Volume 39 Number 3
Effect of tamsulosin on lower urinary tract symptoms related to double-J ureteral stent: a randomised, double-blinded, placebo controlled trial
Fateme Guitynavard, Shahram Gooran, Parvin Kasiri, Farshad Gholipour and Seyed Mohammad Kazem Aghamir
Keywords urethral stent, stent-related symptoms, lower urinary tract symptoms, tamsulosin, double-J
For referencing Guitynavard F et al. Effect of tamsulosin on lower urinary tract symptoms related to double-J ureteral stent: a randomised, double-blinded, placebo controlled trial. WCET® Journal 2019; 39(3):26-31
DOI https://doi.org/10.33235/wcet.39.3.26-31
Abstract
Objective To evaluate the effect of tamsulosin on stent-related symptoms in patients undergoing double-J ureteral stenting.
Methods and materials Seventy patients (47 men and 23 women; mean age 42.5 years) who underwent double-J stent placement in adjunct to urological surgery were prospectively randomised into two groups. Group 1 included 35 patients who received 0.4 mg of tamsulosin once daily for 4 weeks; group 2 included 35 patients who received a placebo for the same protocol. All patients were interviewed by the same physician about the frequency of stent-related symptoms at 4 weeks after stent insertion.
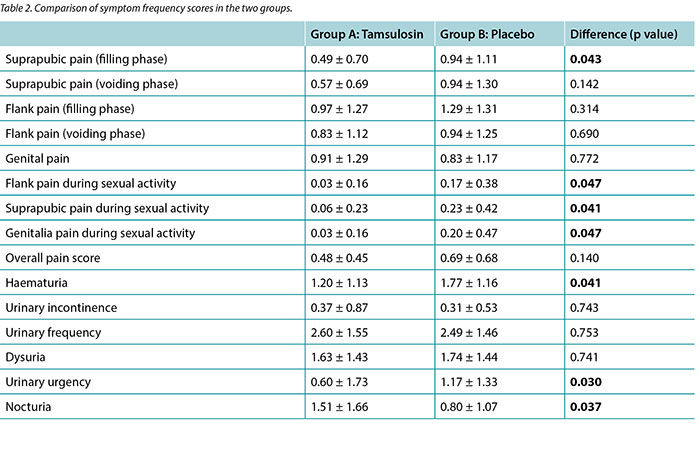
Results Patients in group 1 showed a lower score for suprapubic pain during the filling phase and lower pain during sexual activity than group 2, although the overall pain score was not significantly different between two groups. The mean urinary urgency score was less in group 1 in comparison to the placebo group (p=0.030). No statistically significant differences were found between two groups concerning haematuria, urinary incontinence, frequency nor dysuria.
Conclusion Tamsulosin improves symptoms associated with double-J ureteral stents, especially body pain during sexual activity.
Introduction
Indwelling ureteral stents are a commonly used tool in urologic practices. Zimskind et al.1 described ureteral stent placement for the first time in 1967. However, despite the documented benefits of double-J stents in preventing ureteral obstruction after urologic procedures2, patients may experience stent-related symptoms and some morbidities, including urinary symptoms, stent-related body pain and sexual difficulties which, together, can severely influence the patients’ quality of life and general health substantially3-8.
Authors have hypothesised several aetiologies responsible for stent-related complications such as bladder trigone irritation and renal reflux7,9. Through the years, manufacturers have made innovations in stent design and materials in order to improve stent-caused problems10,11, yet have failed to design an ideal stent which can significantly reduce adverse effects11,12.
Given the effect of α-blockers in alleviating lower urinary tract symptoms associated with overactive bladder and benign prostate hyperplasia13, several studies investigated the role of these agents on stent-related symptoms using the Ureteral Stent Symptom Questionnaire (USSQ)13-17, the International Prostate Symptom Score (IPSS)17-24, the Visual Analog Pain Score (VAPS)19-21,24, or evaluation of clinical symptoms by interviewing the patients25.
Tamsulosin selectively blocks α-1a/1d adrenergic receptors in smooth muscles of distal ureter, bladder trigone, bladder neck and prostate26. Smooth muscle relaxation is believed to reduce bladder, neck and urethral resistance, which can improve stent-related lower urinary tract symptoms17,18. The purpose of this clinical trial was therefore to evaluate the effectiveness of tamsulosin in reducing double-J stent-related symptoms in patients undergoing ureteral stenting.
Methods and Materials
After ethics committee approval, this prospective double-blinded and placebo controlled randomised clinical trial was carried out between June 2014 and July 2015 in Sina Hospital, Tehran, Iran. The current trial was registered at www.clinicaltrials.gov as IRCT2015042221886N2.
Study population and design
Patients who underwent a double-J ureteral stenting by a single surgeon were assessed for eligibility. Stents were routinely placed before extracorporeal shock wave lithotripsy (ESWL) or following percutaneous nephrolithotomy (PCNL), ureterorenoscopy (URS), endoscopic endopyelotomy, or transureteral ureterolithotomy (TUL) for 4 weeks. Patients with at least 18 years of age and with an unilateral double-J ureteral stent were enrolled into the study after obtaining informed consent about random allocation of treatment and potential side effects of tamsulosin. The exclusion criteria included patients younger than 18 years, pregnant women, patients with bilateral stents, forgotten ureteral stent, long-standing ureteral stent placement, benign prostatic hyperplasia, previous prostatic resection, prostatitis, prostate cancer, bladder neoplasms, recent or recurrent urinary tract infection, history of chronic flank pain, patients previously treated with selective α-blockers, patients with severe cardiovascular disease, and patients affected by risk factors for erectile dysfunction.
