Volume 42 Number 1
Nursing care of severe hand foot syndrome caused by the oral anti-tumour drug Xeloda: a case study
Shuangshuang Zhang, Wenxing Zhao and Mengmeng Zhang
Keywords nursing, hand foot syndrome, Xeloda, biocellulose dressing
For referencing Zhang S, Zhao W and Zhang M. Nursing care of severe hand foot syndrome caused by the oral anti-tumour drug Xeloda: a case study. WCET® Journal 2022;42(1):29-33
DOI
https://doi.org/10.33235/wcet.42.1.29-33
Submitted 14 October 2021
Accepted 9 March 2021
Abstract
This case study summarises the nursing experience of a patient with grade III hand foot syndrome (HFS) caused by the oral anti-tumour drug Xeloda. The key points of nursing were a multi-disciplinary team consultation to make a clear diagnosis of grade III HFS, effectively control infection, apply a biocellulose dressing, and promote granulation growth and epidermal cell regeneration on the premise that the anti-tumour drug Xeloda cannot be reduced or stopped. After 98 days of comprehensive nursing intervention the wound healed completely; 6 months later, the ulcer did not reoccur.
Introduction
This case study will summarise the nursing experience of caring for a patient with grade III hand foot syndrome (HFS) caused by the oral anti-tumour drug Xeloda (Capecitabine). The key points of nursing care are the importance of a multi-disciplinary team (MDT) consultation process to provide a definitive diagnosis of a grade III HFS, effectively control infection and apply a biocellulose dressing to the wound to promote the growth of granulation tissue and epidermal cell regeneration on the premise that the anti-tumour drug Xeloda could not be reduced or stopped. After 98 days of comprehensive nursing interventions, the wound had healed completely; 6 months later, the ulcer had not re-occurred.
HFS is also known as palmar-plantar erythrodysesthesia, syndrome, acral erythema and Burgdorf’s syndrome. It is an adverse dermatologic skin reaction that may occur in patients with malignant tumours during treatments with systemic chemotherapy or molecular targeted therapy1–3. Localised tissue injury occurs as a result of a toxic reaction caused by drug accumulation from damage to the eccrine gland system. Further, the drugs used are excreted in sweat in the terminal limb capillaries of the palms of the hand and soles of the feet, making these areas more susceptible to HFS. The vascularity of and increased pressures and skin temperature of the hands and feet may exacerbate this effect2,4. While HFS is not life-threatening, it may severely impact a person’s quality of life, especially older people3. The clinical manifestations of HFS are palmoplantar numbness, insensitivity, a tingling sensation, rapid skin swelling or erythema, desquamation, skin cracking or blisters (usually advanced stage) or severe pain3,5. The palms of the hands are usually affected first. There may be progressive aggravations of any skin lesions.
It is important the HFS is not mistaken for hand-foot skin reaction (HFSR) arising from the use of multikinase or BRAF inhibitors. Skin reactions from the use of these groups of drugs manifest as yellowish, painful hyperkeratotic plaques on the heels, fingertips, within interdigital web spaces and over joints3.
According to National Cancer Institute (NCI)6, HFS is divided into three grades: grade I is dermatitis with skin changes (such as fingerprint disappearance, pigmentation, erythema, skin numbness, insensitivity, paresthesia, desquamation, etc.) with or without pain; grade II is skin change or dermatitis, with pain but without dysfunction; grade III is skin change or dermatitis (such as skin wet desquamation, ulcer, blister), severe pain and dysfunction. Grade III severe ulcers are rare. The pathological manifestations of grade III ulcers are vacuolar degeneration (liquefaction) of basal keratinocytes, infiltration of lymphocytes around skin vessels, apoptosis of keratinocytes and skin oedema7. The Xeloda antithetical couplet named capecitabine tablets, the new generation of oral fluorouracil broad spectrum anti-tumour drugs8, is used for the treatment of advanced breast cancer patients after chemotherapy; tumour cells can be intentionally killed if Xeloda is used independently. Xeloda can also be used in conjunction with other chemotherapy agents9. Further, Xeloda is usually taken orally BD after food and in cases of metastatic breast cancer Xeloda is used for maintenance therapy as long as it remains effective. Adverse reactions to Xeloda mainly include HFS, nausea, diarrhoea and neutropenia. The incidence of HFS is the highest among the adverse reactions to Xeloda, reaching 45–68%8. Furthermore, 20–50% of the patients who used Xeloda were forced to reduce the dosage or even discontinue treatment due to HFS10,11.
