Volume 41 Number 2
WHAM evidence summary: effectiveness of topical coconut products
Robin Watts, Teresa Solomon and Emily Haesler
Keywords skin conditions, topical coconut, coconut oil, wound management, low- and middle-resource countries
For referencing Watts R et al. WHAM evidence summary: effectiveness of topical coconut products. WCET® Journal 2021;41(2):32-35
DOI https://doi.org/10.33235/wcet.41.2.32-35
Clinical Question
What is the best available evidence on the use of topical coconut products in wound management and the treatment of skin conditions?
Summary
Despite the wide use of topical coconut products for medicinal purposes in tropical geographic regions, only a limited number of clinical studies reporting its effectiveness in treating skin conditions and no studies reporting its use in wound management were identified is this rapid review. Level 1 evidence1,2 demonstrated that topical virgin coconut oil (VCO) is associated with improvements in signs and symptoms of xerosis1,2 and psoriasis3 in adults, and mild-to-moderate dermatitis in children4. There is some evidence that VCO improves scores of skin immaturity in preterm neonates5,6. Currently no evidence is available on the use of topical coconut products for healing human wounds.
Clinical practice recommendations
All recommendations should be applied with consideration to the wound, the person, the health professional and the clinical context.
- Topical VCO could be considered for the treatment of mild-to-moderate xerosis (Grade B).
- Topical VCO could be considered for the treatment of psoriasis in the absence of access to topical corticosteroid therapy (Grade B).
- Topical VCO could be considered for the treatment of mild-to-moderate atopic dermatitis in children (Grade B).
Sources of evidence
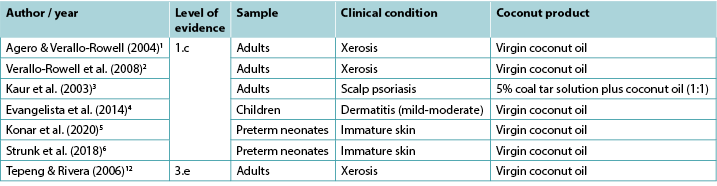
This summary was conducted using methods published by the Joanna Briggs Institute (JBI)7–11. The summary is based on a systematic literature search combining search terms related to wounds and skin conditions with terms related to coconut palm. Searches were conducted in Embase, Medline, Global Health, and Allied and Complementary Medicine databases, and in 10 healthcare journals from low- and middle-income countries for evidence published up to May 2021 in English. Studies were assigned a level of evidence (Table 1) based on JBI’s hierarchy7–11. Recommendations are made based on the body of evidence and are graded according to the system reported by JBI7–11.
Table 1. Levels of evidence

Background
Various parts of the coconut tree have been used for a multitude of purposes in traditional medicine for thousands of years, to the extent that the plant is often called the “tree of life”16. Products derived from Cocos nucifera Linn: Arecaacae include coconut water, oil from coconut milk or copra (dried kernel), dried coconut shell and husk fibre17,18. The most used coconut product, virgin coconut oil (VCO), is extracted directly from coconut flesh and contains medium chain fatty acids that have surfactant qualities1,19,20. Another tested product, coconut shell liquid smoke (CS-LS) is produced by burning coconut shells at 400˚C resulting in a solution arising from condensation of vapour of wood smoke14. Coconut shells contain the highest antioxidant properties of any parts of the coconut14.
Laboratory testing and biochemical analysis of these products have identified a number of useful properties – anti-inflammatory, antimicrobial, antifungal, antioxidant, antineoplastic and analgesic17,18,20–24. When applied topically, VCO provides barrier protection for the stratum corneum and reduces transepidermal water loss (TEWL), promoting skin moisturisation19,20,24,25. When used on wounds, VCO and other coconut-derived products are reported to promote collagen synthesis and faster epithelisation15,20,24.
