Volume 39 Number 4
Comparing fluid handling and microclimate conditions under superabsorbent polymer and superabsorbent foam dressings over an artificial wound
Evan Call, Craig Oberg, Iris Streit and Laurie M Rappl
Keywords Exudate, microclimate, wound, superabsorbent, dressing
For referencing Call E et al. Comparing fluid handling and microclimate conditions under superabsorbent polymer and superabsorbent foam dressings over an artificial wound. WCET® Journal 2019; 39(4):11-23
DOI https://doi.org/10.33235/wcet.39.4.11-23
Abstract
Introduction Superabsorbent foam (SAF) and superabsorbent polymer (SAP) dressings are compared on their abilities to handle moisture exuding from an artificial wound and to affect the microclimate beneath the dressings by measuring: the amount of moisture absorbed; the amount of moisture evaporated through the outer layer; the humidity, both beneath and outside the dressing and the difference between the two; and the temperature, both beneath and outside the dressing and the difference between the two.
Method A thermodynamic indenter was used in a laboratory setting to deliver a steady flow of moisture vapour across a standard wound size to each dressing under the weight of the indenter. Sensors recorded the humidity and temperature inside and outside each dressing over 3 hours and 16 minutes with a 45-second complete unweighting of the dressing at the 3- hour mark to simulate a patient weight shift. Dressings were weighed at test end to determine moisture absorbed and moisture evaporated.
Results There were no significant differences between the SAF and the SAP dressing groups in moisture absorption nor evaporation, nor in humidity nor temperature inside versus outside the dressings.
Conclusion It can therefore be determined that SAF and SAP dressings appear to be equally competent in maintaining a warm and moist microclimate in the wound bed. It should also be noted that these dressings do not dry the wound bed as the term superabsorbent may imply.
Introduction
Wound dressings are selected to manage moisture during wound healing for three reasons: to absorb exudate; to maintain an appropriate microclimate in the wound bed; and to protect the periwound from damage due to maceration from excessive moisture. Broad categories of absorptive technologies are foams, hydrocolloids, calcium alginates, hydrofibres, and moisture-binding polymers1,2. More complex superabsorbent foam (SAF) and superabsorbent polymer (SAP) dressings have been developed recently that claim to go beyond earlier dressings in managing wound moisture; however, these have been untested in comparison to each other. In addition, the term superabsorbent raises questions as to the possibility of over-absorption and therefore drying of the wound bed and inhibition of wound healing.
Exudate management
Since Winters published his pivotal work in 1963, moist wound healing has become accepted as best practice2,3. Cytokines and growth factors require moisture to diffuse throughout the wound bed to stimulate healing via wound closure and re-epithelialisation4. Maintaining the proper moisture level in a wound bed prevents desiccation which allows epithelial cells to migrate freely across the wound surface. Moist wounds granulate, epithelialise and heal two to three times faster than dry wounds5.
Exudate occurs naturally as a part of the wound healing process and keeps the wound bed moist to stimulate closure in most wounds. Some types of wounds are naturally more exudative than others, especially venous wounds, large pressure ulcers, and burns. Chronic wounds are highly exudative due to a sustained state of inflammation and disruption of normal cellular activity. Chronic wound exudate differs markedly from acute wound exudate, consisting of high levels of protein-degrading enzymes, matrix metalloproteases (MMPs), neutrophils and pro-inflammatory cytokines. Exuding wounds therefore require absorptive dressings whose capabilities match the flow of exudate to maintain an optimum moisture level at the wound bed. As these wounds begin to granulate, exudate decreases and dressing needs move from absorption to protection as a primary characteristic2.
The amount of exudate or moisture coming from a wound is commonly documented in a patient’s records with subjective descriptors such mild, moderate, severe and excessive rather than objective measurements. Several validated scales have been proposed for documenting exudate amount, including the Wound Exudate Score, the Bates-Jensen Wound Assessment Tool and the Pressure Ulcer Scale for Healing (PUSH) tool. Each scale provides a descriptor for each level on that scale. A summary and comparison of the available tools and their descriptors can be found in Wound exudate: effective assessment and management, a consensus document on exudate published by the World Union of Wound Healing Societies in 20195.
Wound microclimate
The microclimate has been identified as a key factor in both wound prevention and healing. The microclimate is the combination of temperature and humidity, and sometimes airflow, in a local region as compared to the ambient or surrounding area6. A warm moist environment is conducive to wound healing. Dini et al.7 show wound healing is impaired at <33°C by a decrease in neutrophil, fibroblast and epithelial cell activity. This same study shows a correlation between improvements in the wound bed and wound bed temperatures between 33–35°C. In addition, Salvo et al.8 report two studies showing that temperatures in the range of 36–38°C appear to promote wound healing.
Dressings, however, resist moisture escape, increasing heat and humidity levels beneath them. Advanced absorptive dressings can therefore affect the microclimate of the wound bed by drawing excessive moisture off the wound surface and maintaining warmth and humidity while dissipating excess heat and moisture through a vapour-permeable outer layer. Occlusive dressings do not have these functions.
Periwound skin
Excessive moisture on intact periwound skin weakens the dermis and epidermis by interrupting the arrangement of lipids in the stratum corneum (SC) and the linkages between epidermal cells1. The resulting increase in permeability makes the periwound more susceptible to invasion by contaminants and to the compounding effect of friction and shear. The term moisture-associated skin damage (MASD) refers globally to epidermal injuries resulting from exposure of the skin to moisture (for example perspiration) and irritants (for example urine, stool, ostomy effluent, wound exudate)1. One of the four clinical categories of MASD is periwound skin damage. Compounding the bond-weakening effects of moisture, enzymes in wound exudate that normally degrade contaminants in the wound also degrade proteins in intact skin. The resulting damage can cause pain, an increase in wound size, and decreased keratinocyte migration from the wound edges, therefore impairing wound closure9. As such, clinicians should choose dressings that prevent exudate from coming in contact with the periwound by locking in absorbed exudate. Ideally, the absorptive pad of a dressing should also be sized to that of the open wound bed rather than extending to the periwound.
Dressing constructions
Dressings are a dynamic primary method of either resisting moisture loss from a dry wound bed or absorbing excessive moisture from a wet wound bed while keeping the periwound free from excessive moisture.
Basic dressings such as pads made from cotton gauze or absorbents made from cellulose fibres or foams cover the wound bed and protect it from trauma and from the environment as well as simply absorbing moisture. Island dressings include an adherent border around the absorbent area. Dressings with increased capacities and enhanced features are used to treat highly exudative wounds that can overwhelm basic dressings.
SAP dressings are comprised of multiple layers in order to accommodate more highly exudative wounds by both absorption and evaporation of wound moisture. The superabsorbent core is often a mixture of cellulose and hydrophilic polymers wrapped in a cellulose tissue and/or a non-woven material. Most SAP dressings have an outer low-friction vapour-permeable polyurethane layer that allows moisture to transpire, thereby drawing more exudate from the wound and allowing a longer wear time. Polyurethane with a moisture vapour transmission rate of more than 35g/m2/hr is correlated with faster wound healing under occlusive dressings10. The sum of the fluid absorbed into the dressing and the fluid transpired through the dressing is called the fluid-handling capacity of the dressing, as defined by the European Committee for Standardization11.
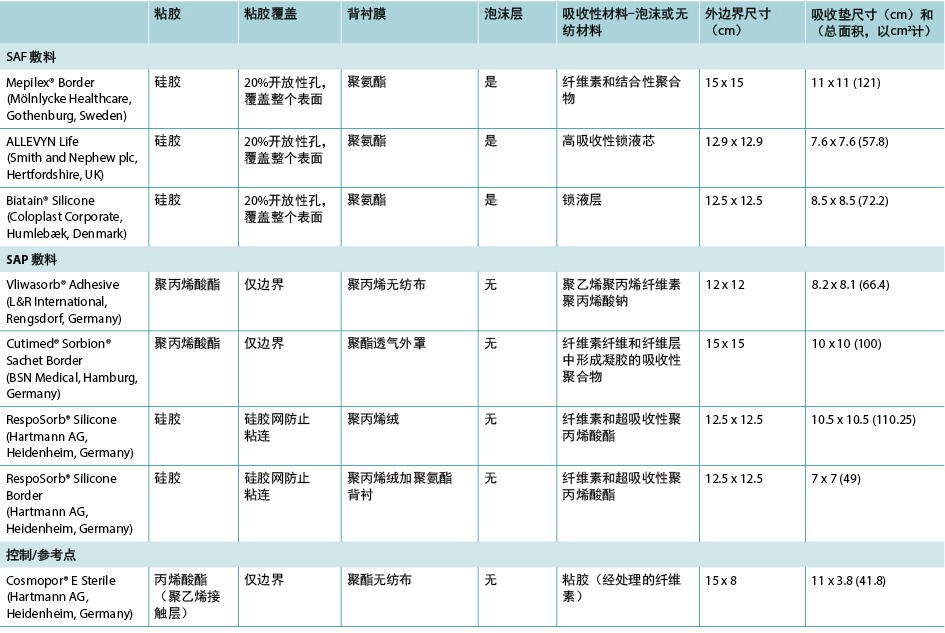
Some SAP dressings have acrylic adhesive on a border around the absorbent island so no additional taping nor fixation of such dressing is required. Some of these dressings also feature a silicone layer across the wound surface of the dressing to minimise trauma to the wound bed – see Table 1 for an examination of the similarities and differences among a representative group of these dressings.
Table 1. Size and construction of representative test dressings.
It should also be noted that the term superabsorbent can imply that a dressing can draw too much moisture from a wound and dry it out, thereby counteracting moist wound healing. One subgroup of SAP dressings – SAF dressings – are constructed in a manner similar to the SAP dressings and include an extra foam layer between the silicone and the polymer that is purported to prevent drying of the wound bed and to optimise the microenvironment of the wound bed (Table 1). Furthermore, removing pressure from a wound and dressing through a pressure release manoeuvre or a repositioning of the patient may affect moisture evaporation and heat dissipation and enhance the function of the dressing, increasing wear time.
