Volume 42 Number 1
WHAM evidence summary: Papaya-based products for treating wounds
Terena Solomons and Emily Haesler
Keywords debridement, wounds, Papaya, pawpaw, papain
For referencing Solomons T and Haesler E. WHAM evidence summary: Papaya-based products for treating wounds. WCET® Journal 2022;42(1):34-39
DOI https://doi.org/10.33235/wcet.42.1.34-39
Clinical question
What is the best available evidence on the effectiveness of papaya-based products for wound healing?
Summary
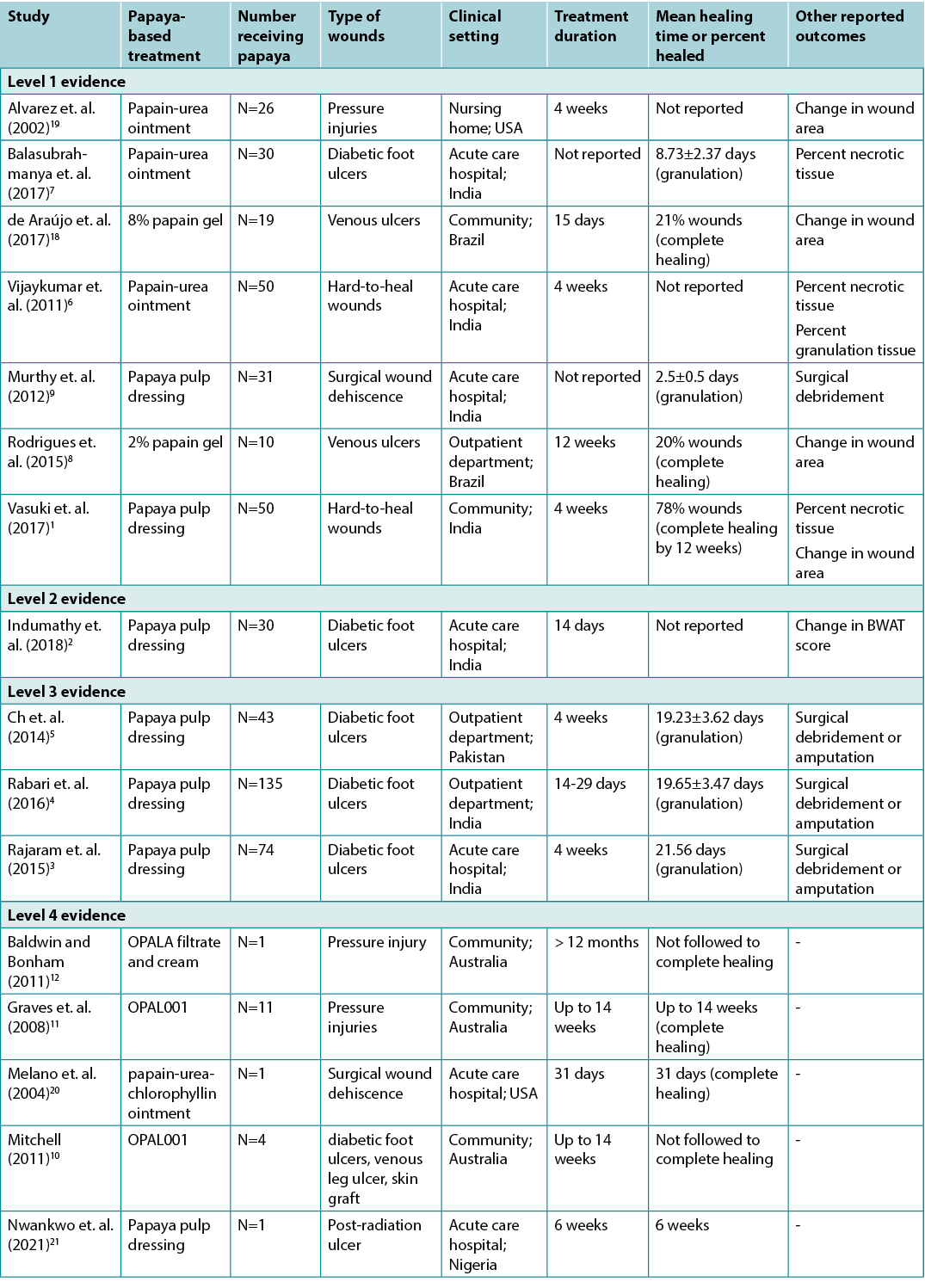
Despite a long history in low-to-middle resource countries of clinical use of papaya for managing wounds, limited high level research has been conducted on the effectiveness of papaya-based products. Evidence was available for natural papaya pulp wound dressings, commercial papain extract products (withdrawn from market in some countries due to the risk of anaphylaxis) and an experimental papaya filtrate product (not commercially available). Most studies were conducted in hard-to-heal wounds requiring debridement and the studies were generally at a high risk of bias.
Level 1 evidence1 and Level 2 evidence2 for papaya pulp dressings demonstrated an improvement in wound tissue type. Level 3 evidence3-5 suggested papaya pulp dressings were associated with improvement in wound tissue type, reasonable healing rates and reduction in requirement for further surgical interventions. Level 1 evidence6-9 for commercial papain products showed improvements in wound tissue type6, 7 and reduction in wound surface area8, 9. Other Level 1 evidence10 failed to demonstrate effectiveness, and Level 4 evidence was mixed.10-12
Clinical practice recommendations
All recommendations should be applied with consideration to the wound, the person, the health professional and the clinical context.
There is insufficient evidence to make a graded recommendation on the effectiveness of papaya-based products for promoting wound healing.
