disease often results in improvement of the skin ulcerations1,2,26. Therefore, apart from local wound care, systemic corticosteroids and cyclosporine are recommended as first-line systemic agents for the management of patients with PG32.
Pain control
Apart from vegetative variants, patients with PG almost entirely experience debilitating pain26. The source of pain may be multifactorial, but in most cases it is associated with the inflammatory process and the subsequent grave ulcer33. Repeated manipulation of the wound, such as wound cleansing and trauma during wound dressing removal, is a source of distress for patient33. Therefore, addressing the patient's pain level is crucial in treatment efficacy. Both our patients in the case studies received analgesic, Tramadol, 50 mg every six hours orally, if necessary with an additional dose during wound dressing changes in order to achieve adequate pain control. Conversely, when patients' disease and inflammation are well controlled by systemic therapy and appropriate wound management, pain may subside gradually.
Conclusion
The pathogenesis of PG still remains uncertain, although current evidence suggested that it has an autoimmune aetiology with defects in immune regulation of the inflammatory response. PG is also associated with various systemic conditions such as inflammatory bowel disease, haematological disorders, and autoimmune arthritis. Pathergy is an exaggerated response to minor trauma in patients with PG. However, chemotherapy is another possible triggering factor, which should be considered, particularly in patients receiving specific drug treatments. The two case studies demonstrated this serious side effect of azacitidine. Early recognition of this complication is important to avoid undue delays in the treatment of the underlying malignancy, but also to initiate appropriate therapy against PG.
Conflict of Interest
The authors declare no conflicts of interest.
Funding
The authors received no funding for this study.
化疗导致的坏疽性脓皮病
Michelle Wai Kuen Lee, Steven Kar Kay Chan, Amy Choi Ching Fong and Kristie Wai Sze Ho