On 26 May 2020, a patient with severe grade III HFS caused by oral Xeloda was admitted to our hospital’s specialist nursing clinic of wound ostomy incontinence. Without stopping the drug, the wound was completely healed in 98 days after comprehensive nursing and medical intervention. The nursing methods are reported as follows.
Case Presentation
Background
A 65-year-old female patient, Ms Li, presented with a history of desquamation and skin peeling of the palms of both hands and the soles of both feet, inflamed dermatitis and occasional blisters for 3 years, and an ulcer on the right heel for 2 months. Her skin conditions and wounds were not healed by moist wound healing methods in two grade III, class A hospitals in the province.
Her past medical history included being diagnosed 13 years ago with breast cancer. Further, axillary lymph node metastasis and bilateral lung metastasis occurred 4 years ago. After six courses of chemotherapy, Xeloda 1.5g BID was taken orally as targeted therapy, and the disease did not progress. After oral administration of Xeloda for 2 weeks, grade I and II HFS appeared, such as dry hands and feet and desquamation.
Systemic evaluation
A systemic evaluation of Ms Li included the following reviews:
- Laboratory examinations undertaken within the last 6 months showed:
- CEA (carcinoembryonic antigen) marker was 5.53–12ng/ml (normal: 0-5).
- Ca (Cancer antigen)-153: 20.04–28.45u/ml (normal: 0–25).
- Pain assessment. Numeric Rating Scale (NRS) score: 8 points (0 represents no pain and 10 represents the worst pain imaginable).
- Mobility: unable to walk affecting normal life, e.g. family members were required to use a wheelchair to push Ms Li to the clinic for dressing changes.
- Nutritional status: average; BMI 19.9kg/m2.
- Psychological status: anxiety and fear were exhibited.
- Local lower limb:
- The dorsalis pedis artery and posterior tibial artery had good pulsation after hand felt without formal assessment with equipment.
- No prior history of lower extremity arteriovenous disease.
- Wound assessment:
- Wound location: the right heel.
- Wound measurement: the size of the wound bed was 5x5cm.
- Wound bed: the wound bed was covered with 100% yellow sloughy non-viable tissue.
- Wound exudate: there was a medium amount of exudate that was purulent and had a slight odour.
- Wound edge: a black hard scab was evident and circumferential around the wound bed.
- The peri-wound skin was dry and a large portion of dry epidermis was peeled away during wound cleansing (Figure 1).
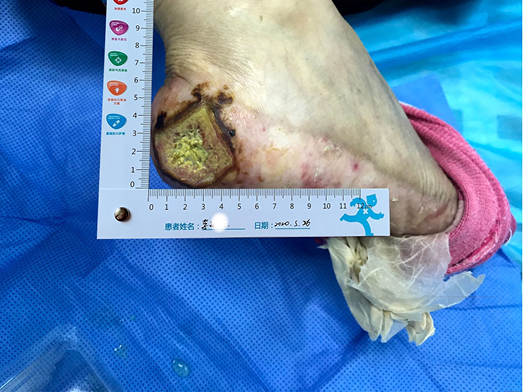
Figure 1. The wound on presentation
Multidiscipinary team and nursing care plan
From a nursing perspective there were a number of difficulties encountered and challenges to be overcome. These included:
- Assessing and managing a chronic non-healing wound. The wound was not healed after 2 months of moist healing treatment in two other grade III, class A hospitals in the province.
- Identifying the cause and type of wound. Was this a cancerous wound, pyoderma gangrenosum or HFS with an accompanying severe ulcer? There was no clear pathology or definitive diagnosis.
- Oral Xeloda therapy can affect wound healing. This raised the question of whether oral Xeloda could be reduced or stopped.