Evaluate the individual’s risk of allergic reaction (e.g., previous latex allergy) and licensing guidance in the geographic region before using topical papaya-based products. Cease use of natural papaya pulp dressings if the person experiences adverse outcomes (Grade B).
Search for evidence
This summary was developed using methods published by the Joanna Briggs Institute (JBI)13-17. The summary is based on a systematic literature search in English combining search terms that describe wounds and papaya. Searches were conducted in Embase, Medline, PubMed, Global Health, the Cochrane Library, Allied Health and Complementary Medicine and Google Scholar databases for dates up to December 2021. Searches were also conducted in ten healthcare journals from low-and-middle resource countries. Evidence was limited to clinical studies in humans. Studies were assigned a level of evidence (see Table One) based on JBI’s hierarchy13-17. Recommendations are made based on the body of evidence and are graded according to the system reported by JBI13-17.
Table One: Levels of evidence

Background
Papaya (Carica papaya, also called pawpaw) is a tropical plant originating from Southern Mexico and Central America that is now cultivated in tropical and subtropical regions worldwide. Many parts of the tree (e.g., fruit, leaves, seeds and bark) have been used in traditional medicine26. Biochemical analysis of papaya has identified several protease enzymes (e.g., papain and chymopapain) with debriding properties that are purported to remove slough and non-viable tissue and prepare the wound bed for healing. Papaya extract has also been reported to have antimicrobial properties9, 23, 24, 26. Papaya-based treatment is reported to be cost-effective7, 11, and papapaya pulp dressings have been successfully applied and managed by patients/unskilled carers in community settings1, 18.
The literature search identified several methods of applying papaya-based products to a wound:
- Natural papaya pulp dressing: Raw pulp from the fruit is prepared and applied directly to the wound bed.
- Commercial processed preparations: Products containing papain enzyme are available in gel, cream, impregnated dressings and other topically applied formulations. Papain is sometimes combined with other active agents including urea and chlorophyllin-copper complex to enhance its action25. Due to the risk of severe allergic response, papain-based topical agents are banned by the (USA) Food and Drug Administration22.
- Experimental processed formulation: A product prepared as papaya and peach (10-1 by volume), with the fruit flesh treated in a series of processes (titled OPAL001) to form two products – a filtrate and a cream11, 12. The mechanism of activity for the product were hypothesised to be related to either proinflammatory response, antioxidant effect and/or vasorelaxation12. The product is not currently listed with the Therapeutic Goods Administration in Australia where it was developed.
Although no serious adverse reactions were identified in the studies in this evidence summary, papaya has been associated with severe allergic reaction and anaphylaxis, including cross-reactivity in people with latex allergy. This has led to withdrawal of commercial papain-based products from the market in some countries, including the USA22, 26. Anaphylactic reaction is reported to occur at a rate of 1%;27 the response might be associated with the concentration of active ingredients, which is generally higher in processed perparations compared with the natural fruit pulp26.
Evidence
Papaya for improving clinical outcomes in chronic wounds
Studies reporting papaya pulp dressing for wound healing outcomes
One RCT1 compared the efficacy of two methods of debridement – enzymatic debridement using papaya pulp dressings and mechanical debridement using wet-to-dry saline dressings. Following randomisation, 128 participants were enrolled in the study. Of these, 93% had a chronic wound (7% hard wound dehiscence following surgery). There was a significant improvement in granulation tissue formation with papaya dressings compared to wet-to-dry dressings in the third and fourth weeks (p < 0.001) and superior reduction in slough/necrotic tissue for the papaya dressing group compared to the wet-to-dry dressing group at each weekly assessment point (week four, p = 0.0082). However, this did not translate to a significant difference in either reduction in mean wound size at four weeks (p = 0.08) or complete wound healing at three months (papaya 78% versus saline 72%, p = 0.488)1 (Level 1.c).
A quasi-experimental study2 assessed papaya pulp dressing prepared using fresh ripe fruit for healing diabetic foot ulcers. A convenience sample of 60 participants was assigned to either an experimental or control treatment (n = 30 in each group). The papaya dressings were changed daily for 14 days, while the control group received unspecified routine treatment. A significant improvement in healing occurred over time in the group receiving papaya dressing, as measured using the mean healing score on the Bates-Jensen Wound Assessment Tool (BWAT; pre-test 26.37 ± 7.73 versus post- test 51.10 ± 6.81, p < 0.001). A significant difference between the experimental and control group was also reported (p < 0.001)2 (Level 2.c).
A prospective study3 followed 94 patients who underwent a surgical procedure to treat a diabetic foot ulcer: amputation (n = 31) or surgical debridement (n = 63). Thereafter and in conjunction with oral antibiotic therapy, papaya pulp dressings were used for 89% (n = 74) of patients. The grated papaya was prepared, applied daily and covered with sterile gauze. Average healing time, defined as achieving healthy granulation tissue with epithelialised wound edges was 21.56 days (range from 17 to 28 days). Further surgery was required for ten patients3 (Level 3.e).
A second prospective study4 reported outcomes for 135 patients receiving papaya pulp dressings for diabetic foot ulcers (Grade 1-3 on Wagner’s classification system). Prior to commencing the second-daily dressing regimen, 96 patients (71.11%) required surgical debridement. Mean healing time, defined as achieving healthy granulation tissue and epithelialised wound edges, was 19.65 ± 3.47 days (range 14 to 29 days)4 (Level 3.e).