Study procedure
Out of 110 patients who were assessed for eligibility, a total of 76 patients (47 men and 29 women, aged 20–74 years) who gave consent were enrolled in the study. Patients were randomly allocated into two groups using a computer randomisation program. In group A, the case group, 38 patients (25 men and 13 women) received a daily 0.4 mg dose of tamsulosin (Maxulosin®, Exir Pharmaceutical Co., Borujerd, Iran). In group B, the control group, 38 patients (25 men and 13 women) received a placebo once a day for 4 weeks. The patients were given numbered containers enclosing unnamed pills. Both participants and physicians were kept blinded to the medication being prescribed.
Before the scheduled operative procedure, routine laboratory tests and imaging were performed. In all patients, an identical flexible double-J ureteral stent was used, although the length and size of stents were individualised for each patient. Stent placement was done under regional or general anaesthesia. The coiled distal end of the stent was the only part of the stent presented in the bladder. Plain abdominal X-ray film was used to assure correct stent positioning. To standardise analgesic consumption, acetaminophen 500 mg was prescribed for pain control. No procedure-related complication occurred. All study procedures were in accordance with the ethical standards of the ethics committee of Tehran University of Medical Sciences on human experimentation and with the Helsinki Declaration of 1975, as revised in 2009.
Patient assessment and outcome measurements
Four weeks after stent placement, patients were interviewed by the same physician about stent-related symptoms including suprapubic pain (filling and voiding phase), haematuria, urinary incontinence, urinary frequency, dysuria, urinary urgency, nocturia, flank pain (filling and voiding phase), genitalia pain, and flank, suprapubic or genitalia pain during sexual activity. Patients were asked to answer the questions regarding the frequency of each symptom in a five-level Likert scale – ‘never’, ‘rarely’, ‘sometimes’, ‘often’, and ‘very often’.
Statistical analysis
Statistical analysis was performed using SPSS ver. 20.0 (SPSS Inc., Chicago, IL, USA). Descriptive statistics were obtained for each study variable. Chi-square, and student’s t-test were used for comparison between the two groups as appropriate; values of p less than 0.05 were considered statistically significant.
Results
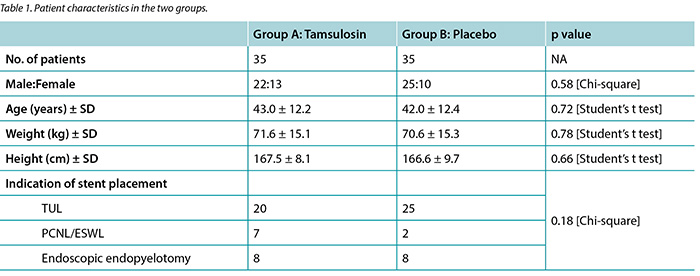
Out of 76 enrolled patients, 70 (92.1%) completed the study. Therapies were well tolerated and no patients had to terminate medication owing to adverse effects, and none underwent stent removal prior to the due date. Characteristics of the study population are presented in Table 1. Group A (35 patients) consisted of 22 men and 13 women (mean age 43.0 ± 12.2 years), and group B consisted of 25 men and 10 women (mean age 42.0 ± 12.4 years). A total of 45 patients (64.2%) underwent transurethral lithotripsy, nine patients (12.8%) underwent percutaneous nephrolithotripsy or ESWL, while endopyelotomy for ureteropelvic junction stricture was performed in 16 patients (22.8%). No statistically significant differences were found regarding gender, age, weight, height, nor indication of stent placement between the case and control groups.
Scores 0 to 4 were attributed respectively to the frequency of scale of symptoms stated by patients, with 0 representing ‘never’ and 4 representing ‘very often’. Table 2 summarises the overall results of our study. Student’s t-test was used to examine the differences of means between groups. As shown in the table, patients receiving tamsulosin expressed higher scores (1.51 ± 1.66) for nocturia than patients in the control group (0.80 ± 1.07) (p=0.037). The mean score for pain in the flank, suprapubic and genital areas during sexual activity was lower in patients receiving tamsulosin in comparison to patients receiving the placebo (p values=0.047, 0.041 and 0.047, respectively). Patients treated with tamsulosin showed lower suprapubic pain in filling phase scores (0.49 ± 0.70) than the control group (0.94 ± 1.11) (p=0.043). The mean urinary urgency score was 0.6 in group 1 and 1.1 in the placebo group (p=0.030). No statistically significant differences were found between the two groups concerning haematuria, urinary incontinence, frequency, dysuria, nor overall pain score (p values >0.05).
Discussion
The results of this double-blind, randomised, placebo-controlled study showed that tamsulosin is effective in relieving stent-related symptoms specially in alleviating pain during sexual activity.
Current indications for ureteral stents in urgent conditions include unbearable acute renal colic, obstructive pyelonephritis and renal failure secondary to ureteral obstruction3. Endoscopic procedures can be followed by stent placement as a safety measure27 in several situations, including ureteral oedema, ureteral perforation, solitary kidney, transplant kidney and history of renal failure. There are also relative indications for indwelling ureteral stents such as passive dilation of ureteral orifice and ureter, before ESWL in stones larger than 2 cm, long-lasting impacted stone, endoscopic procedures lasting over 45 minutes, and pregnancy2,7.