Due to the above nursing assessment and nursing difficulties, a multidisciplinary team (MDT) consultation, including the general surgeon, oncologist and wound care nurse, was quickly convened. Representatives from the relevant departments of the hospital were invited to formulate a diagnosis and treatment plan. The general surgery department suggested that pathology and bacterial culture should be undertaken. The results showed there were dense perivascular and interstitial neutrophilic infiltration of the tissue. However, there were no signs of vasculitis or malignant tumour. The bacteria cultured were identified as Proteus. The diagnosis of grade III HFS with rare severe ulcer was confirmed. Post oncology consultation, the oncologist’s opinion was that, considering the recurrence of breast cancer after 4 years, double lung metastasis as well as multiple unstable tumour markers, oral Xeloda could not be reduced or stopped.
Wound management: initial strategies
The initial goals of wound management were to control infection, promote autolysis of and debride non-viable tissue when required, and to relieve pain. The principles of TIME (Tissue, Infection, Moisture, Exudate) were used to guide local wound management and manage the characteristics of the wound described above. Normal saline and gauze was used to clean and mechanically debride the wound bed. Considering the moderate amount of wound exudate, and dry skin at the edge and around the wound, a sulfadiazine silver lipid hydrocolloid dressing was selected as the primary contact layer to control infection and keep the wound moist. The function of this dressing is to reduce the bacterial burden within the wound and control infection, assist with autolysis and debridement, and reduce pain during dressing changes12. The skin around the wound was dry and partly desquamated. In order to prevent avulsion, a soft silicone foam dressing was used as the secondary or external dressing, which also absorbed the exudate vertically and protected the fragile peri-wound skin.
Evaluation
Initial wound management strategies
After using the sulfadiazine silver lipid hydrocolloid dressing from 26 May to 4 June, the localised infection was controlled. Autolysis of non-viable tissue via the dressing product in conjunction with mechanical debridement had occurred, leaving the wound bed with 100% granulation tissue. The wound edge retained a little dip and within the peri-wound skin part of the epidermis was still exfoliated (Figure 2). The amount of wound exudate remained moderate but the odour had disappeared. Dressing pain was reduced. The size of the wound bed was reduced from 5x5cm to 4.5x4.5cm (Figures 3 & 4). The NRS score was reduced to 6 points, and the patient could be helped by her family to come to the clinic for dressing changes.
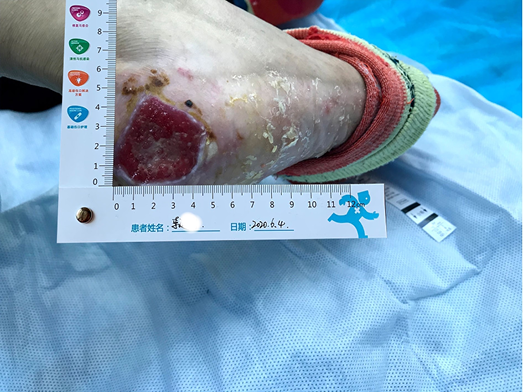
Figure 2. Autolysis of non-viable tissue revealing granulation tissue
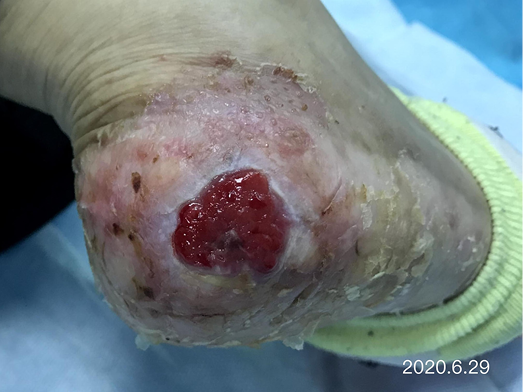
Figure 3. Continued wound healing
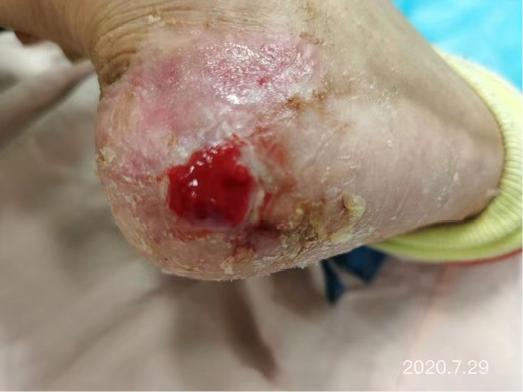
Figure 4. Continued wound healing
Secondary wound management strategies
