Volume 43 Number 3
Current perspectives on pressure injuries in persons with dark skin tones from the National Pressure Injury Advisory Panel
Joyce Black, Jill Cox, Virginia Capasso, Donna Z Bliss, Barbara Delmore, Vignesh Iyer, Jacqueline Massaro, Cassendra Munro, Joyce Pittman, Elizabeth A Ayello
Keywords health equity, pressure injuries, dark skin tones, skin assessment, social determinants of heath
For referencing Black J et al. Current perspectives on pressure injuries in persons with dark skin tones from the National Pressure Injury Advisory Panel. WCET® Journal 2023;43(3):18-29.
DOI
https://doi.org/10.33235/wcet.43.3.18-29
Submitted 30 April 2023
Accepted 26 June 2023
Abstract
Background Pressure injury (PI) development is multifactorial. In patients with dark skin tones, identifying impending PIs by visual skin assessment can be especially challenging. The need for improved skin assessment techniques, especially for persons with dark skin tones, continues to increase. Similarly, greater awareness of the need for inclusivity with regard to representation of diverse skin colours/tones in education materials has been apparent in recent years.
Objective To provide current perspectives from the literature surrounding skin assessment and PI development in patients with dark skin tones.
Methods The following elements will be discussed through the lens of skin tone: 1) historical perspectives of PI staging from the National Pressure Injury Advisory Panel, 2) epidemiology of PI, 3) anatomy and physiology of the skin, 3) skin tone assessment and measurement, 4) augmented visual assessment modalities, 5) PI prevention, 6) PI healing, 7) social determinants of health, and 8) gaps in clinician education.
Conclusions This review highlights the gap in our clinical knowledge regarding PIs in patients with dark skin tones. Racial disparities with regard to PI development and healing are especially highlighted among patients with dark skin tones. Skin tone colour assessment must be standardised and quantifiable in clinical education, practice, and research. This work is urgently needed, and support from private and governmental agencies is essential.
Introduction
Racial diversity across the United States has increased over the past 2 decades. The US Census Bureau reports that the overall diversity index has increased from 54.9% in 2010 to 61.1% in 2020 and is projected to continue to escalate over the next decade.1 Worldwide, people with dark skin tones comprise the majority of the population.2
Over the past 20 years, there has been a growing awareness of and interest in the need for skin assessment that is inclusive of all persons regardless of their skin colour.3-24 The clinical reality is that in the US and worldwide, skin tone demographics have shifted, and awareness of various skin colours has increased, such that the need for accurate skin assessment and diagnosis for all patients has taken on great urgency. Moreover, awareness of the need to include diverse skin colours/tones in education materials (basic professional and ongoing education) for students and clinicians has also increased in recent years.13
In this article, the authors outline current perspectives on pressure injuries (PIs) in patients with dark skin tones. The first section addresses the historical efforts of the National Pressure Injury Advisory Panel (NPIAP; formally the NPUAP) to address this disparity and revise staging definitions to be relevant for all skin tones. Additional topics addressed through the lens of skin tone include the epidemiology of PIs, anatomy and physiology of the skin, visual and augmented skin assessment techniques and modalities, PI prevention and healing, social determinants of health (SDOH) considerations, and professional education gaps.
Historical perspectives on PI staging in persons with dark skin tones
In the mid-1990s, clinicians and NPIAP board members recognised the need for the stage 1 definition to include how these PIs would appear in persons with dark skin tones.1 They noted that assessments that identify a stage 1 PI, such as “erythema” and “non-blanchable erythema,” were not always visible in patients with dark skin tones and, therefore, other indicators of stage 1 PI were needed. A task force was formed to address these issues.2,4
The NPIAP Task Force agreed on the following assumptions:20
- Intact skin has a variety of colour changes
- Very darkly pigmented skin does not have visible blanching
- The person’s race and ethnicity are not predictive of skin pigmentation
- Non-blanchable erythema only reflected one description of change in skin colour seen in early PI; it was not a universal descriptor
- Other objective findings of stage 1 PI could include temperature changes (warmth, coolness, edema, induration).