However, despite widespread use of indwelling ureteral stents, there is considerable controversy over the necessity of stent placement after uncomplicated ureteroscopic lithotripsy28. Even after choosing the correct stent size and proper placement, indwelling ureteral stents are associated with inevitable morbidities in over 80% of patients29, including urinary symptoms, haematuria, pain and sexual difficulties which all lead to reduced health-related quality of life6. Some researchers report that the position and completeness of the stent’s lower loop and proper attention to detail throughout stent placement have an effect on symptom severity30,31. For example, an ureteral stent was better tolerated after periureteral injection of botulinum toxin type A after stent placement by significantly decreasing pain and narcotic requirement32.
However, the exact pathophysiology of stent-related symptoms is not fully understood. It is assumed that lower ureteral smooth muscle spasms, involuntary bladder contraction triggered by neuronal-rich trigone irritation, and urine reflux to the kidneys due to increased bladder outlet resistance are responsible factors11,14,15,21,33. α-blockers are recommended as expulsive therapy for ureteral stones and to decrease recurrent colic episodes. They are believed to relieve stent-related morbidity by different ways34-36. Blocking α-adrenergic receptors reduces muscle tone of prostatic urethra, bladder trigone and ureter which leads to decreased bladder outlet resistance, voiding pressure, and urinary reflux17. However, the effectiveness of these medications and proper therapeutic protocols for alleviation of stent-related symptoms are yet to be discovered by further investigations. Results of the current study showed that tamsulosin (a selective α1A antagonist) can control several stent-related morbidities.
The effectiveness of α-blockers in relieving stent-related symptoms has been investigated by several studies, including patients receiving either alfuzosin 10 mg13,14,16,25,37, tamsulosin 0.4 mg15,17,22,23,17, tamsulosin 0.2 mg19,20, terazosin 4 mg21,38, terazosin 2 mg21, or a placebo for 1–6 weeks’ treatment. Damiano et al.15 released the first report of the benefits of a daily dose of tamsulosin 0.4 mg in a prospective study comparing tamsulosin to placebo in 75 patients using the USSQ. Although this study was not double-blinded nor placebo-controlled, the authors found that patients receiving tamsulosin had their general health better preserved. In another study performed by Wang et al.17 on 154 patients using the same measurement tool, those receiving tamsulosin showed lower stent-related symptoms and better quality of life than patients receiving a placebo.
Using both IPSS and VAPS, the effect of lower dose of tamsulosin (0.2 mg once a day) alone and in combination with tolterodin or solifenacin on stent-related symptoms was investigated by Lee et al.19 and Lim et al.20, respectively. Lee et al. found that only the storage symptom scores were significantly lower in patients receiving either mono- or combination therapy than patients treated with no medication. The author stated that correct placement of the stent is therefore more important than medication in reducing stent-related storage symptoms19. Conversely, Lim showed that combination therapy with tamsulosin and solifenacin can better improve both irritative and obstructive symptoms than tamsulosin alone or compared to receiving no medication20; the results were later confirmed by Shalaby et al.23. Further, improvement of stent-related symptoms by α-blockers was found to be independent to the type of α-blocker, as showed by Dellis et al.37 comparing tamsulosin and alfuzosin. To the best of our knowledge, there is only one study18 on α-blocker (doxazosin) ineffectiveness in reducing stent-related symptoms.
In a recent meta-analysis performed by Lamb et al.35 including 461 patients from five studies, all studies showed a reduction in the USSQ urinary symptom score and body pain scores in patients receiving either tamsulosin or alfuzosin. However, general health and sexual matters improvement were not statistically significant. Results of a further 12 studies including a total number of 946 patients’ data were meta-analysed by Yakoubi et al.36. Analyses showed a significant reduction in urinary symptoms and pain scores as well as general health improvement in patients receiving α-blockers.
In our study, the reduction in pain scores in patients treated with tamsulosin was more evident when compared to during sexual activity in all three areas – flank, genital and suprapubic. Ureteral stents were showed to impair the quality of sexual life in both men and women8. Erectile dysfunction was indicated as the main source of sexual distress in men, probably related to lower urinary tract symptoms and stent permanence. In women, sexuality can be severely impaired as a result of stent-related psychological distresses8.
We also found tamsulosin effective in reducing haematuria and urinary urgency, although, patients treated with tamsulosin had increased rate of nocturia than patients receiving placebo. Nocturia has not reported as an adverse effect of tamsulosin previously, so this finding should be investigated by larger-scale studies. Koseoglu et al.39 found tamsulosin ineffective in reducing nocturia in patients being treated for benign prostatic hyperplasia. In another study, combination of α-blockers with zolpidem was shown to better reduce nocturia than α-blockers alone40.
Our study had several limitations. First, our sample size was statistically small in order to detect small differences between two groups. Second, some patients did not complete the study. Third, we utilised clinical interviews with the patients to evaluate urinary symptoms, whereas Joshi et al.6 has developed a specific tool for assessing stent-related symptoms called the USSQ. Fourth, the quantity of the analgesics used by patients was not reliably reported. Finally, we did not investigate any adverse effects made by α-blocker use, although therapies were well tolerated and no patients had to discontinue medication owing to side effects. Therefore, further large-scale, randomised, prospective studies are needed to obtain more accurate information.