There are few published studies comparing the effects of different dressings on similar wound types in patient use, nor ones examining the claims made by manufacturers in reference to benefits of their products in affecting the wound bed or the microclimate. Clinical research comparing dressings is very difficult to accomplish due to the many variables affecting wound healing and the impossible task of standardising patients. However, well-designed laboratory research can standardise the wound size and the exudate amount so that test dressings can be compared to each other. This standardisation limits applicability of results to the highly variable clinic population, but can provide some evidence to give guidance as to the effectiveness of a dressing in controlling the physical properties of a wound to optimise wound healing.
Study purpose
This in vitro study compares dressings in the SAF and the SAP dressing categories described in Table 1. A basic non-polymer surgical dressing was included as a control or a reference point. The dressings were studied for their abilities to handle moisture exuding from an artificial wound by measuring the:
- Amount of moisture absorbed, reported as total grams per dressing and g/cm2.
- Amount of moisture evaporated through the outer layer, reported as total grams per dressing and g/cm2.
- Humidity, both inside the covered wound bed and at the outer layer and the difference between the two.
- Temperature, both inside the covered wound bed and at the outer layer and the difference between the two.
Additionally, the effect of a pressure release or patient repositioning on each of these parameters was measured.
It is important to note that previous work was reported as simply moisture per dressing12. For this study, due to the large variation in dressings mass and surface area, these values are reported in grams of moisture per cm2.
Method
This study was conducted by an independent laboratory that is a contributor to the development of standards for support surfaces, wheelchair cushions, dressings and other wound-related medical equipment. The laboratory had an ambient temperature of 23°C ±2°C and a relative humidity of 50% ±5% as specified in ISO 554-1976(E)13.
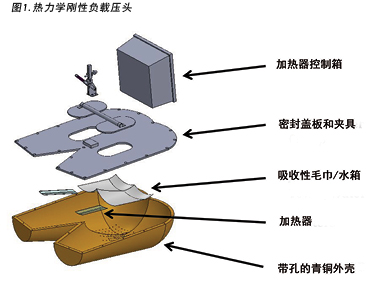
The human model was a bronze thermodynamic rigid cushion loading indenter first described by Ferguson-Pell et al.14 for use in testing wheelchair cushions. The model was developed by a group of researchers from the United Kingdom, Japan and the United States. Since 2009 it has become a standard piece of testing equipment for studying support surfaces, dressings and wheelchair cushions in the international wound care market15 (Figure 1).
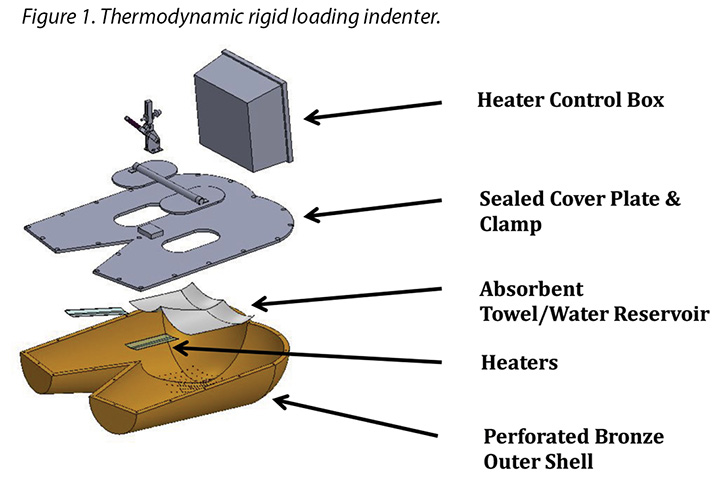
The test indenter was compliant with the American National Standards for Support Surfaces16. The indenter is shaped according to the buttocks and upper thighs of a 50th percentile human male and has pre-drilled holes in the area of the sacrum and ischium. The indenter can be loaded with pre-measured amounts of water, heated and cooled, and weighted. Diffusion of the water through the pre-drilled holes simulates moisture loss through the skin. This indenter mimics the heating, cooling, sweating and drying, weighting and unweighting of the human sacral area. Resulting conditions at the indenter–surface interface are measured, and can be compared between products, as well as in steady-state and transient conditions.
For this study the indenter was weighted with 64lbs ± 2.25lbs (just over 29kgs ± just over 1kg) which is the weight of this segment of the body for a 50th percentile male lying in bed supine. An artificial wound was created by covering all sweating pore holes not covered by the dressing pad; aluminised tape ensured no moisture evaporated outside the artificial wound area (Figure 2). This left an area of 7x7cm holes for moisture vapour; this was used for all dressings tested.

Challenging dressings under these conditions provides a scenario that is clinically relevant because foams and polymers are compressed and moisture vapour escape is reduced by the actual use conditions of the test14,15. For example, temperature and humidity sensors are high accuracy digital sensors manufactured by Sensirion AG (Staefa ZH, Switzerland) which are accurate to ±1.8% at 0–80% relative humidity and ±0.3°C at 20–50°C. The test support surface was an open-cell foam cushion 3x18x18” (7.6x45.7x45.7cm) covered with a breathable liquid moisture-resistant mattress cover by Dartex Coating US (Slaterville, RI) and positioned on a rigid mattress board.
Moisture and humidity were supplied by a weighed amount of distilled water soaked into EnduracoolTM Microfiber Cooling towels. This towel was chosen because the microfibre is a thermo-regulating technology with a moisture release rate compatible with previous published studies on LAL support surfaces17,18. In a laboratory setting, wound exudate is represented by distilled water. While the properties of each fluid differ markedly, the study is designed to demonstrate performance differences and this can be accomplished with a standard fluid. Previous publications characterising the moisture-handling capacity of dressings using this method provide a comparative baseline12.
The wound model used here is not a highly exudating model; it is a water vapour model. This model delivers a low level of moisture to the dressing surface over a period of time in a controlled manner so that a researcher can determine if the moisture is bound to the dressing or transpires through it. Test dressings were chosen from conveniently acquired and commonly utilised multilayer dressings in the European Union and in the United States. The representative dressings are listed in Table 1.
The indenter was suspended over the cushion by an H-frame which was positioned around the bed frame at about the midpoint of the bed frame. The cushion was centred under the indenter so that the ischial tuberosity area of the indenter was located 10–15cm from the rear edge of the cushion (Figure 3). The indenter was set to 37°C. The weights of two dry sets of EnduracoolTM towels were recorded, then 100±1g of distilled water was added to each towel set providing a potential for 200g to be delivered to the dressing. Each towel was folded in thirds and placed inside the indenter so that the towels covered all of the sweating pore holes. Heated weights were placed on top of the towels to facilitate moisture delivery.
Three sensors were placed to sense moisture escaping from the artificial wound area (Figure 4). Sensor locations were modified to accommodate the size of each dressing. The dressing was centred over the open sweating pore holes and covered all three sensors. Sensors placed outside the dressing lined up with those under the dressing (Figure 4). The indenter was lowered so that it settled on the support surface and transferred the full load of 64lbs ± 2.25lbs (just over 29kgs ± just over 1kg) to the support surface. A seventh sensor was used to measure ambient temperature and humidity. An EK-H4 Viewer program was set to log data at 30-second intervals through the test period.

To simulate a pressure release or patient repositioning, the indenter was raised from the support surface for 45 seconds at 3 hours ± 6 minutes. Readings were taken five minutes before the lift, identified as 175 minutes in the results section, to provide an air gap between the indenter and the support surface. It was held fully suspended from the support surface for a total of 45 seconds ± 10 seconds, then lowered back onto the support surface fully loaded for an additional 15 minutes ± 1 minute. Final readings are reported as 196 minutes in results.
At the end of the test the indenter was raised and the towels were removed and weighed. The dressing was removed from the indenter and artificial wound, and its weight recorded. The weight of a dry paper towel was recorded, the towel was used to wipe all moisture remaining in the indenter, and the towel was weighed again.
Between trials the indenter was allowed to return to steady state before the next trial was initiated. Distilled water was added to return the towels and the water mass back to the total weight of the dry towels plus 200±1g of distilled water. Two cushions and two covers were used; these were switched between trials to allow full recovery. A total of three trials were done for each dressing. Three trials were also performed with the indenter hanging in the air for 3 hours and 16 minutes without a dressing applied to measure the moisture vapour output of the artificial wound.
The final weight of the towels plus the paper towel was subtracted from the starting weight of the towels to ascertain the total amount of distilled water delivered to the system. Moisture absorbed in the dressing pad was calculated by subtracting the dressing initial weight from the dressing’s final weight. The amount of water vapour produced from the system was calculated by subtracting the starting weight of the towels from the end weight of the towels and adding the moisture recovered from inside the indenter using the paper towel. The amount of moisture that evaporated the dressing was calculated by subtracting the amount of moisture trapped in the dressings from the amount of water vapour produced by the system. The 95% CI was calculated.
While the artificial wound size was constant throughout the study, the absorbent pads for the dressings varied from 41.8cm2 to 121cm2 (Table 1). As such, the total moisture absorbed and evaporated per dressing was calculated. Calculations for moisture absorbed and moisture evaporated were done on a per cm2 basis as well to account for the variability in dressing sizes and to more accurately compare dressing performance. The average difference in temperature and humidity between the outside or cushion surface of the dressing and the inside or patient side of the dressing at 175 minutes and over the full length of the test was calculated by averaging the difference between the value over the dressing and the value under the dressing for each data point.
Results
The aim of the study was four-fold, namely to measure: the amount of moisture absorbed; the amount of moisture evaporated through the outer layer; the humidity, both beneath and outside the dressing and the difference between the two; and the temperature, both beneath and outside the dressing and the difference between the two.
The amount of moisture each dressing absorbed is shown in Table 2. This shows the total moisture and the moisture absorbed in g/cm2 per dressing as well as showing the average. The range of the SAF dressing group was 0.22–0.34g. The range of the SAP dressing group was 0.24–0.39g if the one outlier at 0.56g is not considered. The reference dressing was the smallest in area and absorbed the least by far. The range for six of the seven study dressings was 0.22–0.39g.
Table 2. Moisture absorbed in absorptive pad and moisture evaporated per dressing in total g and g/cm2 and averages of SAF and SAP.