Evidence
Evidence from animal studies
Evidence on the wound healing effect of coconut comes from animal studies. Results from three studies13–15 are provided as examples of the significant amount of laboratory work on this topic. In the first study13, undertaken in India, VCO was applied daily for 10 days to open dermal wounds in rats. There were three groups of six rats each – a control group, a group treated with 0.5ml VCO, and a third treated with 1ml VCO. Time to complete epithelisation and composition of granulation tissue (e.g., collagen and fibroblasts) were among the outcome measures. In terms of both time to complete epithelisation and total collagen content, groups 2 and 3 were statistically significant compared to the control (p<0.05), 1ml being more effective than 0.5ml13 (Level 5).
The second study14 was conducted in Indonesia to evaluate the healing activity of CS-LS for burns. Thirty-six mice were randomised into three groups (n=12/group) – CS-LS, normal saline 0.9% (NaCl), and 10% povidone iodine. The burn wounds were left open, with treatment applied twice daily for 25 days. Wound contraction was measured on days 1, 5, 10 and 25 after burn induction. The CS-LS group showed the fastest wound contraction of the three groups by day 5 (p<0.001). On day 10 there was a statistically significant difference to the povidone iodine group (p<0.001) and on day 25 there was a statistically significant difference to the NaCl group (p<0.05)14 (Level 5).
In the third study15, VCO for treating diabetic ulcers was explored with a rat population. Rats with ulcers were divided into four groups – non-treated, non-diabetic rats (n=18), non-treated diabetic rats (n=18), diabetic rats receiving 1ml VCO applied daily for 14 days (n=18), and diabetic rats receiving silver sulfadiazine cream applied daily for 14 days (n=18). Wound closure rates were measured on days 5, 10 and 14. Diabetic ulcers treated with VCO had statistically significantly faster closure rates (p<0.05) compared with diabetic ulcers receiving no treatment on all days. On days 5 and 14 there was a statistically significant difference between the VCO and the silver sulfadiazine cream groups (p<0.05), favouring VCO15 (Level 5).
Evidence from human studies
Evidence on effectiveness for treating wounds
No evidence on topical coconut products for use in treating human wounds was identified.
Evidence on effectiveness for treating skin conditions
Xerosis in adults
Two blinded RCTs1,2 provided evidence for using VCO to relieve xerosis (dry skin) in adults. The first RCT1 was conducted on 34 individuals with mild-to-moderate xerosis to determine the effectiveness and safety of VCO compared with mineral oil when used as a therapeutic moisturiser. The solutions were applied to the legs twice daily for 14 days. Skin hydration and skin lipids were tested to measure effectiveness while TEWL and skin pH were the quantitative measures for safety. Xerosis was evaluated for dryness, scaling, roughness and pruritus by both an investigator using Wehr’s Grading and by participants using a visual analogue scale. Data were collected at baseline, day 7 and day 14. Participants also evaluated side effects (e.g., erythema, stinging or itching). Both treatments were comparable in terms of outcome measures for effectiveness and safety. By the end of the study 81% (13/16) of the participants in the VCO group showed improvement of at least one level in xerosis grading compared to 72% (13/18) of the mineral oil group1 (Level 1).
The second RCT2 compared VCO to virgin olive oil (VOO) for relieving xerosis and eliminating Staphylococcus aureus from skin in adults with atopic dermatitis (n=52). One group was treated with VCO and the other with VOO, with oils massaged gently into the skin twice daily at two skin sites displaying no clinical signs of infection. Outcome measures were skin cultures, photography and the objective component of the SCORAD severity index (O-SSI). Assessment occurred at baseline and at 4 weeks. At 4 weeks, the VCO group improved more significantly on the O-SSI compared to the VOO group (p=0.004)2. Of the VCO group, 77% (20/26) were positive for S. aureus on entry to the study compared 46% (12/26) in the VOO group. Following treatment, only 5% (1/12) of the VCO group remained positive versus 50% (6/12) of the VOO group. The relative risk for VCO was 0.1 compared to 10.1 for VOO (p=0.00; 95% confidence interval [CI], 0.01–0.73, number needed to treat [NNT]=2.2) (Level 1).