DOI: https://doi.org/10.33235/wcet.39.1.9-17
摘要
坏疽性脓皮病(PG)是一种顽固性、疼痛、非感染性、溃疡性皮肤炎症。大约50%的PG患者存在既有全身性疾病,例如炎症性肠病、血液疾病、类风湿性疾病或肝脏疾病。某些患者在急性外伤或损伤后出现PG,此过程称为病态反应性。另外一些PG患者的特征性表现是原因不明的分散性皮肤病变,并且归类为突发性。但
是,近数十年的报道表明,接受某些药物治疗的患者出现了PG。本文中,我们报告两例骨髓增生异常综合征(MDS)和慢性髓细胞性白血病(CMML)患者接受化疗所诱发的PG。
前言
坏疽性脓皮病(PG)是一种顽固性、疼痛、非感染性、溃疡性皮肤炎症,由Brocq于19161年首次描述。1930年,Brunstring等人将其命名为坏疽性脓皮病2。PG通常与基础全身性疾病相关,大多发生在40至60岁患者上1,3-6。典型的PG可以出现在任何皮肤表面,但最常见于下肢,伤口愈合后常常留下筛状疤痕2,7。
大约50%的PG患者存在既有全身性疾病,例如炎症性肠病、血液疾病、类风湿性疾病或肝脏疾病1,8。某些患者在急性外伤或损伤后出现PG,此过程称为病态反应性2,9-11。另外一些PG患者的特征性表现是原因不明的分散性皮肤病变,并且归类为突发性12。但是,近数十年的报道表明,接受某些药物治疗的患者出现了PG。Wu等人13在综述中确定了43例药物导致的PG。以下报告两例骨髓增生异常综合征(MDS)和慢性髓细胞性白血病(CMML)患者接受化疗时诱发的PG。
病例 1
一位58岁女性被确诊患有MDS。MDS是一组具有鲜明病理学和细胞遗传学特征的骨髓疾病,特征性表现是外周血中血细胞减少,导致出血和感染并发症的风险增加14。另外,这些患者都有患急性骨髓性白血病(AML)的倾向14,15。阿扎胞苷是胞嘧啶的一种化学类似物,是一种用于治疗血液和骨髓疾病的化疗药物。该药经皮下注射,每周一次。第8天时,患者出现非中性粒细胞减少性败血性休克,并且在她的腹部(注射部位)注意到多发性皮肤病变,需要转入重症监护病房。最初,皮肤病变为红斑,随之迅速进展为水疱,最终发生皮肤坏死。伤口界限清晰,呈一个环形大块溃疡,伴凸起水肿边界(图1)。
伤口培养表明,未见特定细菌、真菌或分枝杆菌。伤口活检证明为炎症性嗜中性皮肤病。经皮肤科专家会诊后最终确诊为PG。开始口服甲泼尼松 50 mg, 每日一次。口服类固醇治疗一个月后,伤口边缘仍呈紫色,证明仍处于PG活动期(图2)。在治疗方案中添加了免疫抑制剂环孢素和四环素类广谱抗生素多西环素。治疗后,伤口紫色减退,边缘出现上皮化(图3)。另外,患者的疼痛有所缓解。随后,甲泼尼松剂量逐渐减至 5 mg,联合环孢素70 mg和多西环素 100 mg,每日一次,作为维持剂量。患者出院后,由社区护士每隔一天实施伤口护理。
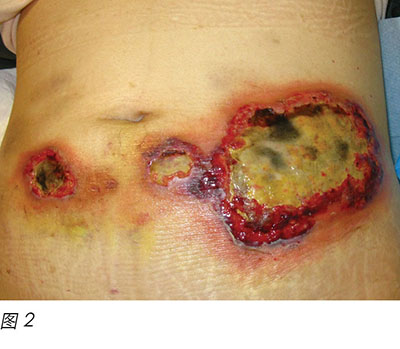
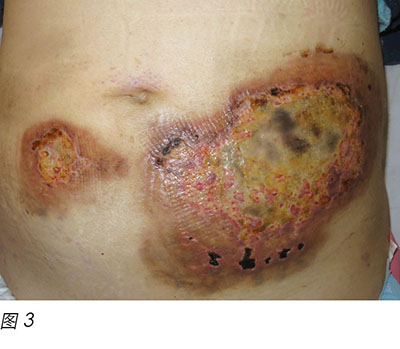
两个月后,患者病情恶化,在血液科门诊随访时发现白细胞呈上升趋势。与患者及其家人讨论后,患者再次入院,接受一个周期地西他滨静脉输注。地西他滨是治疗MDS的另外一种DNA甲基转移酶消耗药物16。不幸的是,在引入这种药物两星期后,患者再次出现发热性中性粒细胞减少,导致PG突然复发(图4)。再次开始甲泼尼松30 mg 每日一次,联合环孢素40 mg 每日两次,伤口出现明显改善(图5)。随着伤口继续改善,两种药物逐渐减量。在给予第2周期地西他滨治疗后的两个月,PG再次突然复发。再次开始高剂量甲泼尼松和环孢素治疗。但是,患者长时间的中性粒细胞减少状态导致其健康状况恶化,在活性治疗两个月后去世。
病例 2
一位72岁男性被确诊患有CMML。CMML是一种病理学异质性疾病,形态学特征与骨髓增生异常综合征和骨髓增生性疾病相同17。CMML伴有骨髓发育不良、血细胞减少和肝脾肿大18。因此,患者出现进行性贫血和血小板减少症,开始阿扎胞苷治疗。患者对第一周期阿扎胞苷耐受良好。第二周期开始四天后,最初在左下肢出现多发性红斑状脓疱,有紫色边界,随后遍及腹部、胸壁和肩部(图6-9)。
对腹部和左下肢伤口进行活组织切口检查发现,真皮和浅表皮下组织内出现弥漫性密集浸润,伴有多形状局部脂肪坏死。总体特征符合嗜中性皮肤病,表明PG。对伤口实施微生物学检测,未见需氧和厌氧菌生长。但患者出现发热性中性粒细胞减少,给予多种抗生素。由于存在免疫系统抑制,脓毒症无法控制,开始治疗后两周时患者去世。
讨论
阿扎胞苷(AZA)是一种DNA甲基转移酶抑制剂,已被证明用于治疗MDS及其亚型疾病时可改善总体生存率19-21。但是,AZA诱导的NDA去甲基化可能会引起表观遗传学改变,这会增加干扰素生成和细胞骨架重排。这些改变可能通过上调炎症和中性粒细胞迁移支持AZA诱导的PG的发病机制13。个案报道和文献已证实此副作用21-23。两份患者的病例研究报告也证明AZA诱导PG。