The amount of moisture evaporated (transpired or evaporated) through the outer polyurethane layer is also shown in Table 2 and in Figure 5. The range of the SAF dressing group was 3.9–5.4g, a difference of 1.5g, whereas the range of the SAP dressing group was 3.4–4.1g, a difference of just 0.7g. Indeed, six of the seven test dressings were within 0.7g of each other (range 3.4–4.1g). There was no significant difference among the dressings at a 95% CI.
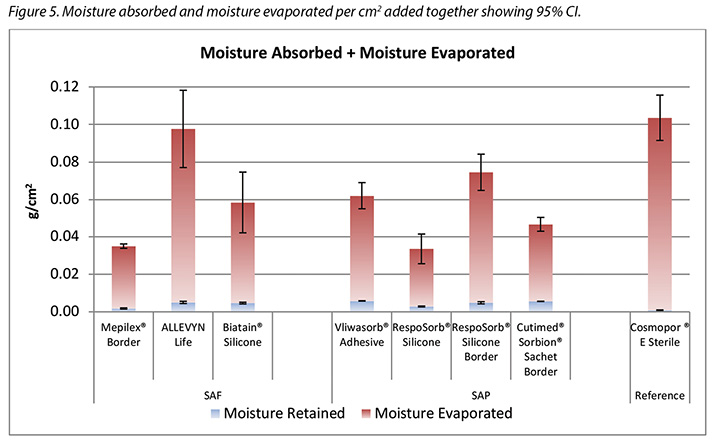
In Table 2 it should be noted that the total amount of moisture delivered by the indenter was 5.9g. Adding together moisture absorbed and moisture evaporated shows that none of the dressings removed all of the moisture.
Figure 5 graphs the total amount of moisture absorbed and moisture evaporated in gcm2 for each dressing. Figures 6a and 6b graph the moisture absorbed and the moisture evaporated per cm2 arranged in order of size of the absorbent pad, from smallest to largest. As the dressing size increased, the amount of moisture absorbed and escaping per cm2 appears to decrease, making the larger pads appear to perform more poorly. However, larger dressings have more area over which to absorb and evaporate moisture, and this graph is not standardised for wound and dressing size.
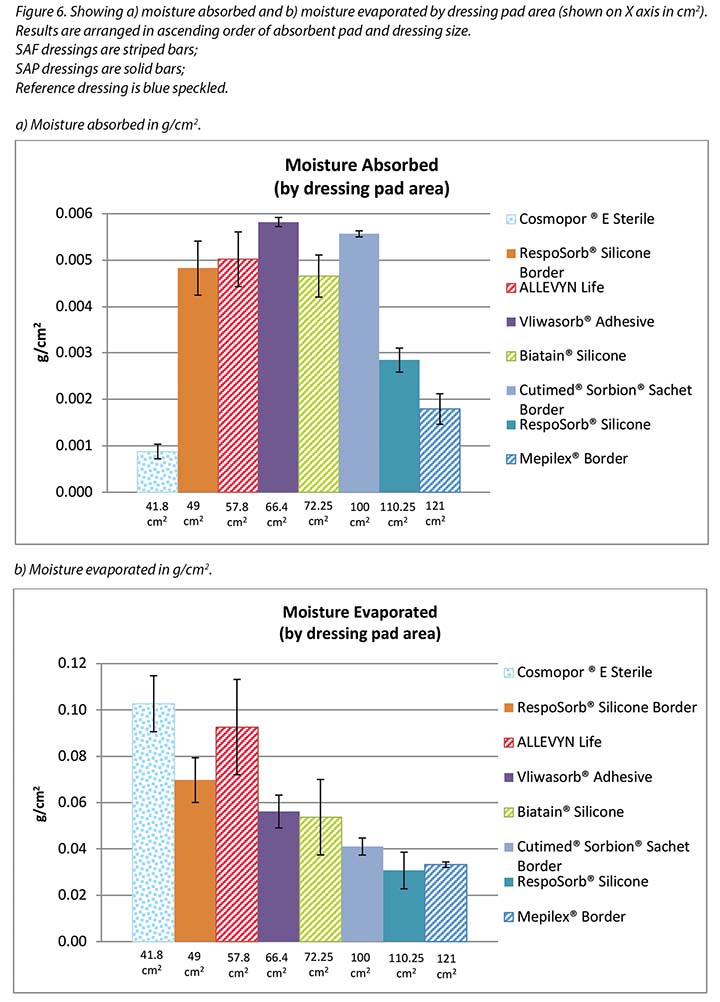
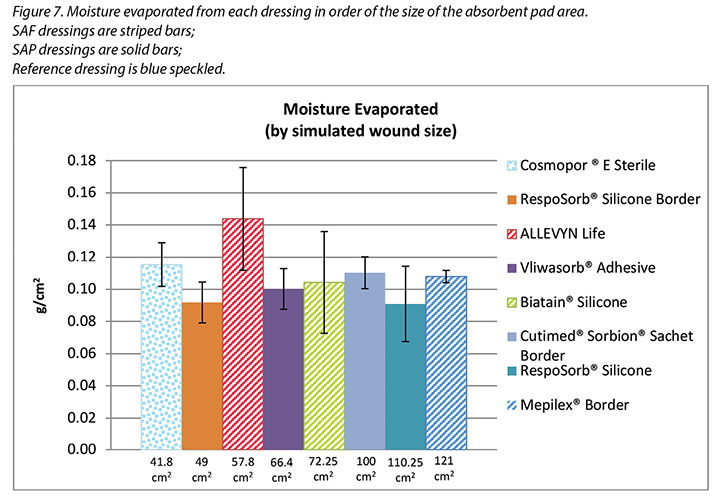
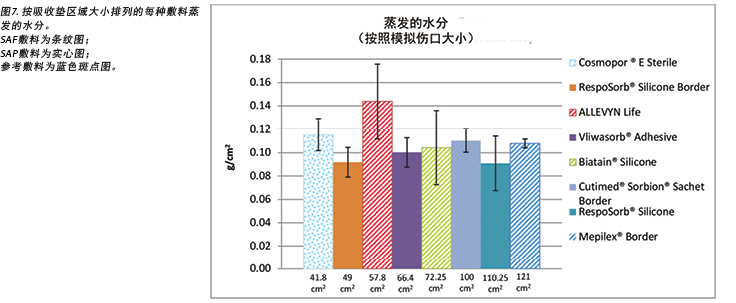
Figure 7 graphs the moisture evaporated per cm2 per wound area. That is, the dressings are compared by using both a standard wound size – the area of the exposed holes in the indenter – and a standard amount of vapour across a standard area. When compared in this way, all dressings manage approximately the same amount of moisture according to their absorbent area.
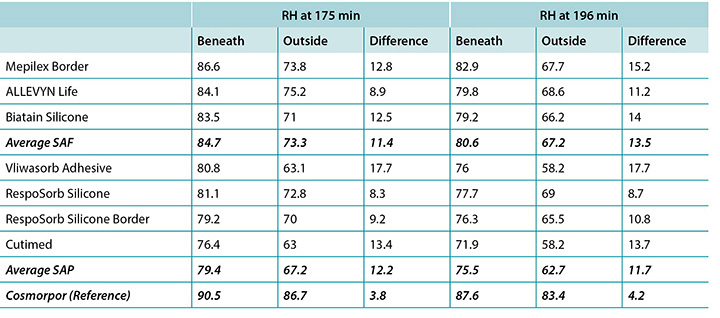
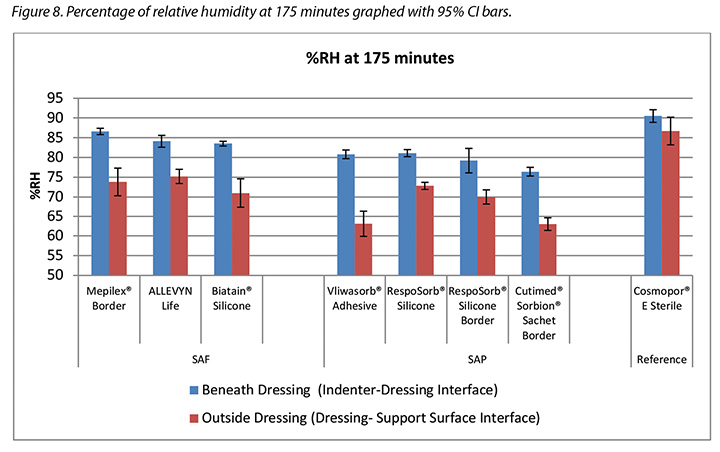
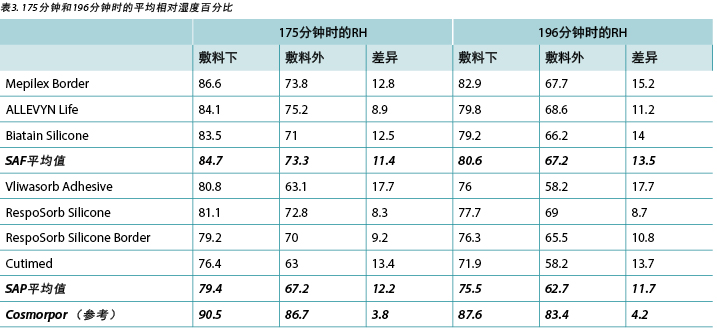
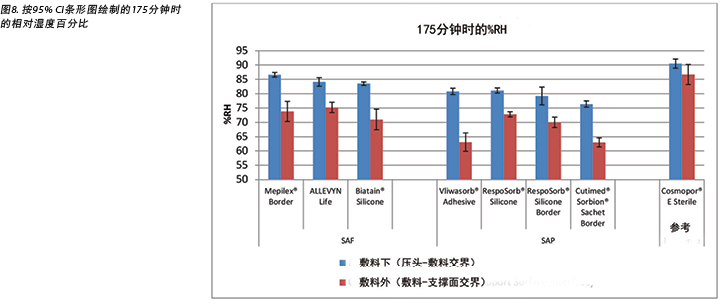
The humidity, both beneath and outside the dressing as well as the difference between the two, was also recorded. Table 3 shows the average percentage of relative humidity at 175 minutes (before pressure release) and at 196 minutes (after pressure had been reapplied for 15 minutes, at test end) inside and outside the dressings, and the difference between the inside and outside dressings, by dressing and by group. At 175 minutes all humidity readings outside the dressings were above ambient (50%), indicating active transpiration across the polyurethane backings. The SAF dressing group had a higher average humidity both inside and outside the dressing than the SAP dressing group, but the difference between the inside and the outside of the two groups was similar, 11.4 and 12.2% respectively. The reference dressing had much higher humidity both inside and outside the dressing, with a difference of only 3.8% (Table 3 and Figure 8). At 196 minutes the SAF dressing group had higher average humidities inside and outside the dressing than the SAP dressing group, but the differences between the inside and the outside were greater in the SAF dressing group. The reference dressing had a very small difference between the inside and the outside (Table 3). Looking at the error bars in Figure 8, there were no significant differences among the dressings at the 95% CI.