A study5 that included patients who were receiving combined therapy for diabetic foot ulcers (n = 43) tested the effect of papaya pulp dressings on healing. The papaya dressings were changed every two days. Healing time, defined as achieving healthy granulation tissue with epithelialised wound edges, ranged from 18 to 29 days (mean 19.23 days ± 3.624) and 88% of the ulcers required no further surgical intervention after papaya dressings commenced5 (Level 3.e).
A case study reported effective use of papaya pulp dressings to heal a post-radiation sacral ulcer. The wound had received surgical debridement, honey dressings, negative pressure wound therapy and failed flap surgery prior to commencing papaya treatment. Second-daily papaya pulp dressing led to healthy granulation after six weeks, allowing the patient to undergo a follow-up successful flap repair21 (Level 4.d).
Studies reporting processed papaya-based preparations for wound healing outcomes
In the largest RCT6 exploring processed papaya-based products, 100 participants with hard-to-heal, sloughy wounds received either papain-urea or collagenase debriding ointment. Treatment was commenced when the wound was stable (no healing observed over the preceding eight weeks) and continued for four weeks, with weekly assessment. The papain-urea group showed statistically significantly superior reduction in slough/necrotic tissue over time (89.22% ± 15.16% versus 82.51% ± 17.45%, p = 0.043). Between-group difference was not statistically significant in the first three weeks, and the small difference observed in week four may not be clinically significant. Percent of granulation tissue was statistically significantly greater for the papain-urea group at every weekly assessment, including baseline (week four: papain-urea 6.82% ± 8.15% versus collagenase 3.58% ± 3.09%, p = 0.01)6 (Level 1.c).
Sixty participants with diabetic foot ulcers were randomly assigned to received either papain-urea or an unidentified conventional wound dressing to explore the effectiveness of a commercially available papaya-based debriding agent.7 Both treatments were applied second-daily. The papain group achieved statistically significantly greater reduction of necrotic tissue (72.27% ± 4.68% versus 24.63% ± 3.74%, p = 0.03) and faster granulation (8.73 ± 2.37 days versus 16.03 ± 4.68 days, p = 0.001). The superior outcome led to faster hospital discharge7 (Level 1.c).
In a small, double-blind RCT18, 8% papain gel was compared to both fibrin gel a non-active gel control for the healing of chronic venous ulcers (n = 55 people with n = 63 ulcers). Individual ulcers were randomised to one of the three groups and assessed at baseline then every 15 days. Neither fibrin gel nor papain gel improved ulcer healing compared to the control. This conclusion was based on the following: complete wound healing rates were similar in all groups (fibrin gel 14.3%, papain gel 21.1% and control 30.4%, p = 0.43) and no statistically significant difference between groups in reduction in wound area (p = 0.62). All groups achieved improvements in exudate levels, signs of local wound infection and edge epithelisation by day 60 (all p > 0.05). Two participants (one in each of the active treatment groups) reported mild pain18 (Level 1.c).
In a small, non-blinded RCT, Rodrigues et. al. (2015)8 reported on the effectiveness of 2% papain gel compared to 2% carboxymethyl cellulose gel for healing venous leg ulcers. Twenty-one participants were randomised, of which 18 participants (n = 28 ulcers) completed the 12-week study. The results showed a statistically significant reduction in wound area for ulcers treated with papain, particularly between the fifth and 12th week of treatment (p = 0.032) and this was statistically significant compared to the control group (p = 0.006). However, the rate of complete healing was low (two ulcers treated with papaya and no control group ulcers completely healed in 12 weeks) and the amount of exudate and devitalised tissue were similar in both groups (p > 0.05 for both)8 (Level 1.c).
Another non-blinded small RCT19 (n = 29 randomised, n = 26 analysed) compared papain-urea to collagenase in non-infected pressure injuries. Participants were treated with moist-to-moist saline dressings in a screening period for up to two weeks prior to commencing the trial. After four weeks of treatment, papain-urea ointment was deemed to be statistically significantly (p < 0.05) superior for reducing wound size, with no pain or discomfort experienced by participants19 (Level 1.c).
Several case studies10-12 reporting use of OPAL001 papaya-based products have been published. In the first report, 11 quadriplegic patients with Category/Stage 2 and 4 pressure injuries received OPAL001 products in conjunction with contemporary wound dressings. Complete healing was achieved for nine of the pressure injuries after 6 days to 14 weeks of treatment11. In the second case report, removal of non-viable tissue and healing was achieved for two diabetic foot ulcers, one venous leg ulcer and an ulcerated skin graft in individuals with impaired vascular function10. The third case report12 detailed reduction in hyperkeratosis and the size of a sacral pressure injury after four weeks of treatment with OPALA filtrate and cream. Ongoing self-treatment with OPALA cream achieved resolution of hyperkeratosis, but the pressure injury deteriorated12 (all Level 4.d).
Table Two: Summary of the evidence for papaya-based treatments
Papaya for treating surgical wound dehiscence
An RCT9 compared the safety and efficacy of papaya pulp dressings with hydrogen peroxide solution in patients with wound dehiscence post-caesarean section (n = 63). Participants received concurrent antibiotics selected following culture and sensitivity. Time required to develop healthy granulation tissue in the hydrogen peroxide group was 6.2 ± 1.6 days compared to the papaya group at 2.5 ± 0.5 days (p < 0.05). Only 3.2% of the papaya dressing group required additional surgical debridement compared with 56% of the hydrogen peroxide group (p < 0.05). Minor adverse events (e.g., local irritation) were reported but not significantly different to those associated with hydrogen peroxide9 (n.b., hydrogen peroxide is not recommended for irrigating wounds) (Level 1.c).