Conclusion
The use of tamsulosin 0.4 mg once daily in patients with unilateral ureteral stenting significantly improved stent-related urinary symptoms, especially the body pain during sexual activity. Therefore, tamsulosin should be considered for patients who complain of stent-related symptoms. In the future, large-scale, prospective and randomised studies will be needed.
Ethics approval and consent to participate
Patient consent was undertaken before surgery based on the ethical code of the Tehran University of Medical Sciences Ethics Committee. This trial was registered at www.clinicaltrials.gov as IRCT2015042221886N2. The information is published without the name of the patients. Information, data and photos can be provided if requested.
Competing interests
All authors claim that there is not any potential competing or conflict of interest.
Funding
None.
Acknowledgements
Special thanks to the Urology Research Center, Tehran University of Medical Sciences.
坦索罗辛对双J输尿管支架管相关下尿路症状的作用:一项随机、双盲、安慰剂对照试验
Fateme Guitynavard, Shahram Gooran, Parvin Kasiri, Farshad Gholipour and Seyed Mohammad Kazem Aghamir
DOI: https://doi.org/10.33235/wcet.39.3.26-31
摘要
目的 评价坦索罗辛对行双J输尿管支架管置入术患者的支架管相关症状的作用。
方法和材料 将在泌尿手术中行双J支架管置入术的70例患者(男性47例,女性23例;平均年龄42.5岁)前瞻性地随机分为2组。第1组包含35例患者,其口服0.4 mg坦索罗辛,每日1次,持续4周;第2组包含35例患者,其按相同方案口服安慰剂。支架管植入4周时,由同一名医生向所有患者询问支架管相关症状的发生频率。
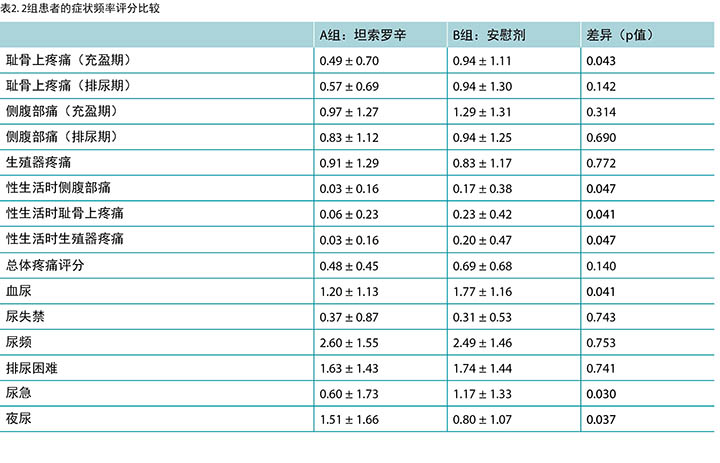
结果 第1组患者的充盈期耻骨上疼痛评分及性生活时的疼痛评分均低于第2组,但两组的总体疼痛评分没有显著差异。第1组的平均尿急评分低于安慰剂组(p=0.030)。未发现两组在血尿、尿失禁、尿频和排尿困难方面存在统计学上的显著差异。
结论 坦索罗辛可改善双J输尿管支架管相关的症状,尤其是性生活时的身体疼痛。
前言
留置输尿管支架管是泌尿科实践中的常用工具。Zimskind等人1在1967年首次描述了输尿管支架管置入术。但是,虽然有文献记录了双J支架管在预防泌尿手术后输尿管梗阻方面的受益2,但患者可能出现支架管相关症状和某些合并症,包括泌尿系症状、支架管相关身体疼痛和性生活困难,这些因素加在一起可能会实质性严重影响患者的生活质量和总体健康3-8。
作者设想支架管相关并发症有若干病因,如膀胱三角受到刺激和肾反流7,9。这些年来,制造商为了改善支架管引起的问题,在支架管设计和材料上进行了创新10,11,但仍然未能设计出能够显著减少不良反应的理想支架管11,12。
鉴于α-阻断剂能有效缓解膀胱过度活动症及良性前列腺增生相关的下尿路症状13,几项研究采用输尿管支架管症状调查问卷(USSQ)13-17、国际前列腺症状评分(IPSS)17-24、视觉模拟疼痛评分(VAPS)19-21,24,或通过对患者进行访谈来开展临床症状评估25,调查了这些药物对支架管相关症状的作用。
坦索罗辛可选择性地阻断下段输尿管、膀胱三角、膀胱颈和前列腺平滑肌中的α-1a/1d肾上腺素受体26。有文献认为平滑肌松驰可以减小膀胱、膀胱颈和尿道阻力,从而改善支架管相关下尿路症状17,18。因此,本临床试验旨在评价坦索罗辛对于减少行输尿管支架管置入术的患者中双J支架管相关症状的有效性。
方法与材料
经伦理委员会批准后,本前瞻性、双盲、安慰剂对照随机临床试验于2014年6月至2015年7月在伊朗德黑兰的Sina医院进行。本试验已在www. clinicaltrials.gov注册,编号IRCT2015042221886N2。
研究人群和设计
对由同一名外科医生行双J输尿管支架管置入术的患者进行资格评估。在行体外冲击波碎石术(ESWL)前,或在经皮肾镜取石术(PCNL)、输尿管肾镜术(URS)、内腔镜肾盂内切开术、或经尿道输尿管取石术(TUL)后4周常规置入支架管。患者至少18岁且植入单侧双J输尿管支架管,在获取其有关随机分配治疗方案和坦索罗辛潜在副作用的知情同意后,入组本研究。排除标准包括不足18岁的患者、孕妇、植入双侧支架管的患者、遗忘输尿管支架管、长期放置输尿管支架管、良性前列腺增生、前列腺切除史、前列腺炎、前列腺癌、膀胱肿瘤、新近或复发性尿路感染、慢性侧腹部痛病史、有选择性α-阻滞剂用药史的患者、重症心血管疾病患者及勃起功能障碍风险因素受累患者。