After the wound infection was controlled and non-viable tissue was removed from the wound bed, the sulfadiazine silver lipid hydrocolloid was stopped and the treatment plan was changed. After considering the delays in wound healing that had occurred over a 2-month period within other hospitals plus our hospital, consideration was given as to what other auxiliary wound management products could be used to promote granulation tissue and epidermal cell regeneration to facilitate wound closure of this chronic wound. Therefore, to guide selection of another primary dressing, the MDT considered the factors under ‘S’ in the acronym MOIST. MOIST was developed as an adjunct to TIME to cater for the development of new wound technologies. MOIST refers to ‘M’ Moisture balance; ‘O’ Oxygen balance; ‘I’ Infection control; ‘S’ Support and ‘T’ Tissue management13. The S refers to modification of the wound environment e.g. inflammatory mediators, pH levels and growth factors. Consequently, a biocellulose wound dressing (Nanoderm® from China Shandong Nameide Biotechnology) was selected. This is a natural biomaterial which is safe and non-stimulating for tumour growth within cancer patients and can effectively promote granulation tissue and epidermal cell regeneration. A soft silicone foam dressing was chosen for the secondary/external dressing.
After dressing changes every 3–4 days, the granulation tissue increased and the size of the wound gradually reduced. By 11 August, the wound had narrowed to 1.5x1.5cm and entered the epithelialisation stage (Figure 5). The primary biocellulose wound dressing was discontinued. The skin around the wound was fragile and so the soft silicone foam dressing was still used. At this time, the NRS score was 2, and the patient could come to the hospital for dressing changes. After almost 3 months’ treatment, the wound had healed completely on 31 August (Figure 6). At follow-up 6 months later, the patient’s ulcer had not re-ocurred. In order to express her gratitude, the patient specifically came to the clinic and said that she had recovered her state of health to that of 4 years ago and had returned to a normal life that included square dancing.
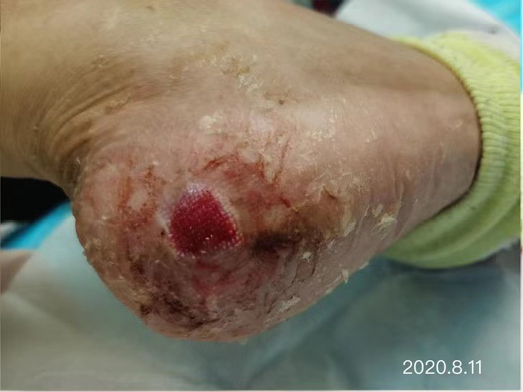
Figure 5. The epithelialisation stage
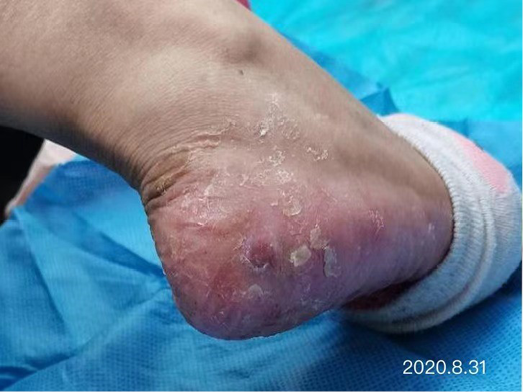
Figure 6. Final stages of healing
Discussion
Many articles were searched for the best evidence to assist with the management of this case. In terms of systemic therapy, Xeloda-targeted therapy combined with high-dose vitamin B6 (300mg/d) and Vit E cream for both hands and feet can reduce the severity of HFS14. Nearly half of the patients treated thought that HFS is the most serious side effect of chemotherapy received, which has a serious impact on the confidence and compliance of treatment. It is important to provide patients who will receive Xeloda therapy relevant knowledge of the benefits and side effects of the treatment and health guidance to manage any adverse effects. Having sufficient psychological preparation may assist patients to overcome the underlying disease and any treatment related side effects. The effective management of chronic wounds is enhanced through a MTD approach to determine the cause of the wound and factors that may complicate wound healing, and to determine evidence-based wound management strategies to facilitate wound healing and improve patients’ quality of life15.