A case study20 reported that the use of a papain-urea-chlorophyllin product applied to post-surgical sternal wound dehiscence was associated with complete healing after 31 days of second-daily treatment. The patient received concurrent negative pressure wound therapy20 (Level 4.d).
Considerations for use
- Papaya-based products facilitate breakdown of necrotic and nonviable tissues that contain protein and the debriding action is from the top downward in the wound. Debridement should be ceased when the wound bed is cleared of slough and necrotic tissue25.
- There is no standardised method of preparing papaya pulp dressing. Studies variably use ripe, semi-ripe or unripe fruit pulp9. Enzymatic content of the pulp is reported to potential decrease as the fruit ripens, suggesting raw or semi-ripe fruit is more effective1, 4, 9. Antimicrobial properties are reported to remain consistent as fruit ripens1, 4, 9.
- The following preparation method for papaya pulp dressings is reported:
- Remove the skin and seeds from papaya fruit2, 5.
- Either grate the fruit pulp9, 21, or mash it to a paste.
- Apply the papaya pulp to wound bed after cleansing the wound.9, 21
- Covered with sterile gauze9.
- Change the papaya pulp dressing daily2, 5 or second daily9, 21.
- Unused papaya paste should be placed in cold storage5.
Conflicts of interest
The author declares no conflicts of interest in accordance with International Committee of Medical Journal Editors (ICMJE) standards.
About WHAM evidence summaries
Wound Healing and management Collaborative (WHAM) evidence summaries are consistent with methodology published in:
Munn Z, Lockwood C, Moola S. The development and use of evidence summaries for point of care information systems: A streamlined rapid review approach, Worldviews Evid Based Nurs. 2015;12(3):131-8.
Methods are outlined in detail in resources published by the Joanna Briggs Institute as cited in this evidence summary, and on the WHAM website: http://WHAMwounds.com. WHAM evidence summaries undergo peer-review by an international multidisciplinary Expert Reference Group.