Psoriasis in adults
Two studies provided evidence on the use of coconut oil for treating psoriasis. In an RCT (n=40)3, adults with scalp psoriasis were randomised into three groups to assess the effectiveness of relatively bland emollients: 5% coal tar solution plus coconut oil (1:1); 10% urea, 10% lactic acid, 10% propylene glycol plus 10% liquid paraffin (in a cream base); and VCO alone. All three groups showed comparable significant improvement over time, showing 57%, 64.4% and 58.3% clearing of symptoms respectively (p<0.01) without adverse effects. The authors noted that topical corticosteroids have demonstrated substantially higher response and clearance rates than this study found3 (Level 1).
An observational study (n=31)12 explored the use of VCO applied twice daily for 8 weeks to psoriasis lesions in adults. Erythema, scaling and plaque elevation were evaluated every second week using photography and a clinical assessment of symptom clearance. At the completion of the study 16% of participants (5/31) had complete clearance. Scaling was observed to be most reduced in the 4–6-week period of treatment, while erythema and plaque elevation were most improved in the 6–8-week period. No adverse effects were experienced12 (Level 3).
Dermatitis in children
One RCT4 (n=117) compared the effectiveness of topical VCO to that of topical mineral oil for children (aged between 1–13 years) with mild-to-moderate atopic dermatitis. For both treatment groups, 5ml of oil was applied twice daily. Impact on epidermal function was measured using a clinical assessment tool (SSI) and by measuring TEWL and skin capacitance, all measured at baseline and 2, 4 and 8 weeks. On the SSI measure the VCO was significantly more effective than the mineral oil (mean reduction in symptoms 68.23% versus 38.13%, p<0.001). The VCO also produced significantly effective results in terms of the TEWL over the 8-week period compared to the mineral oil group (decrease in TEWL 70.7% versus 35.36%). In terms of the emollient effect of the two oils, a statistically significant difference between the two became apparent at 8 weeks of treatment (p=0.03). No adverse effects were reported in the VCO group, while five children in the mineral oil group required “rescue” treatment with topical corticosteroids4 (Level 1).
Treatment of immature skin in preterm neonates
Two non-blinded RCTs5,6 investigated application of VCO to preterm neonates to promote skin maturity. In both studies, skin maturity was assessed on days 7, 14 and 21 using the Neonatal Skin Condition Scale (NSCS) that includes evaluation of dryness, erythema and skin breakdown. In both studies, babies with existing skin conditions (e.g., infection or rash) were excluded5,6.
In the largest RCT (n=2,294)5, preterm neonates (<37 weeks) were randomised to a treatment group receiving 5ml VCO applied four times daily or to a control group receiving massage only (no topical treatment). Babies receiving VCO had statistically significantly better NSCS scores than the control group at days 7, 14, 21 and 28 (p<0.01) and were significantly less likely to experience a decrease in skin maturity (p<0.01) or hypothermia (p<0.01), without increase in adverse events including rash or accidental slippage of the baby. However, parents were significantly more likely to rate the intervention as cumbersome (2% versus 0.3%, p<0.001) (Level 1).
In the second RCT6, 72 preterm babies (n<30 weeks) received either no topical emollient (n=36) or 5ml/kg VCO applied twice daily over the body (excluding face, scalp and around medical devices). After 3 weeks of treatment, NSCS score declined for the babies in the control group but remained stable for those receiving VCO (p=0.01). There was no significant difference in adverse events including skin irritation or temperature instability6 (Level 1).
Due to methodological limitations of these studies, more evidence is required to recommend VCO for routine care of immature neonate skin. However, available research suggests that the practice is safe to explore5,6.
Table 2. Summary of clinical evidence for topical coconut products
Considerations for use
- When used as a skin moisturiser, VCO is applied to adults and children by rubbing directly on skin and/or lesions, usually twice daily1,2,4,12,22.
- Topical application of VCO for mild-to-moderate skin conditions is associated with a lower rate of adverse effects than topical corticosteroids4,12.
- To apply VCO to the immature skin of very preterm neonates, stroke the oil onto skin for 2–3 minutes without massage during routine care to avoid excessive handling6.
Conflict of Interest
The authors declare no conflicts of interest in accordance with International Committee of Medical Journal Editors (ICMJE) standards.