研究流程
经资格评估的110例患者中,共有76例(男性47例,女性29例,年龄20–74岁)提供知情同意的患者入组本研究。使用计算机随机化程序将患者随机分为2组。A组为治疗组,其38例患者(男性25例,女性13例)每日服用0.4 mg坦索罗辛(Maxulosin®,Exir Pharmaceutical Co.,Borujerd,伊朗)。 B组为对照组,其38例患者(男性25例,女性13例)服用安慰剂,每日1次,持续4周。将无名药片装在带编号的容器内交给患者。参与者和医生均对给予的药物不知情。
在开始预计的手术操作前,进行常规实验室检查和影像学检查。所有患者均使用完全相同的柔软双J输尿管支架管,但支架管的长度和尺寸则根据每例患者进行了个性化调整。在局麻或全麻下放置支架管。支架管的卷曲远端是支架管出现在膀胱中的唯一部分。拍摄腹部X光平片以确认支架管放置的位置正确。为了统一镇痛剂的消耗,给予500 mg对乙酰氨基酚用于镇痛。未发生与手术相关的并发症。所有研究流程均符合德黑兰医科大学伦理委员会有关人类实验的伦理标准,以及1975年《赫尔辛基宣言》(2009年修订版)的伦理标准。
患者评估与结果测量
支架管放置4周后,由同一名医生向患者询问有关支架管相关症状的问题,包括耻骨上疼痛(充盈期和排尿期)、血尿、尿失禁、尿频、排尿困难、尿急、夜尿、侧腹部痛(充盈期和排尿期)、生殖器疼痛,以及性生活时的侧腹部、耻骨上和生殖器疼痛。同时要求患者按5级李克特量表回答关于每种症状频率的问题,即“从未”、“很少”、“有 时”、“经常”和“频繁”。
统计分析
使用SPSS 20.0版(SPSS Inc.,美国伊利诺伊州芝加哥)进行统计分析。获取每个研究变量的描述性统计数据。酌情使用卡方检验、student t检验比较两组的结果;p值小于0.05视为具有统计显著性。
结果
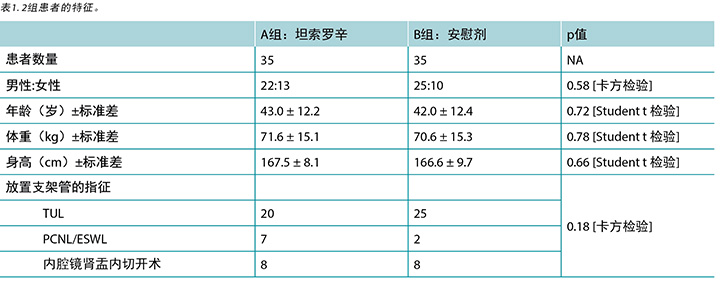
76例入组患者中,70例(92.1%)完成了本研究。治疗的耐受性良好,到期日前没有患者因不良反应终止用药或取出支架管。研究人群的特征如表1所示。A组(35例患者)含男性22例,女性13例(平均年龄43.0 ± 12.2岁),B组含男性25例,女性10例(平均年龄 42.0 ± 12.4 岁)。共计45例(64.2%)患者行经尿道输尿管取石术,9例患者(12.8%)行经皮肾镜取石术或ESWL,而16例患者(22.8%)因肾盂输尿管连接部狭窄而行肾盂内切开术。治疗组和对照组之间在性别、年龄、体重、身高、放置支架管的指征方面均无统计学上的显著差异。
分别对患者报告症状量表中的频率按0至4分赋分,0为“从未”,4为“频繁”。表2汇总了本研究的总体结果。使用Student t检验评估两组之间的平均值差异。如表中所示,服用坦索罗辛的患者对夜尿的评分(1.51 ± 1.66)高于对照组患者(0.80 ± 1.07)(p=0.037)。服用坦索罗辛的患者对性生活时侧腹部、耻骨上及生殖器区域疼痛的平均评分低于服用安慰剂的患者(p值分别为0.047、 0.041和0.047)。接受坦索罗辛治疗的患者对充盈期耻骨上疼痛的评分(0.49 ± 0.70)也低于对照组(0.94 ± 1.11) (p=0.043)。第1组和安慰剂组的平均尿急评分分别为0.6和1.1(p=0.030)。未发现两组之间在血尿、尿失禁、尿频、排尿困难及总体疼痛评分上存在统计学上的显著差异(p值 >0.05)。
讨论
本双盲、随机、安慰剂对照研究的结果显示,坦索罗辛可有效缓解支架管相关症状,尤其是缓解性生活时的疼痛。
目前,输尿管支架管在紧急条件下的适应症包括无法忍受的急性肾绞痛、梗阻性肾盂肾炎和输尿管梗阻继发性肾功能衰竭3。另外,在输尿管水肿、输尿管穿孔、孤立肾、移植肾和肾功能衰竭史等几种情况下,可在放置支架管前进行内镜术,以作为一种安全措施27。此外,还存在几种留置输尿管支架管的相对适应症,如输尿管口和输尿管被动扩张、ESWL前结石大小超过2 cm、长期嵌顿结石、内镜术超过45分钟以及妊娠2,7。
但是,尽管留置输尿管支架管的使用广泛,但对于简单输尿管镜碎石取石术后放置支架管的必要性仍存在大量争议28。即使支架管尺寸选择正确,放置妥当,留置输尿管支架管仍会导致超过80%的患者出现不可避免的合并症29,包括泌尿系症状、血尿、疼痛和性生活困难,这些都降低了健康相关的患者生活质量6。某些研究者报告称,支架管下段环状部分的位置和完整性以及在整个支架管放置过程中对细节的适当关注都对症状严重度有影响30,31。例如,放置支架管后,在输尿管周围注射A型肉毒毒素可显著减少疼痛和麻醉剂需求量,从而提高对输尿管支架管的耐受性32。
但是,支架管相关症状的确切病理生理学机制尚不明确。有人认为,其导致因素是神经元丰富的膀胱三角受到刺激后引起的输尿管下段平滑肌痉挛和膀胱非自主收缩,以及膀胱出口阻力增加导致尿液返流到肾脏11,14,15,21,33。故建议将α-阻滞剂用作输尿管结石的排石疗法,并用于减少复发性绞痛发作。研究者认为这类药物能够通过不同途径缓解支架管相关合并症34-36。阻断α-肾上腺素受体可降低前列腺段尿道、膀胱三角和输尿管的肌肉张力,从而降低膀胱出口阻力和排尿压,减少尿液返流17。但是,这类药物以及适当的治疗方案对于缓解支架管相关症状的有效性尚需进一步研究。本研究结果表明,坦索罗辛(一种选择性α1A拮抗剂)能够控制多种支架管相关合并症。