阿扎胞苷可经静脉或皮下给药。但是,已知多达97%的患者在接受皮下注射后可能会出现皮肤病变、瘀斑、瘀点和皮肤硬结23-24。AZA诱导的注射部位出现PG很罕见,但Roy等人最近曾报道一例21。在病例研究1中,患者经治疗八天后,注射部位出现并发症。某些文献报道,注射前更换不含阿扎胞苷残留物的注射针头可能会降低注射部位产生反应的发生率,但测量此项措施效果的对照研究有限24。
病例研究1中使用的另外一种药物是地西他滨。这是一种DNA甲基转移酶(DNMT1)消耗药物,经批准用于MDS治疗。2017年,Saleh和Saunthararajah报道了使用地西他滨成功治疗MDS诱发的PG25。但是,病例研究1中,PG在地西他滨治疗期间复发。有必要就PG与地西他滨之间的关系开展进一步研究。
伤口处理
对于所有PG患者来说,局部治疗是一个重要问题,但对伤口处理尚未达成共识1,26。治疗主要依靠经验,取决于病变的严重程度和范围。局部伤口处理的总体目标是减轻病变炎症和疼痛,促进伤口愈合1-2。据报道,采用某些局部治疗药物(例如他克莫司)后,伤口不再继续扩大,炎症边界缩小,疼痛减轻。但是,患者的血肌酐增加27-28。因此,应严密监测全身吸收,建议在此领域开展一项临床研究,以便权衡局部药物的风险与益处。
文献表明,湿润伤口处理是PG伤口处理中最重要的部分,因为它可以缓解伤口疼痛、促进自溶清创,改善血管生成8。据记载,在个案PG伤口处理中,诸如聚氨酯泡沫、亲水纤维和海藻酸盐敷料等各类敷料可消退红斑、扁平卷起边缘并缓解疼痛1,29。在第一个病例中,曾尝试使用亲水纤维敷料,但由于疼痛剧烈,患者无法耐受。可能是敷料的亲水作用所致。另外,由于伤口渗出物少,敷料会与伤口床粘连,拆除时会增加疼痛。这也可能增加引发病态反应性的风险30。水凝胶敷料配方含水分、聚合物和其他成分。按设计,这些成分可以为伤口组织补充水分,保持神经末梢湿润以便减少疼痛,为细胞迁移保持湿润的环境31。鉴于本文两例患者的PG伤口组织内未见细菌生长,采用了水凝胶薄纱敷料以期促进自溶清创和缓解疼痛。在另一方面,尽管造成PG的潜在原因是非感染性的,但大多数患者接受皮质类固醇治疗,因此应该注意防止细菌性感染。密切监视了伤口的临床感染迹象,如红斑、温热、疼痛加剧、渗出物增多和异味30。虽然在病例1的后期从伤口中发现皮肤菌群,但是经评估,无需使用局部抗菌伤口敷料。
就病态反应性对于病情加速的潜在作用而言,报道的两例在治疗期间均未接受任何外科干预或保守的锐器伤口清创术1。证据显示,有效管理全身性疾病通常会改善皮肤溃疡1,2,26。因此,除局部伤口护理外,作为PG患者疾病管理的一线全身治疗,建议全身应用皮质类固醇和环孢素32。
疼痛控制
除增殖型PG外,PG患者几乎均出现令人难以忍受的疼痛26。疼痛是多种因素引起的,但大多数病例中,它与炎症过程和随后的凹陷型溃疡有关33。反复处理伤口,如伤口清洗和拆除敷料时引起的创伤,是造成患者疼痛的一个原因33。因此,控制患者的疼痛程度对于保证疗效至关重要。我们病例研究中的两例患者均接受口服镇痛剂曲马多(Tramadol) 50 mg,每六小时一次,如果需要,在更换伤口敷料时添加额外剂量,以便更好地控制疼痛。另一方面,通过全身性疗法和适当的伤口处理较好地控制患者的疾病和炎症后,疼痛可能会逐渐消退。
结论
尽管目前证据表明PG是一种自身免疫性疾病,对炎症反应的免疫调节存在缺陷,但PG的发病机制仍不清楚。PG还与多种全身性疾病有关,如炎症性肠病、血液疾病和自身免疫性关节炎。病态反应性是PG患者对轻微创伤的过激反应。但是,化疗是另外一个可能的触发因素,应该予以考虑,特别是正在接受某些特定药物治疗的患者。本文中的两个病例研究证明了阿扎胞苷的严重副作用。为避免对潜在恶性肿瘤的治疗造成不应有的延误,并开始对PG进行适当的治疗,早期发现此并发症都至关重要。
利益冲突
作者声明没有利益冲突。
资助
作者未因该项研究收到任何资助。
Author(s)
Michelle Wai Kuen Lee
Nurse Consultant, Wound and Stomal Therapy, Department of Surgery, Queen Mary Hospital, Hong Kong
Steven Kar Kay Chan
APN, Wound and Stomal Therapy, Department of Surgery,
Queen Mary Hospital, Hong Kong
Amy Choi Ching Fong
APN, Wound and Stomal Therapy, Department of Surgery,
Queen Mary Hospital, Hong Kong
Kristie Wai Sze Ho
APN, Central Nursing Division,
Queen Mary Hospital, Hong Kong
Correspondence to Michelle Wai Kuen Lee