Summary
HFS with severe skin ulcers is very rare. When Xeloda is unable to be reduced or stopped, the wound is difficult to heal. This presents great challenges to wound specialist nurses in determining wound management strategies. In this case, through MDT consultation and wound specialist nurse evaluation of the patient, the wound and systemic treatments, the wound infection was controlled using a sulfadiazine silver lipid hydrocolloid dressing, and a biocellulose dressing was applied to effectively promote granulation tissue and epidermal cell regeneration. In this case the treatment of primary disease was not affected, the patient’s pain was minimised, and wound healing realised.
口服抗肿瘤药物希罗达致重症手足综合征的护理:病例研究
Shuangshuang Zhang, Wenxing Zhao and Mengmeng Zhang
DOI: https://doi.org/10.33235/wcet.42.1.29-33
摘要
本病例研究总结了1例口服抗肿瘤药物希罗达致Ⅲ级手足综合征(HFS)患者的护理经验。护理重点是在抗肿瘤药物希罗达无法减停的前提下,进行多学科团队会诊,从而对Ⅲ级HFS做出明确诊断,有效控制感染,并应用生物纤维素敷料以促进肉芽生长和表皮细胞再生。经过98天的综合护理干预,创面完全愈合;6个月后溃疡未复发。
引言
本病例研究将总结护理1例口服抗肿瘤药物希罗达(卡培他滨)所致Ⅲ级手足综合征(HFS)患者的护理经验。护理重点为在抗肿瘤药物希罗达无法减停的前提下,多学科团队(MDT)会诊的重要性则突显而出,从而对III级HFS进行明确诊断,有效控制感染,并应用生物纤维素敷料以促进肉芽生长和表皮细胞再生。经过98天的综合护理干预,伤口已完全愈合;6个月后,溃疡未复发。
HFS又称掌跖感觉丧失性红斑综合征、肢端红斑和Burgdorf综合征。这是一种皮肤不良反应,可能发生于恶性肿瘤患者进行全身化疗或分子靶向治疗期间1-3。局部组织损伤是因为外分泌腺系统损伤导致药物蓄积从而引起毒性反应。此外,所用药物会通过手掌和足底的末端肢体毛细血管中的汗液排泄,所以这些部位更易患HFS。手足的血管分布、压力增大以及皮肤温度升高可能会加剧这种影响2,4。虽然HFS不会危及生命,但可能会严重影响人的生活质量,尤其是老年人3。HFS的临床表现为掌跖麻木、感觉迟钝、有刺痛感、皮肤快速肿胀或出现红斑、脱屑、开裂或水疱(通常为晚期)或出现剧烈疼痛3,5。手掌通常先受累。任何皮肤病变均可能逐渐恶化。
重要的是,不能将HFS误诊为使用多激酶或BRAF抑制剂而引起的手足皮肤反应(HFSR)。使用这几类药物引起的皮肤反应表现为脚后跟、趾尖、趾蹼间隙内和关节上出现淡黄色疼痛性角化过度斑块3。
根据美国国家癌症研究所(NCI)6,HFS分为3级:Ⅰ级为伴有皮肤改变的皮炎(如指纹消失、色素沉着、红斑、皮肤麻木、感觉迟钝、感觉异常和脱屑等),伴有或不伴有疼痛;Ⅱ级为皮肤改变或有皮炎,伴有疼痛但无功能障碍;Ⅲ级为皮肤改变或有皮炎(如皮肤湿性脱屑、溃疡和水疱)、出现剧烈疼痛和功能障碍。Ⅲ级重症溃疡较为罕见。Ⅲ级溃疡的病理表现为基底角质细胞空泡变性(液化)、皮肤血管周围淋巴细胞浸润、角质形成细胞凋亡及皮肤水肿7。希罗达的偶联药名为卡培他滨片,是新一代口服氟尿嘧啶类广谱抗肿瘤药物8,用于晚期乳腺癌患者化疗后的治疗;如果单独使用希罗达,可起到杀死肿瘤细胞的作用。希罗达还可联合其他化疗药物一起使用9。此外,希罗达通常在进食后口服(BD),对于转移性乳腺癌患者,只要希罗达仍有效,即可用来维持治疗。希罗达的不良反应主要包括HFS、恶心、腹泻和中性粒细胞减少症。在希罗达的不良反应中,HFS发生率最高,达到45%-68%8。此外,20%-50%使用希罗达的患者因HFS而被迫减少剂量甚至停止治疗10,11。