有几项研究已经研究了α-阻滞剂对于缓解支架管相关症状的有效性,包括给予患者10 mg阿呋唑嗪13,14,16,25,37、0.4 mg坦索罗辛15,17,22,23,17、0.2 mg坦索罗辛19,20、4 mg特拉唑嗪21,38、2 mg特拉唑嗪21,或安慰剂来进行1–6周的治疗。Damiano等人15在一项涉及75例患者的前瞻性研究中,采用USSQ比较坦索罗辛与安慰剂的效果,并发表了第一份服用0.4 mg每日剂量的坦索罗辛的受益报告。尽管该研究不是双盲,也不是安慰剂对照设计,但作者发现服用坦索罗辛的患者保持了更好的总体健康。Wang等人17进行的另一项研究中,对154例患者使用相同的测量工具,其中接受坦索罗辛治疗的患者,其支架管相关症状的发生率低于接受安慰剂治疗的患者,并且生活质量更高。
Lee等人19和Lim等人20同时使用IPSS和VAPS,分别研究了单独使用低剂量坦索罗辛(0.2 mg,每日一次),或者与托特罗定或索利那新联合用药时对支架管相关症状的作用。Lee等人发现,接受单药治疗或联合用药治疗的患者中,只有储尿症状评分显著低于未用药物治疗的患者。作者称,因此,对于减少支架管相关的储尿症状,正确放置支架管的重要性大于药物治疗19。而Lim的研究表明,坦索罗辛与索利那新联合用药治疗在改善刺激和梗阻症状方面优于坦索罗辛单独用药或不接受药物治疗20;Shalaby等人23后来证实了这一结果。另外, Dellis等人37比较坦索罗辛和阿呋唑嗪后,发现α-阻滞剂对支架管相关症状的改善作用与α-阻滞剂的类型有关。据我们所知,只有一项研究18的结果是α-阻滞剂(多沙唑嗪)对减少支架管相关症状无效果。
Lamb等人35近期进行的荟萃分析中包括来自5项研究的461例患者,所有研究均显示接受坦索罗辛或阿呋唑嗪治疗的患者在USSQ泌尿系症状评分和身体疼痛评分上有所降低。但是,总体健康和性事的改善没有统计显著性。另外,Yakoubi 等人36对包含共计946例患者的数据的12项研究结果进行了荟萃分析。分析表明,接受α-阻滞剂治疗的患者的泌尿系症状、疼痛评分显著降低,总体健康有所改善。
本研究中,接受坦索罗辛治疗的患者在性生活时所有三个区域(侧腹部、生殖器和耻骨上)的疼痛评分降低均更加明显。本研究也表明,输尿管支架管可损害男性和女性的性生活质量8。男性性生活困扰的主要来源是勃起功能障碍,这很可能与下尿路症状和长期植入支架管有关。而在女性中,由于支架管相关的心理困扰严重损害了性欲8。
我们还发现,坦索罗辛可有效减少血尿和尿急,不过接受坦索罗辛治疗的患者夜尿发生率高于接受安慰剂的患者。以前没有研究报告夜尿是坦索罗辛带来的不良反应,因此该结果还需通过更大规模的研究予以调查。Koseoglu等人39发现,在接受良性前列腺增生治疗的患者中,坦索罗辛对于减少夜尿无效。另一项研究显示,联合使用α-阻滞剂和唑吡坦减少夜尿的效果优于单独使用α-阻滞剂40。
本研究具有一定局限性。第一,从统计学角度而言,本研究的样本量对于检测两组之间的微小差异而言太少。第二,某些患者没有完成本研究。第三,我们采用对患者进行临床访谈的方法评估泌尿系症状,而Joshi等人6制定了特定工具评估支架管相关症状,即USSQ。第四,患者使用的镇痛剂剂量未得到可靠报告。此外,我们没有调查使用α-阻滞剂造成的不良反应,不过治疗的耐受性良好,没有患者因副作用而中断用药。因此,为获得更准确的信息,还需要进行大规模的随机前瞻性研究。
结论
在单侧输尿管支架管置入术患者中,每日服用一次0.4 mg坦索罗辛的治疗方案可显著改善支架管相关症状,尤其是性生活时的身体疼痛。因此,对于主诉支架管相关症状的患者,应考虑给予坦索罗辛。未来尚需进行进一步的大规模前瞻性随机研究。
伦理批准和参与研究的知情同意
术前根据德黑兰医科大学伦理委员会的伦理规范取得患者知情同意书。本试验在www.clinicaltrials.gov注册,编号为IRCT2015042221886N2。本信息发表时患者匿名。如有要求,可提供信息、数据和照片。
利益冲突
所有作者声明没有任何潜在的冲突利益或利益冲突。
资助
无。
致谢
特别感谢德黑兰医科大学泌尿研究中心。
Author(s)
Fateme Guitynavard
Urology Research Center, Tehran University of Medical Sciences, Tehran, Iran
Shahram Gooran
Urology Research Center, Tehran University of Medical Sciences, Tehran, Iran
Parvin Kasiri
Urology Research Center, Tehran University of Medical Sciences, Tehran, Iran
Farshad Gholipour
Urology Research Center, Tehran University of Medical Sciences, Tehran, Iran
Seyed Mohammad Kazem Aghamir*
Urology Research Center, Tehran University of Medical Sciences, Tehran, Iran
Email mkahamir@yahoo.com
* Corresponding author
References
- Zimskind PD, Fetter TR, Wilkerson JL. Clinical use of long-term indwelling silicone rubber ureteral splints inserted cystoscopically. J Urol 1967;97(5):840–844.
- Knudsen BE, Beiko DT, Denstedt JD. Stenting after ureteroscopy: pros and cons. Urol Clin North Am 2004;31(1):173–180.