The method for development of WHAM (Wound Healing and Management unit at Curtin University, Perth) evidence summaries is consistent with methodology published in Munn Z, Lockwood C, Moola S. The development and use of evidence summaries for point of care information systems: a streamlined rapid review approach. Worldviews Evid Based Nurs 2015;12(3):131–8 and other resources on rapid evidence summaries published by the JBI as cited above. WHAM evidence summaries undergo peer-review by an international multidisciplinary Wound Expert Reference Group. WHAM evidence summaries provide a summary of the best available evidence on specific topics and make suggestions that can be used to inform clinical practice. Evidence contained within this summary should be evaluated by appropriately trained professionals with expertise in wound prevention and management, and the evidence should be considered in the context of the individual, the professional, the geographic and clinical setting and other relevant clinical information.
Funding
The authors received no funding for this evidence summary.
Copyright © 2021 Wound Healing and Management Unit, Curtin University.
![]()
WHAM证据总结:外用椰子产品的有效性
Robin Watts, Teresa Solomon and Emily Haesler
DOI: https://doi.org/10.33235/wcet.41.2.32-35
临床问题
在伤口管理和皮肤病治疗中使用外用椰子产品的现有最佳证据是什么?
总结
虽然外用椰子产品在热带地理区域被广泛用于医疗用途,但在本次快速审查中仅发现了数量有限的临床研究报告了其在治疗皮肤病方面的有效性,没有报告其用于伤口管理的研究。1级证据1,2表明,外用初榨椰子油(VCO)与改善成人干燥病1,2和银屑病3以及儿童轻度至中度皮炎4的体征和症状有关。有证据表明,VCO可改善早产儿的皮肤不成熟度的评
分5,6。目前尚无关于使用外用椰子产品治愈人类伤口的证据。
临床实践建议
采用所有建议时,应考虑伤口、患者、专业医护人员和临床环境。
• 可考虑使用外用VCO治疗轻度至中度干燥病(B级)。
• 在无法获得局部皮质类固醇治疗的情况下,可考虑使用外用VCO治疗银屑病(B级)。
• 可考虑使用外用VCO治疗儿童的轻度至中度特应性皮炎(B级)。
证据来源
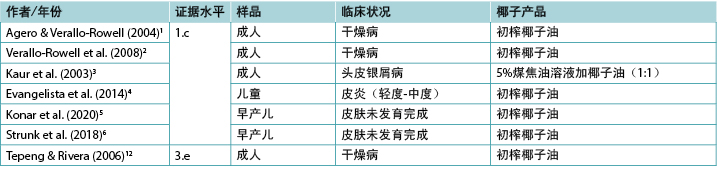
本总结是采用乔安娜•布里格斯研究所(JBI)公布的方法进行7-11。本总结以系统性的文献检索为基础,并结合使用伤口和皮肤病相关的检索术语和与椰子树相关的术语。在Embase、Medline、Global Health、补充医学文献数据库(Allied and Complementary Medicine)等数据库中进行了检索,并在中低收入国家的10种医疗保健杂志中检索了截至2021年5月以英文发表的证据。根据JBI的等级划分,对研究进行了证据水平(表1)的划分7-11。建议是根据大量证据所提出的,并根据JBI报告的系统进行评分7-11。
表1.证据水平

背景