2020年5月26日,我院伤口造口失禁护理专科门诊收治1例口服希罗达致Ⅲ级HFS重症患者。在未停药的情况下,经过98天的综合护理和医疗干预,伤口完全愈合。现将护理方法报告如下。
病例介绍
背景
患者李女士,65岁,因患有双手掌及双足底脱屑和皮肤剥脱、发炎性皮炎并偶有水疱3年,且右足跟患有溃疡2个月而就诊。患者的皮肤和伤口在省内两家三级甲等医院通过湿性伤口愈合方法治疗后均未痊愈。
除此之外,患者的既往病史表明其13年前被诊断出乳腺癌。此外,4年前出现腋窝淋巴结转移和双肺转移。化疗6个疗程后,口服希罗达1.5g BID作为靶向治疗,病情无进展。口服希罗达2周后出现Ⅰ和Ⅱ级HFS,如手足干燥、脱屑等。
系统性评价
李女士的系统性评价包括以下内容:
· 过去6个月内进行的实验室检查:
· CEA(癌胚抗原)指标为5.53 ng/ml-12 ng/ml(正常范围:0-5)。
· Ca(癌抗原)-153:20.04 u/ml-28.45 u/ml(正常范围:0-25)。
· 疼痛评估。数字评定量表(NRS)评分:8分(0分代表无疼痛,10分代表可以想象到的最严重疼痛)。
· 活动能力:无法行走,从而影响正常生活,例如,需要家庭成员使用轮椅将李女士推至门诊更换敷料。
· 营养状况:平均值;BMI 19.9 kg/m2。
· 心理状况:表现出焦虑和恐惧。
· 局部下肢:
· 手感 足背动脉和胫后动脉,发现搏动良好,未用设备进行正式评估。
· 无下肢动静脉疾病既往史。
· 伤口评估:
· 伤口位置:右足跟。
· 伤口测量:创面床大小为5 cm×5 cm。
· 创面床:创面为100%黄色腐肉无活性组织。
· 伤口渗液情况:有中量的渗液,呈脓性,有轻微臭味。
· 伤口边缘:创面床周围有明显的圆周状黑色硬痂。
· 伤口周围皮肤干燥,在伤口清洁过程中,大部分干燥表皮被剥离(图1)。

图1.就诊时伤口
多学科团队和护理计划
从护理的角度来看,需要克服许多困难和挑战。其中包括:
· 评估和管理慢性不愈合伤口。经过省内另外两家三甲医院采用湿性愈合方法治疗后伤口均未愈合。
· 确定伤口产生的原因和伤口类型。属于癌性伤口、坏疽性脓皮病还是HFS伴重症溃疡?无明确病理及明确诊断。
· 口服希罗达治疗会影响伤口愈合。就此提出疑问:口服希罗达是否可以减量或停药。
由于上述护理评估和护理遇到难题,迅速召集了包括普外科医生、肿瘤科医生和伤口护理护士在内的多学科团队(MDT)进行会诊。邀请医院相关科室代表制定诊疗方案。普外科建议应进行病理学研究和细菌培养。结果显示:组织有致密的血管周和间质中性粒细胞浸润。然而,无血管炎或恶性肿瘤体征。培养的细菌经鉴定为变形杆菌。确诊为Ⅲ极HFS,伴罕见重症溃疡。肿瘤科会诊后,肿瘤科医生认为,考虑到患者4年后复发的乳腺癌、双肺转移以及存在多个不稳定的肿瘤标志物,不能减少或停止口服希罗达。
伤口管理:初始策略
伤口管理的最初目标是控制感染,促进非活性组织的自溶和清创(如需要),并缓解疼痛。TIME原则(组织、感染、湿性、渗液)用于指导局部伤口管理,并管理上述伤口的特征。用生理盐水和纱布清洁并机械清创创面床。考虑到伤口渗液量中等,且伤口边缘和周围皮肤干燥,选择磺胺嘧啶银脂质水胶体敷料作为主要接触层,以控制感染并保持伤口湿润。该敷料的功能是降低伤口内的细菌负荷和控制感染,辅助自溶和清创,并减少敷料更换过程中的疼痛12。伤口周围皮肤干燥,部分脱屑。为了防止敷料脱落,使用软聚硅酮泡沫敷料作为二级或外部敷料,同时可垂直吸收渗液,保护伤口周围脆弱的皮肤。
评价
伤口管理初始策略
在5月26日至6月4日使用磺胺嘧啶银脂质水胶体敷料后,局部感染得到控制。使用敷料药品和机械清创后,出现了非活性组织自溶,创面床肉芽组织含量达到100%。伤口边缘有少许渗液,在伤口周围皮肤内,表皮仍剥脱(图2)。伤口渗液量仍为中等,但气味消失。敷料疼痛减轻。创面床大小由5 cm×5 cm缩小至4.5 cm×4.5 cm(图3、4)。NRS评分降至6分,患者可在家人的帮助下来门诊换药。

图2.无活性组织自溶,露出肉芽组织

图3.伤口持续愈合

图4.伤口持续愈合
第二种伤口管理策略
待创面床感染得到控制,去除无活力组织后,停用磺胺嘧啶银脂质水胶体,并改变治疗方案。考虑到在其他医院和我院治疗历时2个多月发生的伤口愈合延迟,我们考虑是否还可以使用其他的伤口辅助管理产品来促进肉芽组织和表皮细胞再生,以促进这种慢性伤口的愈合。因此,为了指导如何选择另一种主要敷料,MDT考虑了MOIST法中“S”所代表的因素。开发MOIST作为TIME的辅助治疗,以迎合新伤口技术的发展。MOIST是指“M”干湿平衡;“O”代表氧气平衡;“I”代表感染控制;“S”代表环境支持,“T”代表组织管理13。S是指伤口环境的改变,例如炎症介质、pH水平和生长因子的改变。因此,选择了生物纤维素伤口敷料(来自中国山东Nameide Biotechnology的Nanoderm®)。这是一种天然生物材料,对癌症患者的肿瘤生长安全且无刺激,可有效促进肉芽组织和表皮细胞再生。次要/外部敷料选择软聚硅酮泡沫敷料。