Email leewkmichelle@gmail.com
References
Butcher M. Pyoderma gangrenosum: a diagnosis not to be missed. Wounds UK 2005. Available from: https://www.wounds-uk.com/resources/details/pyoderma-gangrenosum-a-diagnosis-not-to-be-missed-1. Accessed on 03/07/2018.
- Teagle A & Hargest R. Management of pyoderma gangrenosum. J R Soc Med 2014; 107(6):228–36.
- von den Driesch P. Pyoderma gangrenosum: a report of 44 cases with follow-up. Br J Dermatol 1997; 137:1000.
- Bennett ML, Jackson JM, Jorizzo JL et al. Pyoderma gangrenosum. A comparison of typical and atypical forms with an emphasis on time to remission. Case review of 86 patients from 2 institutions. Medicine (Baltimore) 2000; 79:37.
- Binus AM, Qureshi AA, Li VW & Winterfield LS. Pyoderma gangrenosum: a retrospective review of patient characteristics, comorbidities and therapy in 103 patients. Br J Dermatol 2011; 165:1244.
- Saracino A, Kelly R, Liew D & Chong A. Pyoderma gangrenosum requiring inpatient management: a report of 26 cases with follow-up. Australas J Dermatol 2011; 52:218.
- Brooklyn T, Dunnill G & Probert C. Diagnosis and treatment of pyoderma gangrenosum. BMJ 2006; 333:181–4.
- Wollina U. Pyoderma gangrenosum — a review. Orphanet Journal of Rare Diseases 2007; 2:19. doi:10.1186/1750-1172-2-19.
- Cairns BA, Herbst CA, Sartor BR et al. Peristomal pyoderma gangrenosum and inflammatory bowel disease. Arch Surgery 1994; 129:769.
- Esnault P, Dompartin A, Moreau A, Caraes B & Leroy D. Recurring postoperative pyoderma gangrenosum. Int J Dermatol 1995; 34:647–50.
- Bennett ML, Jackson JM, Jorizzo JL, Fleischer AB Jr, White WL & Callen JP. Pyoderma gangrenosum. A comparison of typical and atypical forms with an emphasis on time to remission. Case review of 86 patients from 2 institutions. Medicine 2000; 79:37–46.
- Konopka CL, Padulla GA, Ortiz MP, Beck AK, Bittencourt MR & Dalcin DC. Pyoderma gangrenosum: a review article. J Vasc Bras 2013; 12(1):25–33.
- Wu BC, Patel ED & Ortega-Loayza AG. Drug-induced pyoderma gangrenosum: a model to understand the pathogenesis of pyoderma gangrenosum. Br J Dermatol 2017; 177:72–83.