Table 3. Average percentage of relative humidity at 175 and 196 minutes.
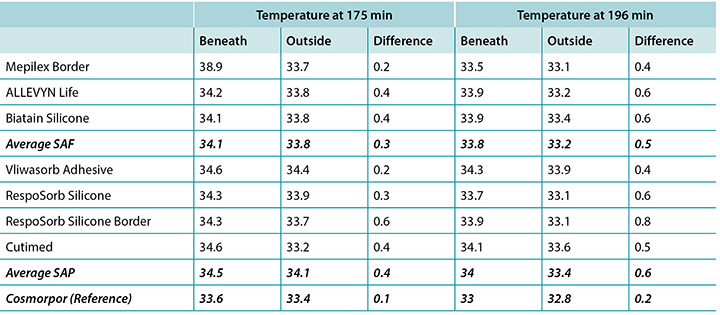
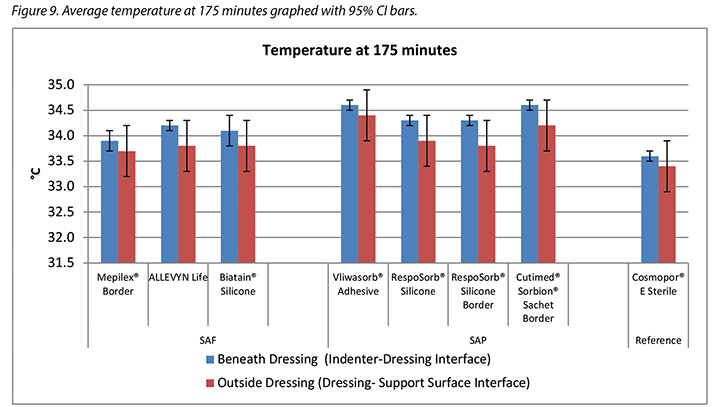
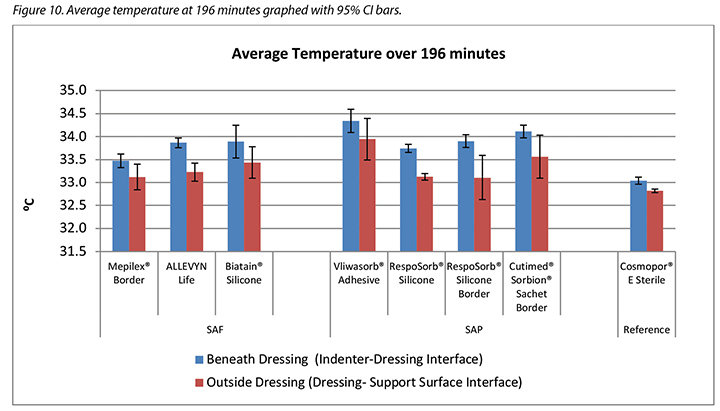
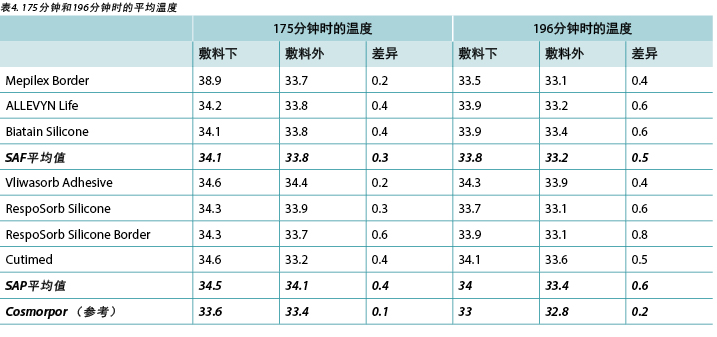
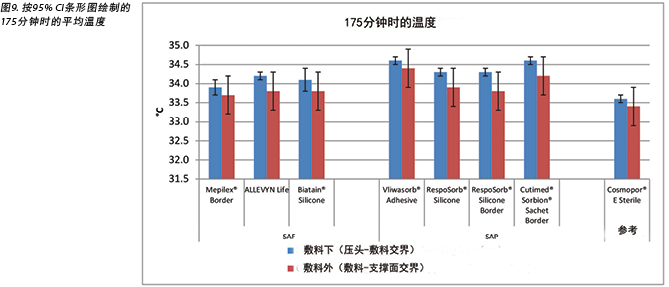
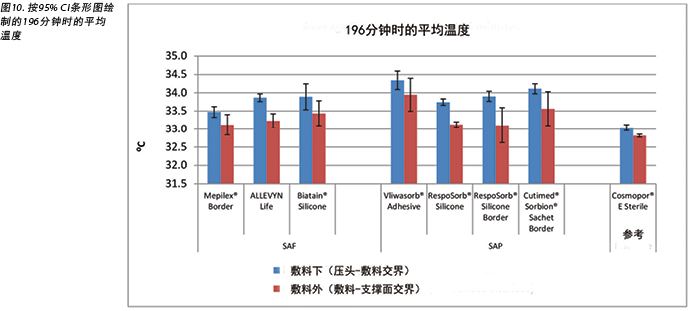
The temperature, both beneath and outside the dressing as well as the difference between the two, was also recorded. At 175 minutes, the average temperatures of the SAF dressing group, the SAP dressing group, and the reference dressing were within 0.9°C. The SAF and the SAP dressing groups had larger differences in temperature between the inside and the outside of the dressings than the reference dressing (Table 4 and Figure 9). At 196 minutes the average temperatures of the SAF and SAP dressings both inside and outside the dressings were within 0.2°C of each other. The reference dressing was only slightly below the two groups (Table 4 and Figure 10). The range of temperatures inside and outside the dressings at 196 minutes was 33.1–34.3°C. The range of temperature difference between the inside and the outside of the dressings was 0.4–0.8°C. The negative control had a temperature difference of only 0.2°C. There were no significant differences among the dressings at the 95% CI.
Table 4. Average temperature at 175 and 196 minutes.
Discussion
This study measures the relative performance of dressings in a laboratory setting by comparing results at specific time points and averaged across time. While the 3+ hour test period is a small segment of time compared to the length of time that a patient normally wears these types of dressings, the test gives an indication of how they may perform relative to each other over longer times.
This method tested how dressings handle moisture from a low steady exuding of vapour. When the integument is compromised in a stage 2, 3 or 4 wound, the regulation of moisture vapour escape is compromised as well, adding to the moisture that a dressing must handle. Total vapour can compromise the integument and must therefore also be considered when evaluating dressings6,19.
As expected, the more advanced SAF and SAP dressings outperformed the control/reference point basic island dressing in most categories.
Moisture absorbed and moisture evaporated
Both SAF and SAP dressing groups absorbed significantly more moisture in the pad than the reference dressing, indicating that their polymer materials absorb and lock in moisture and prevent it from migrating to the periwound or the wound bed. Six of the seven advanced dressings absorbed between 0.24–0.39g, with one outlier absorbing 0.56g. Dressings are expected to absorb enough to prevent maceration of the wound bed but not so much as to dry it. In order to dry a wound, a dressing would have to absorb and transpire all of the moisture provided to it. None of these dressings did, therefore the total of the moisture for each dressing in Table 2 does not equal the total moisture delivered by the indenter, 5.9g. It appears from these results that the SAF and SAP dressings are adept at absorption and will not dry a wound that exudes at the moderate level of the test fixture. The ALLEVYN Life comes closest to absorbing and transpiring all of the fluid supplied.
The same amount of moisture was delivered to all dressings. The smallest dressing had the least moisture absorbed and the most moisture which evaporated; this may be expected because the wound size was constant throughout, leaving the ratio of area of the artificial wound delivering the moisture to the area of the dressing that is available to transpire it dramatically greater for the larger dressings. Larger dressings are able to absorb and retain more, and the polymer locked in that moisture so that it could not – and did not – escape. Larger dressings can also spread and diffuse the moisture over a larger area.
The SAF dressing group, the SAP dressing group and the reference dressing were not significantly different in the amount of fluid that evaporated or transpired from the wound area at the 95% CI (Figure 6b). When moisture evaporated is graphed based on both moisture delivered and pad area in g/cm2, the advanced dressings are very similar (Figure 7). Various sizes of dressing can affect results. However, when results are shown in g/cm2 in order to compare various sized dressings, the differences are very small. This makes interpretation more difficult.
This test was performed consistent with other testing – some published and some unpublished – where striking differences in moisture handling between advanced dressings were shown6,12. This has inspired manufacturers to improve their products and the polymers and vapour-permeable backings used in the dressings, possibly resulting in more uniformity in dressing structures and the similarity in apparent handling capacities among the tested dressings.
Humidity and temperature
Specific temperature and humidity values defining the optimal microclimate conditions have not yet been determined by clinical nor laboratory research. Dressings are expected to keep the wound bed moderately warm and moist rather than too wet or too dry, too warm or too cool relative to the individual patient’s normal subdermal or dermal body conditions and to the external atmosphere. Higher humidity and moisture levels may negatively impact the wound environment just as they weaken intact skin1. The SC is in equilibrium hydration at ambient humidity of 40–60% but absorbs water at disproportionately increasing rates above 60%20–22. Wildnauer’s results show that relative humidity above 60% has the greatest influence in decreasing internal cohesive forces within the SC23.