几千年来,传统医学一直将椰子树的各个部分用于多种用途,以至于人们常将这种植物称为“生命之树”16。源自椰子树的产品:Arecaacae包括椰子汁、椰奶或干椰子肉(干棕仁)、干椰壳和外果壳纤维17,18。初榨椰子油(VCO)是最常用的椰子产品,其直接从椰子肉中提取,含有中链脂肪酸,具有表面活性剂的性质1,19,20。另一种测试产品是椰壳烟熏液(CS-LS),它是通过在400°C下燃烧椰壳产生由木材烟雾的蒸汽冷凝而成的溶液制成14。在椰子的所有部分中,椰壳含有最高的抗氧化性能14。
这些产品的实验室测试和生化分析已确定了许多有用的性能 - 抗炎、抗菌、抗真菌、抗氧化、抗肿瘤和镇痛性能17,18,20-24。外用VCO可为角质层提供屏障保护,减少经皮水分丢失(TEWL),促进皮肤保
湿19,20,24,25。据报告,当用于伤口时,VCO和其他椰子衍生产品可促进胶原蛋白合成,并加快上皮
化15,20,24。
证据
动物研究证据
关于椰子伤口愈合效果的证据来自动物研究。本文提供了三项研究13-15的结果,作为关于这个主题的大量实验室研究工作的示例。在印度进行的第一项研究13中,每天在大鼠的开放性皮肤伤口上涂抹VCO,持续10天。研究分为3组,每组有6只大鼠 - 对照组、使用0.5ml VCO的治疗组,以及使用1ml VCO的治疗组。完成上皮化的时间和肉芽组织(如胶原蛋白和成纤维细胞)的形成是结果指标。在完成上皮化的时间和总胶原蛋白含量方面,与对照组相比,第2组和第3组均具有统计学意义(P<0.05),1 ml比0.5 ml更有效13(5级)。
第二项研究14是在印度尼西亚进行,旨在评价CS-LS对烧伤的愈合活性。36只小鼠被随机分为三组(n=12/组) - CS-LS、0.9%生理盐水(NaCl)和10%聚维酮碘。烧伤伤口保持开放,每日治疗两次,持续25天。在烧伤诱导后的第1、5、10和25天测量伤口收缩情况。到第5天,CS-LS组表现出三组中最快的伤口收缩情况(P<0.001)。在第10天,与聚维酮碘组相比显示出具有统计学意义的差异(P<0.001),在第25天,与NaCl组相比显示出具有统计学意义的差异(P<0.05)14(5级)。
在第三项研究15中,使用大鼠群体探究了VCO治疗糖尿病溃疡的问题。将患有溃疡的大鼠分为4组 - 未治疗的非糖尿病大鼠(n=18),未治疗的糖尿病大鼠(n=18),持续14天每天涂抹1 ml VCO的糖尿病大鼠(n=18),以及持续14天每天涂抹磺胺嘧啶银乳膏的糖尿病大鼠(n=18)。在第5、10和14天测量伤口闭合率。与未接受治疗的糖尿病溃疡相比,在研究期间的所有天数内,接受VCO治疗的糖尿病溃疡均表现出具有统计学意义上更快的闭合率(P<0.05)。在第5天和第14天,VCO组和磺胺嘧啶银乳膏组之间具有统计学意义的差异(P<0.05),VCO效果更佳15(5级)。
人类研究数据
治疗伤口有效性的证据
未发现关于外用椰子产品用于治疗人类伤口的证据。
治疗皮肤病有效性的证据
成人干燥病
两项设盲的随机临床试验(RCT)1,2提供了使用VCO以缓解成人干燥病(皮肤干燥)的证据。为确定VCO用作治疗性润肤膏时与矿物油相比的有效性和安全性,在34例患有轻度至中度干燥病的患者中开展了第一项RCT1。将溶液涂抹在腿上,每日两次,持续14天。为测量有效性,对皮肤水合和皮肤脂质进行了测试,TEWL和皮肤pH值是测量安全性的定量指标。研究者使用Wehr评分法对干燥、脱皮、粗糙和瘙痒进行评价,受试者则使用视觉模拟量表进行评价。在基线、第7天和第14天收集数据。受试者还对副作用(如红斑、刺痛或瘙痒)进行了评价。两种治疗在有效性和安全性的结果指标方面类似。研究结束时,与矿物油组的72%(13/18)相比,VCO组中81%(13/16)的受试者的干燥病分级得到至少一个级别的改善1(1级)。
第二项RCT2比较了VCO与初榨橄榄油(VOO)在缓解干燥病和消除特应性皮炎成人患者皮肤中的金黄色葡萄球菌方面的作用(n=52)。一组使用VCO进行治疗,另一组使用VOO进行治疗,治疗方法为每日两次将油轻轻按揉到两个未显示出临床感染迹象的皮肤部位上。结果指标是皮肤培养物情况、照相术和SCORAD严重程度指数的客观部分(O-SSI)情况。在基线和第4周进行评估。在第4周,与VOO组相比,VCO组的O-SSI改善更为明显(P=0.004)2。在VCO组中,77%(20/26)的受试者在入组研究时其金黄色葡萄球菌呈阳性,而VOO组为46%(12/26)。治疗后,VCO组中仅5%(1/12)的受试者的金黄色葡萄球菌仍呈阳性,而VOO组为50%(6/12)。VCO的相对风险为0.1,而VOO为10.1(P=0.00;95%置信区间[CI],0.01-0.73,需要治疗的人数[NNT]=2.2)(1级)。