- Sekeres MA, Schoonen WM, Kantarjian H et al. Characteristics of US patients with myelodysplastic syndromes: results of six cross-sectional physician surveys. J Natl Cancer Inst 2008; 100:1542–1551.
- Montalban-Bravo G & Garcia-Manero G. Myelodysplastic syndromes: update on diagnosis, risk-stratification and management. Am J Hematol 2018; 93(1):129–147. doi: 10.1002/ajh.24930.
- Saleh MFM & Saunthararajah Y. Severe pyoderma gangrenosum caused by myelodysplastic syndrome successfully treated with decitabine administered by a noncytotoxic regimen. Clin Case Rep 2017; 5(12):2025–2027.
- Harrington AM, Schelling LA & Ordobazari A. Immunophenotypes of chronic myelomonocytic leukemia (CMML) subtypes by flow cytometry. Am J Clin Pathol 2016; 146:170–181.
- Abramson Cancer Center. Chronic Myelomonocytic Leukemia. Retrieved from: https://www.pennmedicine.org/cancer/types-of-cancer/leukemia/chronic-myelomonocytic-leukemia/what-is-cmml. Accessed on 15 October, 2018.
- Fenaux P, Mufti GJ, Hellstrom-Lindberg E et al. Efficacy of azacitidine compared with that of conventional care regimens in the treatment of higher-risk myelodysplastic syndromes: a randomised, open-label, phase III study. Lancet Oncol 2009; 10(3):223–32.
- Leone G, Teofili L, Voso MT & Liiubbert M. DNA methylation and demethylating drugs in myelodysplastic syndromes and secondary leukemias. Haematological 2002; 87:1324–41.
- Roy C, Adam JP, Morin F et al. Azacitidine-induced pyoderma gangrenosum at injection sites in a patient with myelodysplastic syndrome. Curr Oncol 2018; 25(1):e103–e105.
- Tseng E, Alhusayen R, Sade S, Buckstein R & Prica A. Pyoderma gangrenosum secondary to azacitidine in myelodysplastic syndrome. Br J Haem 2015; 169(4):461.
- Gravina GL, Festuccia C, Marampon F et al. Biological rationale for the use of DNA methyltransferase inhibitors as new strategy for modulation of tumor response to chemotherapy and radiation. Molecular Cancer 2010; 9:305.
- Santini V, Fenaux P, Mufti GJ et al. Management and supportive care measures for adverse events in patients with myelodysplastic syndromes treated with azacitidine. Eur J Haematol 2010; 85(2):130–8.
- Saleh MFM & Saunthararajah Y. Severe pyoderma gangrenosum caused by myelodysplastic syndrome successfully treated with decitabine administered by a noncytotoxic regimen. Clin Case Rep 2017; 5(12):2025–2027.
- Miller J, Yentzer BA, Clark A, Jorizzo JL & Feldman SR. Pyoderma gangrenosum: A review and update on new therapies. J Am Acad Dermatol 2010; 62:646–54.
- Pitarch G, Torrijos A, Mahiques L, Sanchez-Carazo JL & Fortea JM. Systemic absorption of topical tacrolimus in pyoderma gangrenosum. Acta Derm Venereol 2006; 86:64–5.
- Ghislain PD, De Decker I & Lachapelle JM. Efficacy and systemic absorption of topical tacrolimus used in pyoderma gangrenosum. Br J Dermatol 2004; 150:1052–3.
- Conwell P, Mikulski L, Moran D, Tramontozzi M & William W. Pyoderma gangrenosum treatment: a steroid-free option. Ostomy Wound Manage 2004; 50(5):26–8.
- Angel DE & van Rooyen JL. The challenges of managing patients with pyoderma gangrenosum: three case reports. Wound Practice & Research 2016; 24(1):48–58.
- Carville K. Wound Care Manual. 6th edn. Australia: Silver Chain Foundation, 2012, pp. 218–219.
- Ahn C, Negus D & Huang W. Pyoderma gangrenosum: a review of pathogenesis and treatment. Expert Rev Clin Immunol 2018; 14(3):225–233.
- Dunwoody CJ, McCann SA & Zumbo M. Pyoderma gangrenosum: a case study for pain management in dermatology nursing. Dermatol Nurs 2000; 12:313–4.