WHAM evidence summaries provide a summary of the best available evidence on specific topics and make suggestions that can be used to inform clinical practice. Evidence contained within this summary should be evaluated by appropriately trained professionals with expertise in wound prevention and management, and the evidence should be considered in the context of the individual, the professional, the clinical setting and other relevant clinical information.
Copyright © 2021 Wound Healing and Management (WHAM) Collaborative, Curtin University.

WHAM证据总结:木瓜制品应用于伤口治疗
Terena Solomons and Emily Haesler
DOI: https://doi.org/10.33235/wcet.42.1.34-39
临床问题
关于木瓜制品用于伤口愈合相关有效性的最佳可用证据是什么?
总结
虽然在中低收入国家临床上使用木瓜治疗伤口历史悠久,但对木瓜制品的有效性开展的高水平研究仍有限。天然木瓜浆伤口敷料、市售木瓜蛋白酶提取物产品(由于过敏反应风险已在一些国家撤市)和实验木瓜滤液产品(非市售)的有效性有据可循。大多数研究在需要清创术治疗、难以愈合的伤口中进行,通常存在较高的偏倚风险。
木瓜浆敷料相关的1级证据1和2级证据2证明,伤口组织类型有所改善。3级证据3-5表明,木瓜浆敷料与伤口组织类型改善、愈合率合理升高以及进一步手术干预的需求减少存在关联。市售木瓜蛋白酶产品相关的1级证据6-9表明,伤口组织类型有所改善6,7,伤口面积有所减少8,9。其他1级证据10未能证明其有效性,4级证据混杂。10-12
临床实践建议
采用任何建议时,应考虑到伤口情况、患者情况、专业医护人员和临床环境。
木瓜制品的有效性证据不足,无法就其在促进伤口愈合的有效性方面提出分级建议。
使用外用木瓜制品前,评估个体的过敏反应风险(例如,乳胶过敏史)和所在地理区域的许可指南。如果患者出现不良结局(B级),停止使用天然木瓜浆敷料。
证据检索
本总结是采用乔安娜·布里格斯研究所(JBI)公布的方法而得出13-17。本总结以系统性的英文文献检索为基础,并结合描述伤口和木瓜的检索术语。在Embase、Medline、PubMed、Global Health、Cochrane Library、Allied Health和Complementary Medicine以及Google Scholar数据库中进行检索,检索日期截至2021年12月。并在中低收入国家的10种医疗保健期刊中进行了检索。证据仅限于人体临床研究。根据JBI的等级划分,对研究的证据水平(见表1)进行了划分13-17。建议根据大量证据而提出,并根据JBI报告的系统进行评分13-17。
背景
木瓜(Carica Papaya,也称为pawpaw)是一种起源于墨西哥南部和中美洲的热带植物,目前在全球热带和亚热带地区种植。树的许多部分(比如果实、叶片、种子和树皮)已被用于制作传统药物26。木瓜的生化分析已经确定了几种具有清创特性的蛋白酶(例如木瓜蛋白酶和木瓜凝乳蛋白酶),据称,这些酶可清除腐肉和无活性组织,并为创面床愈合做准备。报告显示,木瓜提取物还具有抗菌特性9,23,24,26。报告显示,基于木瓜的治疗具有成本效益7,11,在社区环境中患者/未受过训练的护理人员已成功应用木瓜浆敷料并达到一定效果1,18。
文献检索识别了将木瓜制品应用于伤口治疗的几种方法:
• 天然木瓜浆敷料:从水果中制备原浆,直接用于创面床。
• 商业加工制剂:含有木瓜蛋白酶的产品有凝胶、乳膏、浸渍敷料和其他局部应用制剂。木瓜蛋白酶有时联合其他活性药物(包括尿素和叶绿素铜复合物)一同使用,以増强其作用25。由于存在严重过敏反应的风险,(美国)食品药品监督管理局禁止使用基于木瓜蛋白酶的外用药物22。
• 实验处理制剂:以木瓜和桃(体积比为10:1)为原料进行制备,用一系列工艺(名为OPAL001)处理果肉,生成滤液和乳膏两种产品11,12。假设产品的活性机制与促炎反应、抗氧化作用和/或血管舒张有关12。该类产品目前尚未被列入澳大利亚药品管理局清单之中,而澳大利亚正是其研发地。
尽管在本证据总结的研究中未发现严重不良反应,但木瓜与重度过敏反应和速发严重过敏反应相关,包括乳胶过敏患者的交叉反应。这导致市售木瓜蛋白酶产品在一些国家(包括美国)撤市22,26。报告显示,过敏反应的发生率为1%;27反应可能与活性成分的浓度有关,与天然果浆相比,加工后制剂中活性成分的浓度通常较高26。
证据
木瓜改善慢性伤口的临床结局
报告木瓜浆敷料用于伤口愈合结局的研究
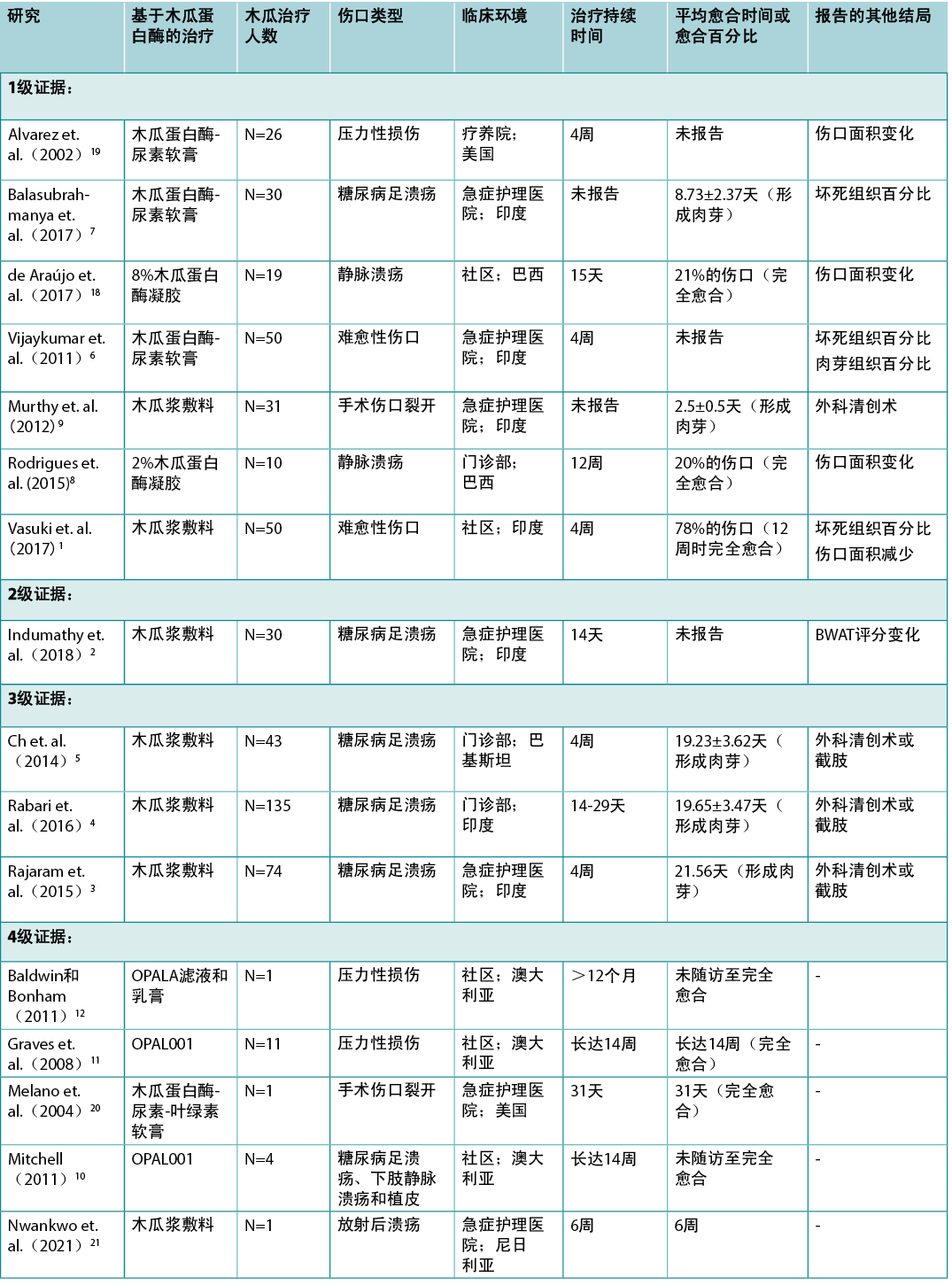
一项RCT1研究比较了两种清创术的疗效Å\Å\使用木瓜浆敷料的酶清创和使用湿-干盐水敷料的机械清创。随机分组后,128例受试者入组研究。其中,93%的受试者有慢性伤口(7%术后硬伤口裂开)。在第3周和第4周,与湿-干敷料相比,木瓜敷料显著改善了肉芽组织形成(p<0.001),而且在每周的评估节点,木瓜敷料组的腐肉/坏死组织减少情况优于湿-干敷料组(第4周,p=0.0082)。但是,在第4周时在促使平均伤口面积减小(p=0.08)或3个月时促使伤口完全愈合(木瓜78%与生理盐水72%,p=0.488)方面未出现显著差异11(1.c级)。