成人银屑病
两项研究提供了关于使用椰子油治疗银屑病的证据。在一项RCT(n=40)3中,为评估相对温和的润肤剂的有效性,头皮银屑病成人患者被随机分为三组:5%煤焦油溶液加椰子油(1:1);10%尿素、10%乳酸、10%丙二醇加10%石蜡油(在膏基中);以及单独的VCO。随着时间的推移,所有三个组均显示出类似的明显改善,分别显示出57%、64.4%和58.3%症状清除率(P<0.01),未出现不良反应。作者指出,与本研究的研究结果相比,局部皮质类固醇显示出明显更高的应答率和清除率3(1级)。
一项观察性研究(n=31)12对使用VCO治疗成人银屑病皮损进行了探究,治疗方法为每日涂抹两次VCO,持续8周。每隔一周使用照相术和症状清除率临床评估来评估红斑、脱屑和斑块凸起的情况。研究结束时,16%的受试者(5/31)已完全清除。观察到脱屑在4-6周的治疗期间减少最多,而红斑和斑块凸起在6-8周期间改善最大。未出现不良反应12(3级)。
儿童皮炎
一项RCT4(n=117)比较了外用VCO与外用矿物油对患有轻度至中度特应性皮炎的儿童(年龄在1-13岁之间)的有效性。在两个治疗组中,受试者每日涂抹两次5 ml的油。使用临床评估工具(SSI)并通过测量TEWL和皮肤电容测量对表皮功能的影响,所有测量均在基线以及第2、4和8周进行。在SSI测量方面,VCO明显比矿物油更有效(症状平均减少了68.23%与38.13%,P<0.001)。在8周内,与矿物油组相比,VCO在TEWL方面也产生了显著有效的效果(TEWL分别减少了70.7%与35.36%)。就两种油的润肤效果而言,两者之间具有统计学意义上的差异在治疗8周后变得明显(P=0.03)。VCO组无不良反应报告,而矿物油组有5名儿童需要使用局部皮质类固醇进行“修复”治疗4(1级)。
早产儿皮肤发育不成熟的治疗
两项未设盲的RCT5,6研究了在早产儿中使用VCO促进皮肤成熟度的情况。在这两项研究中,在第7、14和21天使用新生儿皮肤状况量表(NSCS)评估皮肤成熟度,该量表包括对干燥、红斑和皮肤破损的评价。在这两项研究中,现有皮肤病(如感染或皮疹)的婴儿被排除在外5,6。
在最大型的RCT(n=2,294)5中,早产儿(<37周)被随机分配至每日四次涂抹5 ml VCO的治疗组或仅接受按摩(无外用治疗)的对照组。接受VCO治疗的婴儿在第7、14、21和28天的NSCS评分在统计学上显著优于对照组(P<0.01),并且出现皮肤成熟度下降(P<0.01)或体温过低(P<0.01)的可能性明显降低,包括皮疹或婴儿意外滑倒在内的不良事件没有增加。但家长们认为干预措施较为麻烦的可能性更高(2%与0.3%,P<0.001)(1级)。
在第二项RCT6中,72名早产儿(n<30周)接受了不使用外用润肤剂(n=36)或每日两次在全身(不包括面部、头皮和医疗器械周围)涂抹5 ml/kg VCO的治疗。治疗3周后,对照组中婴儿的NSCS评分有所下降,但接受VCO治疗的婴儿的评分保持稳定(P=0.01)。不良事件(包括皮肤刺激或温度不稳定性)无显著差异6(1级)。
由于这些研究在方法上的局限性,仍需更多证据来推荐VCO用于新生儿皮肤发育不成熟的常规护理。但现有研究表明,这种实践是安全的,值得我们进行进一步探索5,6。
表2.外用椰子产品的临床证据总结
使用注意事项
• 当使用VCO作为皮肤润肤膏时,VCO适用于成人和儿童,方法是直接将VCO涂抹在皮肤和/或病变处,通常每日涂抹两次1,2,4,12,22。
• 与局部使用皮质类固醇相比,外用VCO治疗轻度至中度皮肤病的不良反应发生率更低4,12。
• 若要将VCO涂抹在极早产儿的未成熟皮肤上,请在常规护理期间将油轻轻涂抹在皮肤上2-3分钟,请勿进行按摩,以避免过度处理6。
利益冲突
根据国际医学期刊编辑委员会(ICMJE)的标准,作者声明没有利益冲突。
WHAM(珀斯科廷大学伤口愈合与管理课程)证据总结的开发方法与Munn Z, Lockwood C, Moola S发表的现场医护信息系统(一种简化的快速审查方法)证据总结的开发和使用,Worldviews Evid Based Nurs 2015;12(3):131-8,以及上文引用的JBI发表的关于快速证据总结的其他资源的方法一致。WHAM证据总结经过国际多学科伤口专家参考小组的同行评审。WHAM证据总结提供了关于特定主题的最佳可用证据的总结,并提出了可用于指导临床实践的建议。本总结中包含的证据应由经过适当培训的具有伤口预防和管理专业知识的专业人士员进行评价,并应根据个人、专业人士、地理和临床环境以及其他相关临床信息考虑证据。
赞助
作者未因该证据总结收到任何资助。