At 175 minutes, all dressings were above 75% relative humidity beneath the dressing with a range of 83.5–86.6% for SAF dressings and 76.4–81.1% for the SAP dressings. It is reasonable to consider these values as moist. Dressings with higher inside humidities – the SAF dressing group and the reference dressing at 90.5% – may be cause for concern in potentially over-hydrating the wound bed or periwound. At 175 minutes, all dressings also had lower humidity readings outside the dressing than inside, as would be expected. All of the humidity levels outside the dressings were higher than ambient, indicating that the polyurethane backing does evaporate or transpire fluid. There was also a correlation between relative humidity beneath the dressing and moisture absorbed by the dressing pad. Lower moisture absorbed by the pad correlated with higher humidity beneath the dressing (Pearson’s correlation coefficient = –0.83).
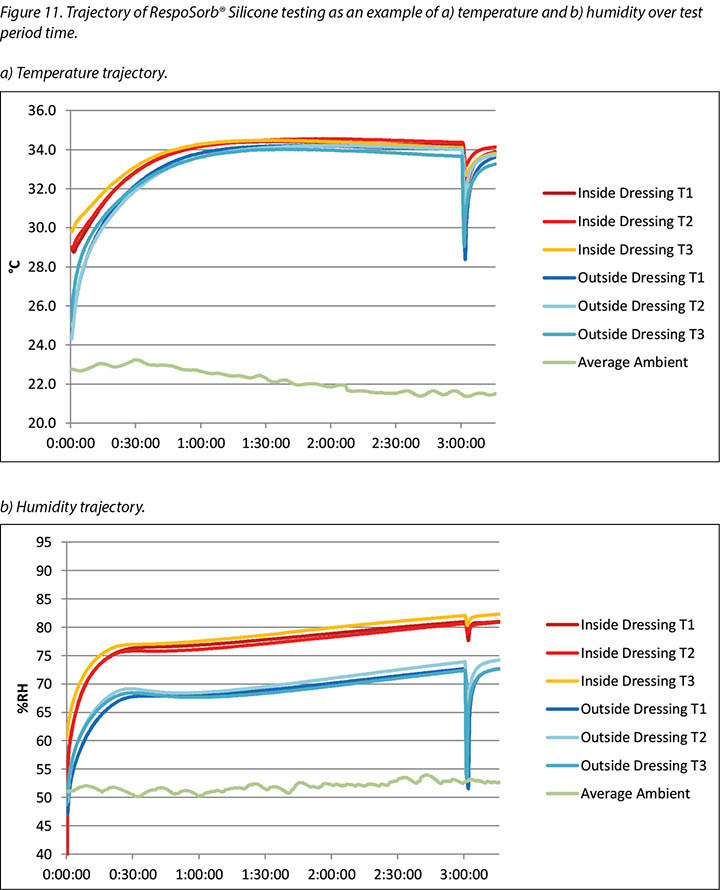
The complete release of pressure at 176 minutes suspended the indenter and the test dressing in air and allowed exchange of heat and moisture with the environment. Figure 11 shows the graphed results for one of the dressings, RespoSorb® Silicone, across the 196 minutes of the test. This is illustrative of a similar path seen for all dressings. Temperatures and humidities rose early in data collection, then temperatures eventually plateaued while humidities continued to rise. Both values dropped sharply when the indenter was lifted for the 45 second pressure release, then increased to or near pre-lift levels. It was impressive that a pressure release can have a rapid, measurable and significant affect on both heat and moisture which demonstrates the benefits of frequent position changes beyond addressing simply pressure and shear. Fifteen minutes after the pressure relief lift, at 196 minutes, all humidity levels were above 70%, with the reference dressing the highest at 87.6%. It appears that moisture vapour escapes more readily than it is trapped as, within 15 minutes of the lift, all values returned to the 175-minute levels.
Both dressing groups kept the wound bed relatively warm as indicated by lower temperatures outside the dressing than inside. This is conducive to wound healing as all were within the recommended 33–35°C range7. Yusuf et al.24 reported that a skin temperature difference of only 0.3°C predicted pressure ulceration. While this measurement is not in a wound bed, it does indicate that small differences in skin temperature can relate to damage. The range of inside dressing temperatures among the dressings was 1°C at 175 minutes and 0.8°C at 196 minutes, so these ranges could be problematic. While Yusuf’s study does not prove causation, we need to be aware of a possible effect of temperature on tissue integrity.
Temperature differences are cumulative. The rate at which temperature drops after a pressure release could affect this accumulation. A small difference accumulated over time can make a large difference. The drops in temperatures at 175 and 196 minutes per dressing were greatest for both the RespoSorb® dressings.
Limitations
It would be of interest to perform this study on a heavily exuding wound model rather than a vapour-exuding intact skin model in order to examine the performance of the dressings under conditions in which they are normally used more accurately.
Conclusion
This in vitro study concludes that SAF dressings and SAP dressings appear to be equally competent in maintaining a warm and moist microclimate at the wound bed level to enhance wound healing. They absorb more moisture per cm2 and evaporate or transpire more moisture than more basic dressings. The foam layer in SAF dressings does not appear to improve microclimate conditions at the wound bed over SAP dressings without the foam layer. Neither the SAF nor the SAP dressings dry the wound bed as the term superabsorber may imply. It was also shown that periodic relief of pressure markedly decreases temperature and humidity at the wound bed which enhances the function of the dressing and may increase wound healing.
Conflict of Interest
The authors declare no conflicts of interest.
Funding
This study was funded by Paul Hartmann AG, Heidenheim, Germany, manufacturer of RespoSorb® Silicone and RespoSorb® Silicone Border.
人造伤口中超吸收性聚合物和超吸收性泡沫敷料下的液体处理和微气候条件比较
Evan Call, Craig Oberg, Iris Streit and Laurie M Rappl
DOI: https://doi.org/10.33235/wcet.39.4.11-23
摘要
前言 通过测量相关参数,本文比较了超吸收性泡沫(SAF)和超吸收性聚合物(SAP)敷料在处理人造伤口渗液和影响敷料下微气候方面的能力,测量参数包括:水分吸收量;通过外层蒸发的水分量;敷料下和敷料外的湿度以及两者之间的差异;敷料下和敷料外的温度以及两者之间的差异。