- Chew BH, Knudsen BE, Denstedt JD. The use of stents in contemporary urology. Curr Opin Urol 2004;14(2):111–115.
- Damiano R, Oliva A, Esposito C, De Sio M, Autorino R, D’Armiento M. Early and late complications of double pigtail ureteral stent. Urologia Int 2002;69(2):136–140.
- Haleblian G, Kijvikai K, de la Rosette J, Preminger G. Ureteral stenting and urinary stone management: a systematic review. J Urol 2008;179(2):424–430.
- Joshi H, Newns N, Stainthorpe A, MacDonagh R, Keeley F, Timoney A. Ureteral stent symptom questionnaire: development and validation of a multidimensional quality of life measure. J Urol 2003;169(3):1060–1064.
- Miyaoka R, Monga M. Ureteral stent discomfort: etiology and management. IJU 2009;25(4):455.
- Sighinolfi M, Micali S, De Stefani S, Mofferdin A, Grande A, Giacometti, M, et al. Indwelling ureteral stents and sexual health: a prospective, multivariate analysis. J Urol 2007;178(1):229–231.
- Ecke TH, Bartel P, Hallmann, S, Ruttloff J. Evaluation of symptoms and patients’ comfort for JJ-ureteral stents with and without antireflux-membrane valve. Urol 2010;75(1):212–216.
- Lingeman JE, Preminger GM, Goldfischer ER, Krambeck AE, Team CS. Assessing the impact of ureteral stent design on patient comfort. J Urol 2009;181(6):2581–2587.
- Thomas R. Indwelling ureteral stents: impact of material and shape on patient comfort. J Endourol 1993;7(2):137–140.
- Candela J, Bellman G. Ureteral stents: impact of diameter and composition on patient symptoms. J Endourol 1997;11(1):45.
- Park SC, Jung SW, Lee JW, Rim JS. The effects of tolterodine extended release and alfuzosin for the treatment of double-J stent-related symptoms. J Endourol 2009;23(11):1913–1917.
- Beddingfield R, Pedro RN, Hinck B, Kreidberg C, Feia K, Monga M. Alfuzosin to relieve ureteral stent discomfort: a prospective, randomized, placebo controlled study. J Urol 2009;181(1):170–176.
- Damiano R, Autorino R, De Sio M, Giacobbe A, Palumbo IM, D’Armiento M. Effect of tamsulosin in preventing ureteral stent-related morbidity: a prospective study. J Endourol 2008;22(4):651–656.
- Deliveliotis C, Chrisofos M, Gougousis E, Papatsoris A, Dellis A, Varkarakis IM. Is there a role for alpha1-blockers in treating double-J stent-related symptoms? Urology 2006;67(1):35–39. doi:10.1016/j.urology.2005.07.038
- Wang C-J, Huang S-W, Chang C-H. Effects of specific α-1A/1D blocker on lower urinary tract symptoms due to double-J stent: a prospectively randomized study. Urol Res 2009;37(3):147–152.