一项准实验研究2对使用新鲜成熟水果制备的木瓜浆敷料治疗糖尿病足溃疡进行了评估。将60例受试者的便利样本分配到试验治疗组或对照组(每组n=30)。每天更换木瓜敷料,持续14天,而对照组进行非特定的常规治疗。使用Bates-Jensen伤口评估工具(BWAT;测试前26.37Å}7.73与测试后51.10Å}6.81,p<0.001)的平均愈合评分测定发现,随着时间推移,使用木瓜敷料的受试者组伤口愈合效果显著。报告也显示了实验组和对照组之间的显著差异(p<0.001)2(2.c级)。
一项前瞻性研究3随访了94例接受外科手术治疗糖尿病足溃疡的患者:截肢(n=31)或外科清创术
(n=63)。此后,对89%(n=74)的患者使用木瓜浆敷料联合口服抗生素治疗。准备磨碎的木瓜,每天涂抹并用无菌纱布覆盖。平均愈合时间(定义为形成健康肉芽组织以及伤口边缘上皮化)为21.56天(范围为17-28天)。10例患者需要进一步手术治疗3(3.e级)。
第二项前瞻性研究4报告了135例患者接受木瓜浆敷料治疗糖尿病足溃疡的结局(Wagner分级系统1-3级)。在开始每日第二次敷料治疗之前,96例患者(71.11%)需要进行外科清创术。平均愈合时间(定义为形成健康肉芽组织以及伤口边缘上皮化)为19.65Å}3.47天(范围:14-29天)4(3.e级)。
一项纳入接受糖尿病足溃疡联合治疗患者(n=43)的研究5测试了木瓜浆敷料对伤口愈合的影响。木瓜敷料每两天更换一次。愈合时间(定义为形成健康肉芽组织以及伤口边缘上皮化)的范围为18-29天(平均19.23
天Å}3.624),而且88%的溃疡患者在使用木瓜敷料后不需要进一步的手术干预5(3.e级)。
一项病例研究报告了木瓜浆敷料可有效愈合放射后骶部溃疡。在接受木瓜治疗前,对伤口进行了外科清创术、蜂蜜敷料、负压伤口治疗和皮瓣手术(失败)治疗。每天使用两次木瓜浆敷料,6周后长出健康肉芽,患者从而成功进行了后续皮瓣修复21(4.d级)。
报告加工木瓜制剂用于伤口愈合相关结局的研究
在探索加工木瓜制剂的最大规模RCT6研究中,对100名例有难以愈合的伤口或腐肉伤口的受试者使用了木瓜蛋白酶-尿素或胶原酶清创软膏。当伤口稳定(在前8周内未观察到愈合)时开始进行治疗,并持续4周,每周评估一次。随着时间推移,木瓜蛋白酶-尿素组腐肉/坏死组织的减少在统计学上更具显著性(89.22%Å}15.16%与82.51%Å}17.45%,p=0.043)。前3周组间差异无统计学显著性,第4周观察到的微小差异可能也无临床意义。在每周评估时,木瓜蛋白酶-尿素组肉芽组织百分比的上升在统计学上更具显著性,基线含括在内(第4周:木瓜蛋白酶-尿素组6.82%Å}8.15%与胶原酶组3.58%Å}3.09%,
p=0.01)6(1.c级)。
60例糖尿病足溃疡受试者被随机分配接受木瓜蛋白酶-尿素或非特定的传统伤口敷料治疗,以探索市售木瓜蛋白酶清创剂的有效性。7两种治疗均应每日进行2次。木瓜蛋白酶组坏死组织的减少在统计学上更具显著性(72.27%Å}4.68%与24.63%Å}3.74%,p=0.03),肉芽也形成得更快(8.73Å}2.37天与16.03Å}4.68天,p=0.001)。因结局更优,因此出院时间提前7(1.c级)。
在一项小型双盲RCT18研究中,比较了8%木瓜蛋白酶凝胶与两种纤维蛋白凝胶(一种非活性凝胶对照)治愈慢性静脉性溃疡的情况(n=55人,n=63处溃疡)。将溃疡患者随机分成3组,进行基线评估并在之后每15天进行一次评估。与对照组相比,纤维蛋白凝胶和木瓜蛋白酶凝胶均未能促进溃疡愈合。该结论基于:所有组的伤口完全愈合率相似(纤维蛋白凝胶组14.3%,木瓜蛋白酶凝胶组21.1%,对照组30.4%,p=0.43),组间伤口缩小的面积在统计学上无显著差异(p=0.62)。到第60天,所有组的渗出液水平、局部伤口感染体征和边缘上皮形成均有所改善(所有p>0.05)。2例受试者(每活性药物治疗组各1例)报告出现轻度疼痛18(1.c级)。
表1:证据等级

在一项小型双盲RCT研究中,Rodrigues等人
(2015)8报告了2%木瓜蛋白酶凝胶与2%羧甲基纤维素凝胶相比在治疗腿部静脉溃疡方面的有效性。21例受试者随机接受试验,其中18例受试者(n=28处溃疡)完成了为期12周的研究。结果显示,木瓜蛋白酶治疗组溃疡伤口面积的减少具有统计学显著性,尤其是在治疗的第5周和第12周之间(p=0.032),与对照组相比,更具统计学显著性(p=0.006)。但是,完全愈合率较低(木瓜治疗组2例溃疡患者在12周内完全愈合,对照组无患者愈合),两组的渗出液和失活组织量相似(均为p>0.05)8(1.c级)。
另一项小型非盲RCT研究19(n=29例随机分组,n=26例进行分析)在非感染性压力性损伤中比较了木瓜蛋白酶-尿素与胶原酶。在开始试验前,受试者在长达2周的筛选期内接受湿润盐水敷料治疗。治疗4周后,认为木瓜蛋白酶-尿素软膏在伤口面积减小方面更具统计学显著性(p<0.05),且受试者未经历疼痛或感到不适19
(1.c级)。
有数项已发表的病例研究10-12是关于应用OPAL001木瓜制品进行治疗的研究。在第一份报告中,对11例类别为/2期和4期压力性损伤的四肢瘫痪患者联合使用了OPAL001产品与常规伤口敷料。其中9例患者的压力性损伤在治疗6天-14周后完全愈合11。第二份病例报告中,在血管功能受损的患者中,对2例糖尿病足溃疡患者、1例下肢静脉溃疡患者和1例皮肤移植物溃疡患者进行了无活性组织清除,之后患者痊愈10。第三份病例报告12介绍了使用OPALA滤液和乳膏治疗4周后,角化过度患病率减少和骶骨压力性损伤面积减小方面的详细情况。使用OPALA乳膏进行持续自我治疗,解决了角化过度的问题,但压力性损伤情况出现恶化12(均为4.d级)。