方法 在实验室环境中使用热力学压头,在压头的重量下,通过标准伤口尺寸向每个敷料输送稳定的水蒸气流。传感器在3小时16分钟内记录了每个敷料内外的湿度和温度,并在第3小时标记处完全移除敷料上的重量,持续45秒,以模拟患者体重的变化。在测试结束时称量敷料的重量,以确定水分吸收量和水分蒸发量。
结果 SAF和SAP敷料组之间的水分吸收量、水分蒸发量、敷料内外的湿度或温度均无显著差异。
结论 因此,可以确定SAF和SAP敷料在保持伤口床的温暖和湿润微气候方面同样有效。还应注意,这些敷料不会像术语“超吸收性”所暗示的那样干燥伤口床。
前言
选择伤口敷料在伤口愈合期间进行水分管理的原因有以下三个:吸收渗出液;在伤口床上保持适当的微气候;并保护伤口周围免受过多水分浸软造成的损害。吸收性技术的大类包括泡沫、水胶体、藻酸钙、水纤维,以及与水分结合的聚合物1,2。最近研发出了更复杂的超吸收性泡沫(SAF)和超吸收性聚合物(SAP)敷料,声称在伤口水分管理方面超越了早期的敷料;但是,尚无研究对其进行比较测试。另外,术语“超吸收性”引起了关于过度吸收的可能性、由此导致的伤口床干燥和抑制伤口愈合的问题。
渗出液管理
自从Winters在1963年发表其重要著作以来,湿润伤口愈合已被视为最佳实践2,3。细胞因子和生长因子需要水分来扩散到整个伤口床中,从而通过伤口闭合和再上皮化来刺激愈合4。在伤口床上保持适当的水分含量可防止干燥,从而使上皮细胞在伤口表面自由迁移。湿润伤口形成肉芽、上皮化和愈合的速度比干燥伤口快2至3倍5。
渗出液作为伤口愈合过程的一部分而自然出现,并保持伤口床湿润,以刺激大多数伤口闭合。某些类型的伤口比其他伤口更易产生自然渗出液,尤其是静脉伤口、大面积压疮和烧伤。由于炎症的持续状态和正常细胞活性的破坏,慢性伤口具有高度渗出性。慢性伤口渗出液与急性伤口渗出液明显不同,其由高水平的蛋白质降解酶、基质金属蛋白酶(MMP)、嗜中性粒细胞和促炎细胞因子组成。因此,渗出性伤口需要吸收性敷料,其吸收能力应与渗出液的流量相匹配,以保持伤口床处的最佳水分含量。随着这些伤口开始形成肉芽,渗出液减少,对敷料的需求从吸收变为以保护为主要特征2。
来自伤口的渗出液或水分量通常记载在患者的记录中,并带有诸如轻度、中度、严重和过度等主观描述语言,而不是客观的测量。目前已有几种用于记录渗出液量的有效量表,包括伤口渗出液评分、Bates-Jensen伤口评估工具和压疮愈合量表(PUSH)。每个量表为该量表中的每个级别提供一个描述符。可用的工具及其描述符的总结和比较可参见《伤口渗出液:有效评估和管理》,这是世界伤口愈合学会联合会于2019年出版的关于渗出液的共识性文件5。
伤口微气候
微气候已被确定为伤口预防和愈合的关键因素。与环境或周围区域相比,微气候是局部区域的温度和湿度(有时还包括气流)的结合6。温暖湿润的环境有利于伤口愈合。Dini等人7的研究表明,温度低于33°C时,由于中性粒细胞、成纤维细胞和上皮细胞的活性降低,伤口愈合受到阻碍。该研究还表明,伤口床的改善与33-35°C的伤口床温度之间存在相关性。此外,Salvo等人8报告的两项研究表明,36-38°C的温度似乎可以促进伤口愈合。
但是,敷料会防止水分逸出,从而导致敷料下的热量和湿度增加。因此,先进的吸收性敷料可通过从伤口表面吸收过多的水分并保持温暖和湿润,同时通过透气性外层散发过多的热量和水分,从而影响伤口床的微气候。闭合性敷料不具有这些功能。
伤口周围皮肤
如果完整的伤口周围皮肤上的水分过多,则会干扰角质层(SC)中脂质的排列以及上皮细胞之间的联系,从而削弱真皮和表皮1。所导致的渗透性增加使得伤口周围更容易受到污染物的侵袭,以及摩擦和剪切力的复合作用。术语“潮湿环境相关性皮肤损伤(MASD)”总体上是指由于皮肤暴露于水分(例如出汗)和刺激物(例如尿液、粪便、造口流出物、伤口渗出液)而引起的表皮损伤1。MASD的四个临床类别之一是伤口周围皮肤损伤。伤口渗出液中的酶通常会降解伤口中的污染物,这些酶会进一步削弱水分的键合减弱作用,还会破坏完整皮肤中的蛋白质。由此产生的损害会导致疼痛、伤口扩大以及伤口边缘中角质形成细胞的迁移减少,从而阻碍伤口闭合9。因此,临床医生应选择能通过锁定吸收的渗出液来防止渗出液与伤口周围皮肤接触的敷料。理想情况下,敷料的吸收垫尺寸也应与开放伤口床的尺寸相同,而不是延伸至伤口周围皮肤。
敷料结构
敷料是一种动态的主要治疗方法,既可以抵抗干燥伤口床的水分流失,也可以吸收湿伤口床的多余水分,同时保持伤口周围皮肤不会存在过多的水分。
基本敷料(例如由棉纱布制成的垫片,或由纤维素纤维或泡沫制成的吸收性敷料)会覆盖伤口床,可以保护伤口免受创伤和环境的伤害,并且可以简单地吸收水分。岛型敷料由吸收区域及其周围的粘附性边缘组成。具有高吸收量和增强功能的敷料可用于治疗高度渗出性伤口,这些伤口可能会使基本敷料不堪重负。
SAP敷料由多层组成,通过吸收和蒸发伤口水分来适应渗出性更高的伤口。超吸收芯通常是纤维素和包裹在纤维素纸和/或无纺材料中的亲水性聚合物的混合物。大多数SAP敷料都具有低摩擦、透气性的聚氨酯外层,允许水分蒸发,从而从伤口吸收更多的渗出液,并且延长使用时间。湿气透过率超过35 g/m2/hr的聚氨酯可加速闭合性敷料下的伤口愈合速度10。根据欧洲标准化委员会的定义,敷料吸收的液体和通过敷料蒸发的液体之和称为敷料的液体处理能力11。
一些SAP敷料在吸收岛周围的边界上具有丙烯酸粘胶,所以此类敷料不需要额外的胶带或固定装置。其中一些敷料在接触伤口表面处还具有一层有机硅胶,以最大程度地减少对伤口床的伤害 - 有关此类敷料的代表性产品组之间的异同之处,请参见表1。
还应注意,术语“超吸收性”可能意味着敷料会从伤口吸收过多的水分并使其彻底干燥,从而抵消了湿润的伤口愈合环境。SAP敷料的一个子类是SAF敷料,它与SAP敷料的结构类似,并且在硅胶和聚合物之间包括一个额外的泡沫层,据称该泡沫层可防止伤口床干燥并优化伤口床的微环境(表1)。此外,通过手动释放压力来移除伤口压力和敷料或患者重新摆位时,可能会影响水分蒸发和热量散发,并增强敷料的功能,从而延长使用时间。
已发表的研究很少比较不同敷料在患者使用期间对类似伤口类型的影响,也没有研究专门针对制造商关于其产品在影响伤口床或微气候方面的益处的主张。比较敷料的临床研究非常难以进行,因为许多因素会影响伤口的愈合,而且无法对患者进行标准化。但是,精心设计的实验室研究可以对伤口大小和渗出液量进行标准化,以便对测试敷料进行相互比较。这种标准化将结果的适用性限制在高度可变的临床人群中,但是可以针对敷料在控制伤口的物理特性以优化伤口愈合方面的有效性提供一定的证据指导。
学习目的
本项体外研究比较了表1中描述的SAF和SAP敷料类别中的敷料。本研究纳入了基本的非聚合物手术敷料作为对照或参考点。通过测量以下参数来研究敷料处理人造伤口渗出水分的能力:
• 水分吸收量,报告为每种敷料中的总克数和g/cm2。
• 通过外层蒸发的水分量,报告为每种敷料中的总克数和g/cm2。
• 湿度,包括已覆盖伤口床的内部和外层湿度,以及两者之间的差异。
• 温度,包括已覆盖伤口床的内部和外层温度,以及两者之间的差异。
此外,还测量了压力释放或患者重新摆位对上述每种参数的影响。
应注意,以前的研究仅报告每种敷料中的水分12。对于本研究,由于敷料的质量和表面积存在较大差异,该数值以每cm2的水分克数表示。
表1. 代表性测试敷料的尺寸和结构
方法
本研究由一个独立的实验室进行,该实验室为支撑面、轮椅坐垫、敷料和其他伤口相关医疗设备的标准制定做出了贡献。根据ISO 554-1976(E)中的规定,实验室的环境温度为23°C±2°C,相对湿度为50%±5% 13。
人体模型为Ferguson-Pell等人14首次描述用于测试轮椅坐垫的青铜热力学刚性坐垫负载压头。该模型由来自英国、日本和美国的一组研究人员开发。自2009年以来,它已成为在国际伤口护理市场上研究支撑面、敷料和轮椅坐垫的标准测试设备15(图1)。
测试压头符合美国国家支撑面标准16。压头的形状与第50个百分位男性的臀部和大腿上部相吻合,并且在骶骨和坐骨区域设有预钻孔。压头可以装满预定量的水,加热和冷却后再称重。水通过预钻孔进行扩散,从而模拟皮肤水分的流失。该压头模拟了人体骶骨区域的加热、冷却、出汗和干燥、称重和不称重。测量压头-表面交界处的最终状态,并在产品之间以及稳态和瞬态条件下进行比较。
在本研究中,压头的重量为64 lb±2.25 lb(略高于29 kg±略高于1 kg),这是仰卧在床的第50个百分位男性的相应部位体重。覆盖所有敷料垫未覆盖到的汗毛孔,形成人工伤口;使用镀铝胶带确保人工伤口区域外无水分蒸发(图2)。由此留出了一个7×7 cm的带孔区域用于水分蒸发;对所有测试的敷料进行此操作。

在所述条件下,为挑战敷料提供了一种与临床相关的情况,因为在测试的实际使用条件下,泡沫和聚合物受到压缩,并且水分蒸发逸散减少14,15。例如,温度和湿度传感器是由Sensirion AG(Staefa ZH, Switzerland)生产的高精度数字传感器,在0-80%相对湿度下精度为±1.8%,在20-50°C下精度为±0.3°C。测试支撑面为3×18×18”(7.6×45.7×45.7 cm)的开孔泡沫垫,表面覆盖由Dartex Coating US(Slaterville, RI)提供的透气防潮垫套,并置于刚性垫板上。
通过吸收了一定量蒸馏水的Enduracool TM超细纤维冷却毛巾来提供水分和湿度。选择该毛巾是因为微纤维是一种温度调节技术,其水分释放速率与关于LAL支撑面的已发表研究相符17,18。在实验室环境中,伤口渗出液以蒸馏水表示。尽管每种液体的特性明显不同,但该研究旨在证明性能差异,并且可以使用标准液体来完成。已有出版物对使用此方法表征敷料的水分处理能力进行了研究,并提供了比较基线12。
本研究使用的伤口模型不是高度渗出模型;而是水蒸气模型。该模型在一段时间内以受控方式将少量水分输送到敷料表面,以便研究人员可以确定水分是否吸附在敷料上或经由敷料渗出。测试敷料选自在欧盟和美国可方便获取且常用的多层敷料。代表性敷料列于表1中。
压头通过H形框架悬挂在垫子上,该H形框架围绕床架放置,并定位在床架的中点附近。垫子的中心位于压头下方,因此压头的坐骨结节区域位于距垫子后边缘10-15 cm处(图3)。压头设定为37°C。记录两组干燥Enduracool TM毛巾的重量,然后向每个毛巾组中添加100±1 g蒸馏水,以便将200 g的水输送到敷料上。将每条毛巾折叠三分之二后放在压头内,使毛巾覆盖所有汗毛孔。将加热的重物放在毛巾上以促进水分的输送。

放置三个传感器,以感应从人造伤口区域逸出的水分(图4)。修改传感器的位置,以适应每种敷料的大小。敷料居中位于开放汗毛孔的上方,并覆盖了所有三个传感器。敷料外的传感器与敷料下的传感器对齐(图4)。降低压头,使其位于支撑面上,并将64 lb±2.25 lb(略高于29 kg±略高于1 kg)的全部负载转移到支撑面。第七个传感器用于测量环境温度和湿度。设置EK-H4 Viewer程序,从而在测试期间以30秒的间隔记录数据。