版权所有© 2021 科廷大学伤口愈合和管理课程。
![]()
Author(s)
Robin Watts AM, PhD, MHSc, BA, Dip NEd, FRCNA
Emeritus Professor, School of Nursing, Midwifery and Paramedicine, Wound Healing and Management (WHAM) unit, Curtin University, Perth, WA, Australia
Teresa Solomon BA, Grad Dip Lib Sc ALIA
Curtin University, Perth, WA, Australia
Emily Haesler* PhD, BN, P Grad Dip Adv Nurs, FWA
Adjunct Associate Professor, School of Nursing, Midwifery and Paramedicine, Wound Healing and Management (WHAM) unit, Curtin University, Perth, WA, Australia
Email Emily.haesler@curtin.edu.au
* Corresponding author
References
- Agero AL, Verallo-Rowell VM. A randomized double-blind controlled trial comparing extra virgin coconut oil with mineral oil as a moisturizer for mild to moderate xerosis. Dermatitis 2004;15(3):109–16.
- Verallo-Rowell VM, Dillague KM, Syah-Tjundawan BS. Novel antibacterial and emollient effects of coconut and virgin olive oils in adult atopic dermatitis. Dermatitis 2008;19(6):308–15.
- Kaur I, Saraswat A, Kumar B. A comparison of three therapeutic modalities in scalp psoriasis and a review of literature. Indian J Dermatol 2003;48.
- Evangelista MT, Abad-Casintahan F, Lopez-Villafuerte L. The effect of topical virgin coconut oil on SCORAD index, transepidermal water loss, and skin capacitance in mild to moderate pediatric atopic dermatitis: a randomized, double-blind, clinical trial. Int J Dermatol 2014;53(1):100–8.
- Konar MC, Islam K, Roy A, Ghosh T. Effect of virgin coconut oil application on the skin of preterm newborns: a randomized controlled trial. J Trop Pediatr 2020;66(2):129–35.
- Strunk T, Pupala S, Hibbert J, Doherty D, Patole S. Topical coconut oil in very preterm infants: an open-label randomised controlled trial. Neonatol 2018;113(2):146–51.
- Munn Z, Lockwood C, Moola S. The development and use of evidence summaries for point of care information systems: a streamlined rapid review approach. Worldviews Evid Based Nurs 2015;12(3):131–8. doi:10.1111/wvn.12094. PMID: 25996621.