木瓜治疗外科伤口裂开
一项RCT研究9在剖腹产术后伤口裂开的患者(n=
63)中比较了木瓜浆敷料与过氧化氢溶液的安全性和有效性。受试者同时接受根据培养基和敏感性选择的抗生素。过氧化氢组形成健康肉芽组织所需的时间为6.2Å}1.6天,木瓜组为2.5Å}0.5天(p<0.05)。木瓜敷料组仅有3.2%的患者需要额外的外科清创,而过氧化氢组则为56%(p<
0.05)。报告有轻微不良事件(例如:局部刺激),但与过氧化氢相关不良事件无显著差异9(注意,不建议用过氧化氢冲洗伤口)(1.c级)。
一项病例研究20报告称,使用木瓜蛋白酶-尿素-叶绿素产品治疗术后胸骨伤口裂开,每日治疗2次,31天后伤口完全愈合。患者同时接受负压伤口治疗20(4.d级)。
表2:木瓜治疗证据总结
使用注意事项
• 木瓜制品有助于分解含有蛋白质的坏死和无活性组织,清创从伤口顶部向下进行。创面床清除腐肉和坏死组织后,应停止清创术25。
• 目前还没有制备木瓜浆敷料的标准化方法。研究使用了成熟、半成熟或未成熟果肉9。有报告称,随着果实成熟,果肉的酶含量可能会降低,这表明使用未成熟或半成熟的木瓜果实更有效1,4,9。有报告称,果实成熟过程中,其抗菌特性不变1,4,9。
• 现对木瓜浆敷料的以下制备方法进行介绍:
• 去除木瓜的皮和籽2,5。
• 将果肉9,21碾碎,或捣碎成糊状。
• 清洁伤口后将木瓜浆涂在创面床上9,21。
• 覆上无菌纱布9。
• 每天更换一次木瓜浆敷料2,5或隔天更换一次9,21。
• 未使用的木瓜敷料应冷藏5。
利益冲突
根据国际医学期刊编辑委员会(ICMJE)标准,作者声明无利益冲突。
关于WHAM证据总结
伤口愈合和管理协作组织(WHAM)证据总结与以下文献中公布的方法一致:
Munn Z, Lockwood C, Moola S. The development and use of evidence summaries for point of care information systems: A streamlined rapid review approach, Worldviews Evid Based Nurs.2015;12(3):131-8.
本证据总结中引用的乔安娜·布里格斯研究所发表的资源中详细概述了方法,详情见WHAM网站:http://WHAMwounds.com。WHAM证据总结经过国际多学科专家参考小组的同行评审。
WHAM证据总结了关于特定主题的最佳可用证据,并提出了可用于指导临床实践的建议。本总结中包含的证据应由经过适当培训的具有伤口预防和管理专业知识的专业人士进行评价,并应根据个人、专业人士、临床环境以及其他相关临床信息考虑证据。
Copyright ˝ 2021 Wound Healing and Management (WHAM) Collaborative, Curtin University.

Author(s)
Terena Solomons
BA Grad Dip Lib Sc AALIA (CP) Health
Western Australian Group for Evidence Informed Healthcare Practice, Curtin University
Emily Haesler*
PhD Post Grad Sip Adv Nurs (Gerontics) BNurs Fellow Wounds Australia
Wound Healing and Management Collaborative, Curtin Health Innovation Research Institute, Curtin University
* Corresponding author
References
- Vasuki V, Thanmaran N, Vimalakaran B, Madan K. Comparative study of papaya dressing versus normal saline dressing in healing of ulcers. 2017, 2017;4(4):8.
- Indumathy S, Thenmozhi PA, Gowri PM. Effectiveness of papaya pulp dressing on diabetic foot ulcer International Journal of Research in Ayurveda and Pharmacy, 2018;9(5):75-8.
- Rajaram B, Venkanna M, Kumaraswamy BV, Kumar D, Maripeddi K, Puligilla S, Reddy S. The role of papaya dressings in the management of diabetic foot ulcers: A propsective study. Journal of Evidence Based Medicine and Healthcare, 2015;2(42):7365-71.