每3-4天换1次药,肉芽组织增多,且创面逐渐缩小。到8月11日,伤口缩小至1.5 cm×1.5 cm,并进入上皮化阶段(图5)。停用主要生物纤维素伤口敷料。伤口周围皮肤脆弱,因此仍使用软聚硅酮泡沫敷料。此时NRS评分为2分,患者可自行来院换药。治疗近3个月后,伤口于8月31日完全愈合(图6)。6个月后随访时,患者溃疡未复发。为表达感激,患者特地来到门诊,表示她的健康状况已经恢复到4年前的水平,并恢复了正常生活,包括跳广场舞。

图5.上皮再生阶段

图6.愈合最后阶段
讨论
对诸多文章进行了检索以寻找最佳证据来协助管理该病例。在全身治疗方面,希罗达靶向治疗联合大剂量维生素B6(300 mg/天)和手足Vit E乳膏可以减轻HFS的严重程度14。近半数接受治疗的患者认为HFS是接受化疗所致最严重的副作用,因此对治疗的信心和依从性产生严重影响。向接受希罗达治疗的患者提供治疗获益和副作用的相关知识以及为处理任何不良反应提供健康指导非常重要。有足够的心理准备可能有助于患者克服基础疾病和任何与治疗相关的副作用。使用MTD法加强慢性伤口的有效管理,以确定伤口产生的原因和可能使伤口愈合复杂化的因素,并确定促进伤口愈合和改善患者生活质量的循证伤口管理策略15。
总结
HFS伴随重症皮肤溃疡非常罕见。在希罗达无法减量或停用的情况下,伤口难以愈合。这对伤口专科护士在确定伤口管理策略方面提出了巨大挑战。在该病例中,通过MDT会诊、伤口专科护士评估患者、伤口以及全身治疗,应用磺胺嘧啶银脂质水胶体敷料后,伤口感染得到控制,应用生物纤维素敷料后,有效促进了肉芽组织和表皮细胞再生。在该病例中,原发疾病的治疗未受影响,患者的疼痛降至最低,且伤口实现愈合。
Author(s)
Shuangshuang Zhang MSN, ET
The First Affiliated Hospital of Shandong First Medical University, Shandong Province Qianfoshan Hospital, Jinan, Shandong, China
Wenxing Zhao* MSN, ET
Central Hospital Affiliated to Shandong First Medical University, Jinan Central Hospital Affiliated to Shandong University, Jinan, Shandong, China
Mengmeng Zhang MSN, ET
Central Hospital Affiliated to Shandong First Medical University, Jinan Central Hospital Affiliated to Shandong University, Jinan, Shandong, China
* Corresponding author
References
- Zhang YH, Shao CHY. Nursing experience of 26 cases of hand foot syndrome caused by anti-tumor treatment. Jilin Medical Science 2014;35(7):1568.
- Inokuchi M, Ishikawa I, Furukawa H et al. Treatment of capecetibane-induced hand-foot syndrome using a topical retinoid: a case report. Oncology Letters 2014;7(2):444–448.
- Kwakman JJM, Elshot YS, Punt CJA, Koopman M. Management of cytotoxic chemotherapy-induced hand-foot syndrome. Oncology Reviews 2020;14(1):57–63.