- Aromataris E, Munn Z, editors. JBI manual for evidence synthesis. Joanna Briggs Institute; 2021. Available from: https://synthesismanual.jbi.global
- Joanna Briggs Institute (JBI) Levels of Evidence and Grades of Recommendation Working Party. New JBI grades of recommendation; 2013. Available from: https://jbi.global/sites/default/files/2019-05/JBI-grades-of-recommendation_2014.pdf
- Joanna Briggs Institute (JBI) Levels of Evidence and Grades of Recommendation Working Party. Supporting document for the Joanna Briggs Institute Levels of Evidence and Grades of Recommendation; 2014. Available from: https://jbi.global/sites/default/files/2019-05/JBI%20Levels%20of%20Evidence%20Supporting%20Documents-v2.pdf
- Joanna Briggs Institute (JBI) Levels of Evidence and Grades of Recommendation Working Party. JBI levels of evidence; 2013. Available from: https://jbi.global/sites/default/files/2019-05/JBI-Levels-of-evidence_2014_0.pdf
- Tepeng K, Rivera F. Virgin coconut oil for psoriasis. J Am Acad Dermatol 2006;54(3):AB210.
- Nevin KG, Rajamohan T. Effect of topical application of virgin coconut oil on skin components and antioxidant status during dermal wound healing in young rats. Skin Pharmacol Physiol 2010;23(6):290–7.
- Tarawan VM, Mantilidewi KI, Dhini IM, Radhiyanti PT, Sutedja E. Coconut shell liquid smoke promotes burn wound healing. J Evid Based Complement Altern Med 2017;22(3):436–40.
- Soliman AM, Lin TS, Ghafar NA, Das S. Virgin coconut oil and diabetic wound healing: histopathological and biochemical analysis. Eur J Anat 2018;22(2):135–44.
- DebMandalm M, Mandalm S. Coconut (Cocos nucifera L.: Arecaceae): in health promotion and disease prevention. Asian Pacific J Trop Med 2011;4(3):241–7.
- Dua K, Sheshala R, Ling T, Ling S, Gorajana A. Anti-inflammatory, antibacterial and analgesic potential of Cocos nucifera Linn.: a review. Med Chem (Los Angeles) 2013;12(2):158–64.
- Vaughn AR, Clark AK, Sivamani RK, Shi VY. Natural oils for skin-barrier repair: ancient compounds now backed by modern science. Am J Clin Dermatol 2018;19(1):103–17.
- Ayanlowo O, -Adeife OC, Ilomuanya M, Ebie C, Adegbulu A, Ezeanyache O, Odiase O, Ikebudu V, Akanbi B. African oils in dermatology. Dermatol Ther 2021 Apr 30:e14968. doi:10.1111/dth.14968. PMID: 33928725.
- Chew YL. The beneficial properties of virgin coconut oil in management of atopic dermatitis. Pharmacog Rev 2019;13(25):24–7.
- Lima E, Sousa C, Meneses L, Ximenes N, Santos Junior G, Vasconcelos G, Lima N, Patroninio M, Macedo D, Vasconcelos S. Cocos nucifera (L.) (Arecaceae): a phytochemical and pharmacological review. Braz J Med Biol Res 2015;48(11):953–64.
- Alves AQ, da Silva VA, Goes AJS, Silva MS, de Oliveira GG, Bastos IVGA, de Castro Neto AG, Alves AJ. The fatty acid composition of vegetable oils and their potential use in wound care. Adv Skin Wound Care 2019;32(8):1–8.
- Kh H, Kuttinath S, Rammohan R. First description of antibacterial and in vitro wound healing properties of Cocos nucifera tomentum. Asian J Pharm Clin Res 2019;12(5):118–22.
- Lin TK, Zhong L, Santiago JL. Anti-inflammatory and skin barrier repair effects of topical application of some plant oils. Int J Mol Sci 2018;19(1).
- Karagounis TK, Gittler JK, Rotemberg V, Morel KD. Use of “natural” oils for moisturization: review of olive, coconut, and sunflower seed oil. Pediatr Dermatol 2019;36(1):9–15.