- Rabari Y, Singh R, Prasad D, Abraham A. The role of papaya (Carica papaya) dressings in the management of chronic ulcers. National Journal of Medical and Dental Research, 2016;4(4):329-32.
- Ch I, Shaikh S, ur Rashid H. The role of papaya dressings in the management of diabetic foot ulcers: A prospective study. Journal of Evidence Based Medicine and Healthcare, 2015;18(1):87-9.
- Vijaykumar H, Pai SA, Pandey V, Kamble P. Comparative study of collagenase and papain-urea based preparations in the management of chronic nonhealing limb ulcers. Indian J Sci Technol, 2011;4:1096-100.
- Balasubrahmanya KS, Praveen MP, Srinidhi M, Shruthi S, Jinumon KV, Rahul DK. A prospective study on effectiveness of use of papain urea based preparation in dressings compared with regular conventional dressings in diabetic foot ulcers Int Surg J, 2017;4(6):1984-87.
- Rodrigues AL, de Oliveira BG, Futuro DO, Secoli SR. Effectiveness of papain gel in venous ulcer treatment: Randomized clinical trial. Revista Latino-americana de Enfermagem, 2015;23(3):458‐65.
- Murthy MB, Murthy BK, Bhave S. Comparison of safety and efficacy of papaya dressing with hydrogen peroxide solution on wound bed preparation in patients with wound gape. Indian J Pharmacol, 2012;44(6):784-87.
- Mitchell GK. Clinical observations supporting a vasodilatory effect of the modified papaya extract OPAL001. Wound Practice & Research, 2011;19(4):190-5.
- Graves N, Ashby A. The use of OPAL001 filtrate and cream in the treatment of chronic pressure ulcers. Wound Practice & Research, 2008;16(2):22-9.
- Baldwin C, Bonham S. Treatment of a sacral pressure ulcer and extensive hyperkeratosis with OPAL A filtrate and cream: A case study. Wound Practice & Research, 2011;19:196.
- Munn Z, Lockwood C, S. M. The development and use of evidence summaries for point of care information systems: A streamlined rapid review approach. Worldviews Evid Based Nurs, 2015;12(3):131-8.
- Aromataris E, Munn Z, editors. JBI Manual for Evidence Synthesis. 2021. https://synthesismanual.jbi.global: Joanna Briggs Institute.
- Joanna Briggs Institute (JBI) Levels of Evidence and Grades of Recommendation Working Party. New JBI Grades of Recommendation. 2013. https://jbi.global/sites/default/files/2019-05/JBI-grades-of-recommendation_2014.pdf: JBI.
- Joanna Briggs Institute (JBI) Levels of Evidence and Grades of Recommendation Working Party. Supporting Document for the Joanna Briggs Institute Levels of Evidence and Grades of Recommendation. 2014. https://jbi.global/sites/default/files/2019-05/JBI%20Levels%20of%20Evidence%20Supporting%20Documents-v2.pdf: JBI.
- Joanna Briggs Institute (JBI) Levels of Evidence and Grades of Recommendation Working Party. JBI Levels of Evidence. 2013. https://jbi.global/sites/default/files/2019-05/JBI-Levels-of-evidence_2014_0.pdf: JBI.
- de Araújo IC, Defune E, Abbade LP, Miot HA, Bertanha M, de Carvalho LR, Ferreira RR, Yoshida WB. Fibrin gel versus papain gel in the healing of chronic venous ulcers: A double-blind randomized controlled trial. Phlebology, 2017;32(7):488-95.
- Alvarez OM, Fernandez-Obregon A, Rogers RS, Bergman L, Black M. A prospective, randomized comparative study of collagenase and papain-urea for pressure ulcer debridement. Wounds, 2002;14(8):293-301.
- Melano E, Rodriguez HL, Carrillo R, Dillon L. The effects of Panafil when using topical negative pressure to heal an infected sternal wound. J Wound Care, 2004;13(10):425-6.
- Nwankwo EU, Maduba CC, Modekwe VI, Nnadozie UU. The use of unripe pawpaw for wound bed preparation following radiation-induced sacral ulcer: A case report and review of literature. Niger J Med 2021;30(339-41).
- US Food Drug Administration (FDA). 2015. Questions and Answers about FDA’s Enforcement Action Regarding Unapproved Topical Drug Products Containing Papain. Available from: http://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/EnforcementActivitiesbyFDA/Selected
EnforcementActionsonUnapprovedDrugs/ucm119646.htm. [Accessed January 2021]. - Hakim R, Fakhrurrazi, Dinni. Effect of Carica papaya extract toward incised wound healing process in mice (Mus musculus) clinically and histologically. Evidence-Based Complementary and Alternative Medicine, 2019;2019:8306519.
- Tumpa SI, Hossain MI, Ishika T. Antimicrobial activities of Psidium guajava, Carica papaya and Mangifera indica against some gram positive and gram negative bacteria. J Pharmacogn Phytochem, 2015;3(6):125-9.
- Kravitz S, McGuire J, Zinszer K. Management of skin ulcers: Understanding the mechanismand selection of enzymatic debriding agents. Adv Skin Wound Care, 2008;21(2):72-4.
- Haesler E, Watts RR, J., Carville K. Local resource botanicals used in wound care. Wound Practice & Research, 2016;24(2):85-90.
- Pieper B, Caliri MH. Nontraditional wound care: A review of the evidence for the use of sugar, papaya/papain, and fatty acids. J Wound Ostomy Continence Nur, 2003;30(4):175–83.