- Xu L, Xue M, Wang J, Zhang W. Analysis of risk factors of hand foot syndrome induced by apatinib mesylate and nursing countermeasures. Nursing Research 2019;33(23):4049–4054.
- Nikolaou V, Syrigos K, Saif MW. Incidence and implications of chemotherapy related hand-foot syndrome. Journal of Expert Opinions of Drug Safety 2016;15(12):1625–1633.
- Dong Y, Lu Z, Yang Y. Nursing research progress of hand foot syndrome caused by capecitabine. Nursing Research 2016;30(1):275–278.
- Janusch M,Fischer M, Marsch WC, et al. The hand-foot syndrome a frequent secondary manifestation in antineoplastic chemotherapy. European Journal of Dermatology 2006;16(5):494–499.
- Li X, Liu D, Wu D, Fan X, Zhang J. Research progress on the relationship between capecitabine related gene polymorphism and hand foot syndrome. Journal of Practical Oncology 2018;32(2):149–153.
- Liu SL, Yu ZY, Liu HF. Xeloda maintenance therapy in the treatment of advanced breast cancer. Chinese and Foreign Women Health 2020;7(13):68,91.
- Guo QH, Ma JL, Zhang J, et al. Clinical observation of hand foot syndrome caused by capecitabine and 5-fluorouracil. Journal of Chinese Medical Guide 2013;11(31):36–37.
- Gao J, He Q, Hua D, et al. Polymorphism of TS 3’ - UTR predicts survival of Chinese advanced gastric cancer patients receiving first-line capecitabine plus paclitaxel. Clinical and Translational Oncology 2013;15(8):619–625.
- Sood A, Granick MS, Tomaselli NL. Wound dressings and comparative effectiveness data. Advances in Wound Care 2014;(3):511–529.
- Dissemond J, Assenheimer B, Engels B et al. Clinical letter, MOIST. Journal of the German Society of Dermatology 2017;(4):443–445.
- Chen Z, Zhou S, Xu S, Ding P, Zhang H. Clinical observation of hand foot soaking formula in the prevention and treatment of capecitabine related hand foot syndrome. China Science and Technology of Traditional Chinese Medicine 2016;23(3):329–330.
- Lei J, Sun L, Li P, Zhu C, Lin Z, et al. The wound dressings and their applications in wound healing and management. Health Science Journal 2019;13(4):662–668.