为了模拟压力释放或患者重新摆位,在3小时±6分钟时,将压头从支撑面升起45秒。在升起压头前五分钟(在结果章节中认定为第175分钟)进行读数,从而在压头和支撑面之间留出一定的气隙。将其从支撑面上完全悬挂起来,总共保持45秒±10秒,然后再下降到支撑面上实现完全负载,继续保持15分钟±1分钟。结果章节中的最终读数报告为196分钟。
在测试结束时,将压头升起,取出毛巾并称重。从压头和人工伤口上去除敷料,并记录其重量。记录干燥纸巾的重量,用该纸巾擦拭压头中残留的所有水分,并再次称重该纸巾。
在两次测试之间,压头可以在下一次测试之前回到稳定状态。加入蒸馏水,使毛巾和水重恢复为干毛巾加上200±1 g蒸馏水的总重量。测试中使用了两个垫子和两个铺盖;在测试之间进行更换,允许其完全恢复至初始状态。每种敷料总共进行了三次测试。此外,还进行了三次测试,在不使用敷料的情况下,将压头在空中悬挂3小时16分钟,以便测量人造伤口的水蒸气输出。
用毛巾的初始重量减去毛巾和纸巾的最终重量,以确定输送到系统中的蒸馏水总量。用敷料的最终重量减去敷料的初始重量,计算出敷料垫中吸收的水分。用毛巾的最终重量减去毛巾的起始重量,并加上使用纸巾从压头内部回收的水分,计算出系统产生的水蒸气量。用系统产生的水蒸气量减去敷料吸收的水分量,计算出敷料蒸发的水分量。计算出95% CI。
尽管在整个研究过程中人造伤口的大小恒定,但敷料的吸收垫从41.8 cm2到121 cm2不等(表1)。因此,计算出每个敷料吸收和蒸发的总水分。基于每cm2计算水分的吸收量和蒸发量,以考虑敷料尺寸的差异并更准确地比较敷料性能。在175分钟以及整个测试过程中,通过计算每个数据点中敷料上方和下方数值之间的平均差值,获得敷料外或垫子表面与敷料内或患者侧之间的平均温度和湿度差值。
结果
该研究旨在研究四个方面,即测量:水分吸收量;通过外层蒸发的水分量;敷料下和敷料外的湿度以及两者之间的差异;敷料下和敷料外的温度以及两者之间的差异。
表2显示了每种敷料吸收的水分量。表中显示了每种敷料吸收的总水分和以g/cm2为单位的水分吸收量,以及平均值。SAF敷料组的范围为0.22-0.34 g。如果不考虑离群值0.56g,则SAP敷料组的范围为0.24-0.39 g。参考敷料面积最小,吸收最少。七种研究敷料中有六种的范围为0.22-0.39g。
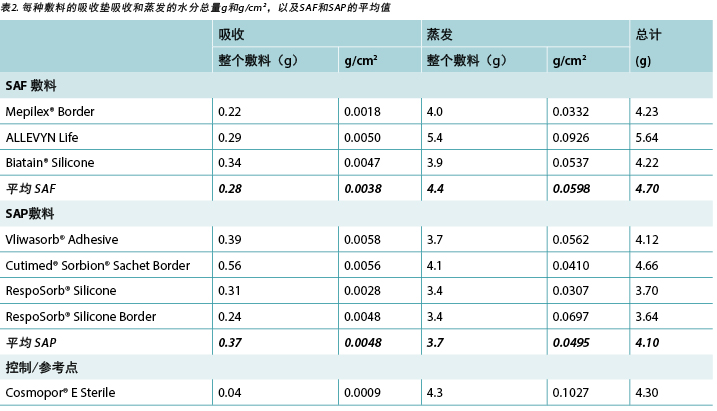
表2和图5也显示了通过聚氨酯外层蒸发(散发或蒸发)的水分量。SAF敷料组的范围为3.9-5.4 g,相差1.5 g;而SAP敷料组的范围为3.4-4.1 g,相差仅为0.7 g。事实上,七种测试敷料中有六种互相之间的重量差在0.7 g以内(范围为3.4-4.1g)。在95% CI时,敷料之间无显著差异。
在表2中,应注意,压头输送的水分总量为5.9 g。将吸收和蒸发水分相加后,发现所有敷料均未去除所有水分。
图5绘制了每种敷料吸收和蒸发的水分总量,单位为gcm2。图6a和6b绘制了按吸收垫的尺寸升序排列的每cm2吸收和蒸发的水分。随着敷料尺寸的增加,每cm2吸收和逸出的水分似乎相应减少,因此较大吸收垫的表现更差。但是,较大敷料具有更大的水分吸收和蒸发区域,该图未针对伤口和敷料的尺寸进行标准化。
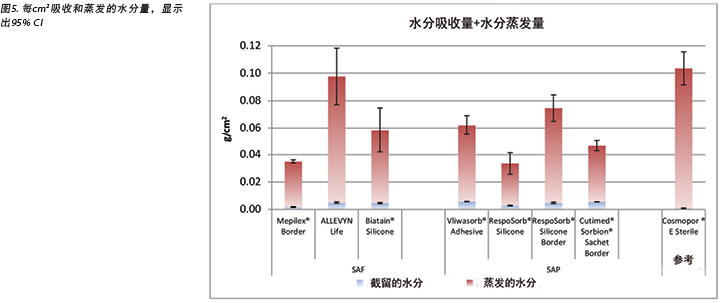
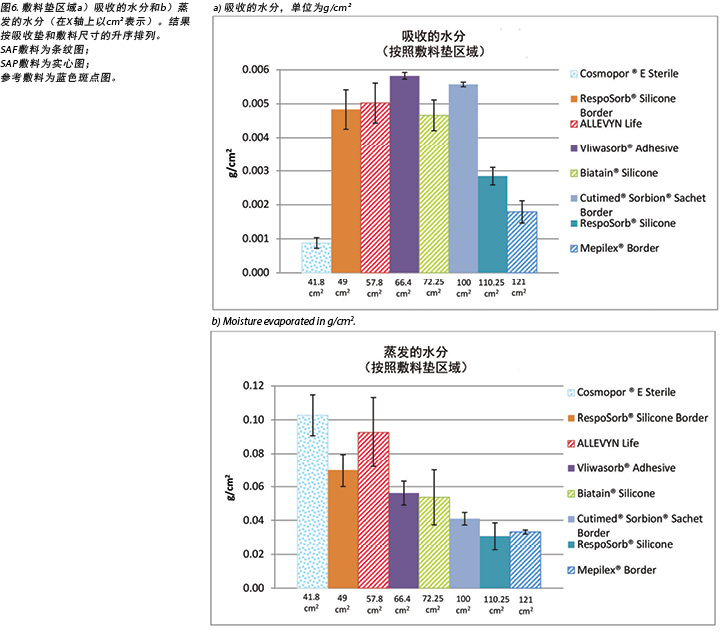
图7绘制了每个伤口区域中每cm2蒸发的水分。即,通过使用标准伤口尺寸(压头中裸露孔的面积)和整个标准区域中的标准蒸气量对敷料进行比较。使用该方式进行比较时,根据其吸收面积,所有敷料的水分管理量几乎相同。
本研究还记录了敷料下和敷料外的湿度以及两者之间的差异。表3显示了敷料内和敷料外在175分钟(释放压力之前)和196分钟(在测试结束后重新施加压力15分钟之后)时的平均相对湿度百分比,以及敷料内和敷料外湿度之间的差异(按敷料和分组)。在175分钟时,敷料外的所有湿度读数均高于环境温度(50%),表明在整个聚氨酯背衬上都存在有效的散发作用。SAF敷料组的敷料内外平均湿度均高于SAP敷料组,但两组敷料的内外湿度差异相似,分别为11.4%和12.2%。参考敷料的内外湿度均更高,仅相差3.8%(表3和图8)。在196分钟时,SAF敷料组的敷料内外平均湿度高于SAP敷料组,但SAF敷料组的内外湿度差异更大。参考敷料的内外湿度差异很小(表3)。根据图8中的误差线,在95% CI时,敷料之间并无显著差异。
本研究还记录了敷料下和敷料外的温度以及两者之间的差异。在175分钟时,SAF敷料组、SAP敷料组和参考敷料的平均温度差异都在0.9°C之内。SAF和SAP敷料组的敷料内外温度差异大于参考敷料(表4和图9)。在196分钟时,SAF和SAP敷料组的内外平均温度差异都在0.2°C之内。参考敷料仅略低于两组(表4和图10)。在196分钟时,敷料内外的温度范围为33.1-34.3°C。敷料内外的温差范围为0.4-0.8°C。阴性对照的温差仅为0.2°C。在95% CI时,敷料之间并无显著差异。
讨论
本研究通过比较特定时间点的结果并跨时间取平均值,来测量实验室环境中敷料的相对性能。与患者正常使用敷料的时间相比,3小时以上的测试时间很短,但该测试为敷料在较长时间内的相对表现提供了提示。
该方法测试了敷料如何处理低水平稳定渗出蒸汽中的水分。当在第2、3或4阶段伤口处出现皮层损伤时,水蒸气逸出调节也会受到损害,从而增加了敷料必须处理的水分量。总蒸气会损害皮层,因此在评价敷料时也必须予以考虑6,19。
不出所料,在大多数类别中,更先进的SAF和SAP敷料均优于控制/参考点的基本岛型敷料。
水分吸收量和水分蒸发量
SAF和SAP敷料组的水分吸收量均明显高于参考敷料,这表明它们的聚合物材料吸收并锁住了水分,并阻止了水分迁移到伤口周围皮肤或伤口床。七种先进敷料中有六种的吸收量在0.24-0.39 g之间,另有一个离群值为0.56 g。敷料应吸收足够的水分以防止伤口床浸软,但不能使其干燥。为了使伤口干燥,敷料必须吸收并散发提供给它的所有水分。测试敷料均未出现该情况,因此表2中每种敷料的总水分不等于压头输送的5.9 g总水分。根据上述结果可知,SAF和SAP敷料擅长吸收水分,同时不会使中等渗出量的测试装置中的伤口干燥。ALLEVYN Life吸收和蒸发的水分量最接近所提供的液体量。
对所有敷料输送的水分量相同。最小的敷料吸收的水分最少,蒸发的水分最多;这是预料之中的情况,因为伤口的 大小在整个过程中保持恒定,对于较大的敷料而言,输送水分的人造伤口面积与蒸发水分的可用敷料面积之比明显更大。较大的敷料能够吸收和保留更多的水分,并且聚合物能够锁住该水分,使其无法(也并未)逸出。较大的敷料还会使水分散布并扩散到更大的区域。
在95% CI时,SAF敷料组、SAP敷料组和参考敷料中,从伤口区域蒸发或散发的液体量无显著差异(图6b)。当基于输送的水分和吸收垫面积,以g/cm2为单位绘制蒸发的水分时,先进敷料的情况非常相似(图7)。敷料的不同尺寸会影响效果。但是,当以g/cm2表示结果来比较各种尺寸的敷料时,差异很小。这使得数据解释更加困难。
本测试的执行与其他测试(某些已发表和某些未发表)一致,在这些测试中,先进敷料之间的水分处理差异显著6,12。这激励了制造商改进其产品以及敷料中使用的聚合物和透气性背衬,这可能会使敷料结构更加均匀,并且所测试敷料之间的处理能力明显相似。
湿度和温度
临床和实验室研究尚未确定最佳微气候条件的特定温度和湿度值。相对于患者个体的正常皮下或真皮身体状况以及外界环境,期望敷料使伤口床保持适度的温暖和湿润,而不是太湿或太干、太热或太冷。较高的湿度和水分含量可能会对伤口环境造成不利影响,因为它们会削弱完整的皮肤1。SC在环境湿度为40-60%时处于平衡水合作用状态,但在湿度超过60%时,吸水率会大幅增加20-22。Wildnauer的结果表明,60%以上的相对湿度对降低SC内部的内聚力影响最大23。
在175分钟时,所有敷料的相对湿度均高于敷料下的75%相对湿度,SAF敷料的相对湿度为83.5-86.6%,SAP敷料的相对湿度为76.4-81.1%。将上述值视为潮湿是合理的。内部湿度较高的敷料(SAF敷料组和参考敷料为90.5%)可能是导致伤口床或伤口周围水分过多的原因。在175分钟时,预计所有敷料的敷料外湿度读数也低于敷料内。敷料外的所有湿度均高于环境湿度,表明聚氨酯背衬确实蒸发或散发了液体。敷料下的相对湿度和敷料垫吸收的水分之间也存在相关性。敷料垫吸收的较低水分与敷料下的较高湿度相关(Pearson相关系数=-0.83)。
在176分钟时完全释放压力,使压头和测试敷料悬于空中,并允许与环境进行热量和水分交换。图11显示了RespoSorb® Silicone在整个196分钟测试期间的结果图。这说明了观察到所有敷料的路径相似。在数据收集初期,温度和湿度上升,然后温度最终稳定,而湿度继续上升。当升起压头进行45秒的压力释放后,两个值均急剧下降,然后升高或接近提升前的水平。令人印象深刻的是,压力释放可以迅速对热量和水分产生可测量且显著的影响,这证明了频繁进行位置变换的好处不仅是解决压力和剪切力问题。压力释放提升后的15分钟(即196分钟),所有湿度水平均高于70%,其中参考敷料的湿度最高,达到87.6%。相比于截留在敷料中,湿气似乎更容易逸出,因为在提升压头的15分钟内,所有值都返回到175分钟的水平。
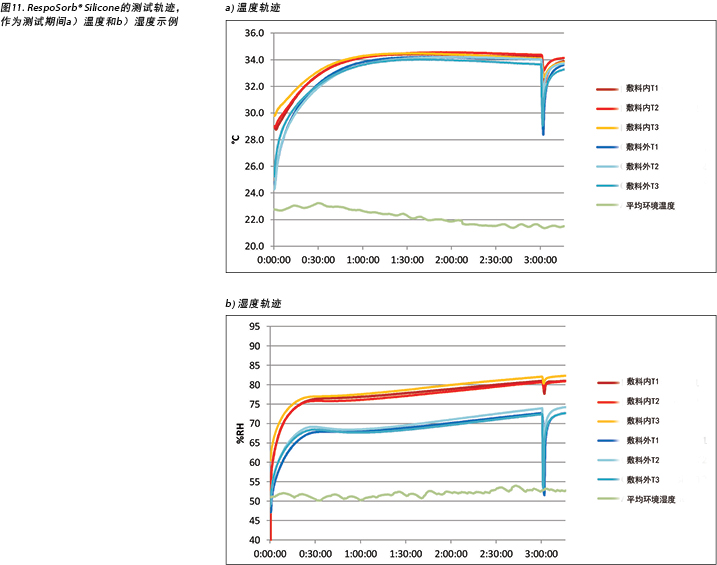
由于敷料外温度低于敷料内温度,这说明两个敷料组都能使伤口床保持相对温暖。这有助于伤口愈合,因为所有伤口温度均在建议的33-35°C范围内7。Yusuf等人24报告称,皮肤温差仅需达到0.3°C即可预测压疮。尽管此测量不在伤口床上进行,但确实表明皮肤温度的微小差异可能导致损伤。上述敷料的内部温差范围为175分钟的1°C至196分钟的0.8°C,因此这些范围可能会引发问题。尽管Yusuf的研究没有证明因果关系,但我们需要意识到温度可能会对组织完整性产生影响。
温差具有累积性。压力释放后温度下降的速率可能会影响这种累积。随着时间的推移,累积的细微差异会产生很大的差异。在175分钟和196分钟时,敷料温度降幅最大的两种敷料均为RespoSorb®敷料。
局限性
为了更准确地检查敷料在常规使用情况下的性能,有必要对重度渗出的伤口模型进行研究,而不仅是对蒸气渗出的完整皮肤模型进行研究。
结论
本项体外研究得出的结论是,SAF敷料和SAP敷料在保持伤口床层的温暖和湿润微气候以促进伤口愈合方面同等有效。与其他基本敷料相比,每cm2的该敷料可吸收更多的水分,并且蒸发或散发更多的水分。与不含泡沫层的SAP敷料相比,SAF敷料中的泡沫层并未改善伤口床的微气候条件。SAF和SAP敷料都不会像术语“超吸收性”所暗示的那样使伤口床干燥。研究还表明,周期性的压力释放显著降低了伤口床处的温度和湿度,这不仅增强了敷料的功能,还可以促进伤口愈合。
利益冲突
作者声明没有利益冲突。
资助