- Kuyumcuoglu U, Eryildirim B, Tuncer M, Faydaci G, Tarhan F, Ozgül A. Effectiveness of medical treatment in overcoming the ureteral double-J stent related symptoms. Can Urol Assoc J 2012;6(6):e234–237.
- Lee SJ, Yoo C, Oh CY, Lee YS, Cho, ST, Lee SH, et al. Stent position is more important than α-blockers or anticholinergics for stent-related lower urinary tract symptoms after ureteroscopic ureterolithotomy: a prospective randomized study. Korean J Urol 2010;51(9):636–641.
- Lim KT, Kim YT, Lee TY, Park SY. Effects of tamsulosin, solifenacin, and combination therapy for the treatment of ureteral stent related discomforts. Korean J Urol 2011;52(7):485–488.
- Mokhtari G, Shakiba M, Ghodsi S, Farzan A, Heidari Nejad S, Esmaeili S. Effect of terazosin on lower urinary tract symptoms and pain due to double-J stent: a double-blind placebo-controlled randomized clinical trial. Urologia Int 2011;87(1):19–22.
- Navanimitkul N, Lojanapiwat B. Efficacy of tamsulosin 0.4 mg/day in relieving double-J stent-related symptoms: a randomized controlled study. J Int Med Res 2010;38(4):1436–1441.
- Shalaby E, Ahmed A.-f, Maarouf A, Yahia I, Ali M, Ghobish A. Randomized controlled trial to compare the safety and efficacy of tamsulosin, solifenacin, and combination of both in treatment of double-j stent-related lower urinary symptoms. Adv Urol 2013.
- Wang C-J, Huang S-W, Chang C-H. Effects of tamsulosin on lower urinary tract symptoms due to double-J stent: a prospective study. Urologia Int 2008;83(1):66–69.
- Nazim SM, Ather MH. Alpha-blockers impact stent-related symptoms: a randomized, double-blind, placebo-controlled trial. J Endourol 2012;26(9):1237–1241.
- Shibasaki M, Sudoh K, Inagaki O, Uchida W, Honda K. Effect of the optical isomers of YM‐12617 on increased intra‐urethral pressure induced by phenylephrine in anaesthetized dogs. J Autonom Pharmacol 1992;12(4):263–268.
- Jeong H, Kwak C, Lee S. Ureteric stenting after ureteroscopy for ureteric stones: a prospective randomized study assessing symptoms and complications. BJU Int 2004;93(7):1032–1034.
- Nabi G, Cook J, N’Dow J, McClinton S. Outcomes of stenting after uncomplicated ureteroscopy: systematic review and meta-analysis. BMJ 2007;334(7593):572.
- Joshi H, Stainthorpe A, MacDonagh R, Keeley F, Timoney A. Indwelling ureteral stents: evaluation of symptoms, quality of life and utility. J Urol 2003;169(3):1065–1069.
- Jeon SS, Choi YS, Hong JH. Determination of ideal stent length for endourologic surgery. J Endourol 2007;21(8):906–910.
- Rane A, Saleemi A, Cahill D, Sriprasad S, Shrotri N, Tiptaft R. Have stent-related symptoms anything to do with placement technique? J Endourol 2001;15(7):741–745.
- Gupta M, Patel T, Xavier K, Maruffo F, Lehman D, Walsh R, Landman J. Prospective randomized evaluation of periureteral botulinum toxin type A injection for ureteral stent pain reduction. J Urol 2010;183(2):598–602.
- Duvdevani M, Chew BH, Denstedt JD. Minimizing symptoms in patients with ureteric stents. Curr Opin Urol 2006;16(2):77–82.
- Dellis A, Joshi HB, Timoney AG, Keeley FX. Relief of stent related symptoms: review of engineering and pharmacological solutions. J Urol 2010;184(4):1267–1272.
- Lamb AD, Vowler SL, Johnston R, Dunn N, Wiseman OJ. Meta‐analysis showing the beneficial effect of α‐blockers on ureteric stent discomfort. BJU Int 2011;108(11):1894–1902.
- Yakoubi R, Lemdani M, Monga M, Villers A, Koenig P. Is there a role for α-blockers in ureteral stent related symptoms? A systematic review and meta-analysis. J Urol 2011;186(3):928–934.
- Dellis AE, Keeley FX, Manolas V, Skolarikos AA. Role of α-blockers in the treatment of stent-related symptoms: a prospective randomized control study. Urol 2014;83(1):56–62.
- Tehranchi A, Rezaei Y, Khalkhali H, Rezaei M. Effects of terazosin and tolterodine on ureteral stent related symptoms: a double-blind placebo-controlled randomized clinical trial. Int Braz J Urol 2013;39(6):832–840.
- Koseoglu H, Aslan G, Ozdemir I, Esen A. Nocturnal polyuria in patients with lower urinary tract symptoms and response to alpha-blocker therapy. Urol 2006;67(6):1188–1192.
- Song YS, Ku JH. Zolpidem pharmacotherapy combined with alpha-blocker therapy for nocturia unresponsive to alpha-blocker monotherapy in men with lower urinary tract symptoms: a preliminary study. Int Urol Nephrol 2007;39(4):1147–1152.