本研究由RespoSorb® Silicone和RespoSorb® Silicone Border的制造商Paul Hartmann AG, Heidenheim, Germany提供资金。
Author(s)
Evan Call
MS, CSM (NRM)
Department of Microbiology, College of Science, Weber State University, Ogden, UT, USA
Craig Oberg
PhD
Department of Microbiology, College of Science, Weber State University, Ogden, UT, USA
Iris Streit
Senior Manager, Global Product Marketing Advanced Wound Management,
HARTMANN GROUP, Heidenheim, Germany
Laurie M Rappl*
PT, DPT, CWS
Rappl & Associates, Simpsonville, SC, USA
Email Lrappl630@gmail.com
* Corresponding author
References
- Woo KY, Beeckman D, Chakravarthy D. Management of moisture-associated skin damage: a scoping review. Adv Skin Wound Care 2017;30(11):494–501.
- Seaman S. Dressing selection in chronic wound management. J Am Podiatr Med Assoc 2002;92(1):24–33.
- Winter GD, Scales JT. Effect of air drying and dressings on the surface of a wound. Nature 1963;197:91–2.
- Rippon MG, Ousey K, Cutting KF. Wound healing and hyper-hydration: a counterintuitive model. J Wound Care 2016;25(2):68, 70–5.
- World Union of Wound Healing Societies. Wound exudate: effective assessment and management: WUWHS consensus document. London (UK): Wounds International; 2019.
- Kottner J, Black J, Call E, Gefen A, Santamaria N. Microclimate: a critical review in the context of pressure ulcer prevention. Clin Biomech;2018;59:62–70.
- Dini V, Salvo P, Janowska A, Di Francesco F, Barbini A, Romanelli M. Correlation between wound temperature obtained with an infrared camera and clinical wound bed score in venous leg ulcers. Wounds 2015;27(10):274–8.
- Salvo P, Dini V, Kirchhain A, Janowska A, Oranges T, Chiricozzi A, et al. Sensors and biosensors for C-reactive protein, temperature and pH, and their applications for monitoring wound healing: a review. Sensors;2017;17(12).
- Woo KY, Sibbald RG. The ABCs of skin care for wound care clinicians: dermatitis and eczema. Adv Skin Wound Care 2009;22(5):230–6; quiz 7–8.
- Bolton LL, Johnson CL, van Rijswijk L. Occlusive dressings: therapeutic agents and effects on drug delivery. Clin Dermatol 1992;9.
- European Standards. Test methods for primary wound dressings. Part 1: aspects of absorbency. Brussels (Belgium): European Committee for Standardization; 2002.
- Call E, Pedersen J, Bill B, Oberg C, Ferguson-Pell M. Microclimate impact of prophylactic dressings using in vitro body analog method. Wounds 2013;25(4):94–103.
- ISO. ISO 554:1976E Standard atmospheres for conditioning and/or testing: specifications. Geneva (Switzerland): International Organization for Standardization; 1976.
- Ferguson-Pell M, Hirose H, Nicholson G, Call E. Thermodynamic rigid cushion loading indenter: a buttock-shaped temperature and humidity measurement system for cushioning surfaces under anatomical compression conditions. J Rehabil Res Dev 2009;46(7):945–56.
- Call E, Pedersen J, Bill B, Black J, Alves P, Brindle CT, et al. Enhancing pressure ulcer prevention using wound dressings: what are the modes of action? Int Wound J 2015;12(4):408–13.
- ANSI/RESNA. ANSI/RESNA SS-1-2019 for support surfaces volume 1: requirements and test methods for full body support surfaces. Arlington (USA): Rehabilitation Engineering and Assistive Technology Society of North America; 2019.
- Figliola RS. A proposed method for quantifying low-air-loss mattress performance by moisture transport. Ostomy Wound Manage 2003;49(1):32–42.
- Reger SI, Adams TC, Maklebust JA, Sahgal V. Validation test for climate control on air-loss supports. Arch Phys Med Rehabil 2001;82(5):597–603.
- Phipps L, Gray M, Call E. Time of onset to changes in skin condition during exposure to synthetic urine: a prospective study. J Wound Ostomy Continence Nurs 2019;46(4):315–20.
- Imhof RE, De Jesus ME, Xiao P, Ciortea LI, Berg EP. Closed-chamber transepidermal water loss measurement: microclimate, calibration and performance. Int J Cosmet Sci 2009;31(2):97–118.
- Li X, Johnson R, Kasting GB. On the variation of water diffusion coefficient in stratum corneum with water content. J Pharm Sci 2016;105(3):1141–7.
- Petrofsky JS, Berk L, Alshammari F, Lee H, Hamdan A, Yim JE, et al. The interrelationship between air temperature and humidity as applied locally to the skin: the resultant response on skin temperature and blood flow with age differences. Med Sci Monit 2012;18(4):CR201–8.
- Wildnauer RH, Bothwell JW, Douglass AB. Stratum corneum biomechanical properties: I. Influence of relative humidity on normal and extracted human stratum corneum. J Investig Dermatol 1971;56(1):72–8.
- Yusuf S, Okuwa M, Shigeta Y, Dai M, Iuchi T, Rahman S, et al. Microclimate and development of pressure ulcers and superficial skin changes. Int Wound J 2015;12(1):40–6.