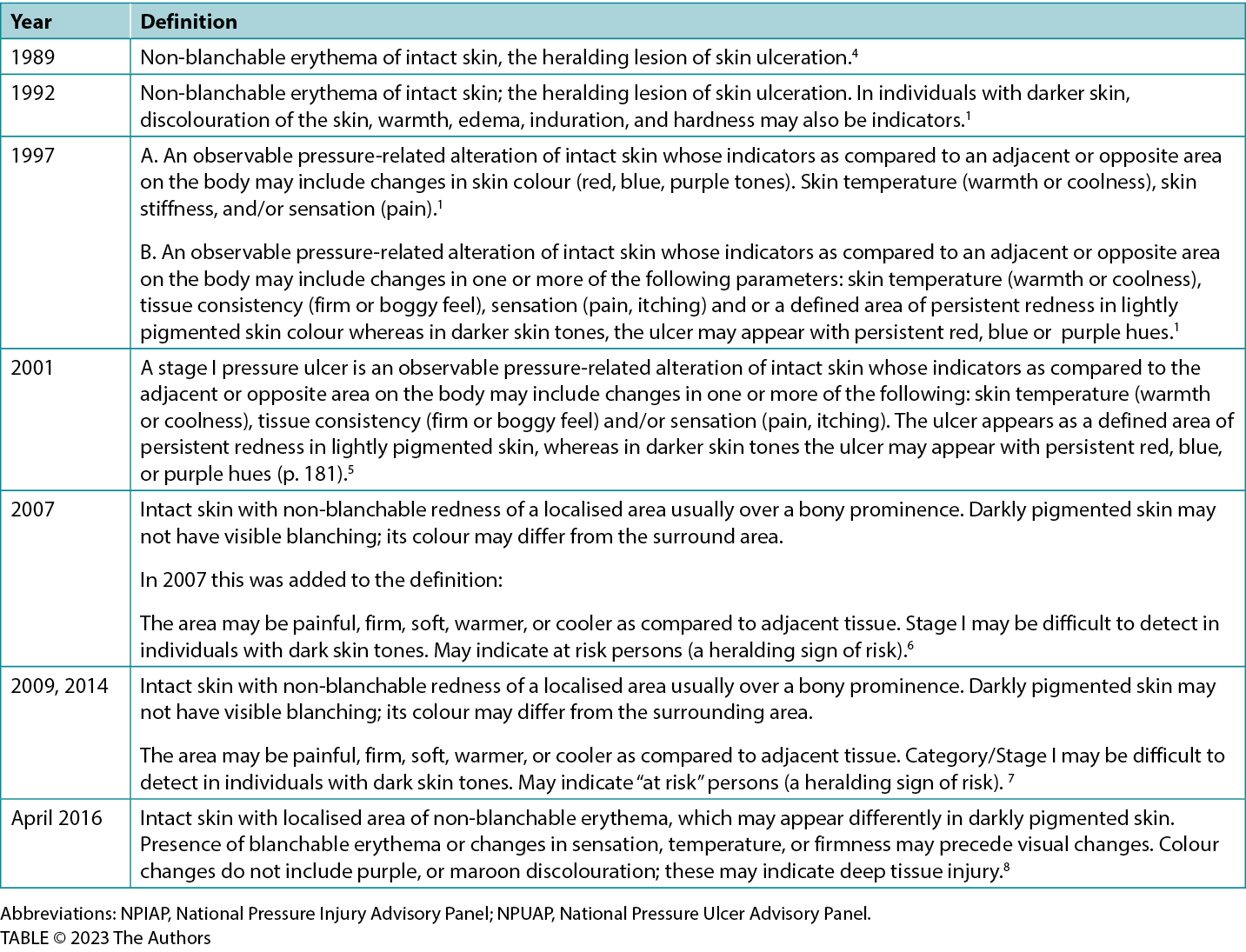
Table 1 lists the various iterations of the definition over the years.1,5,9,17,18,20
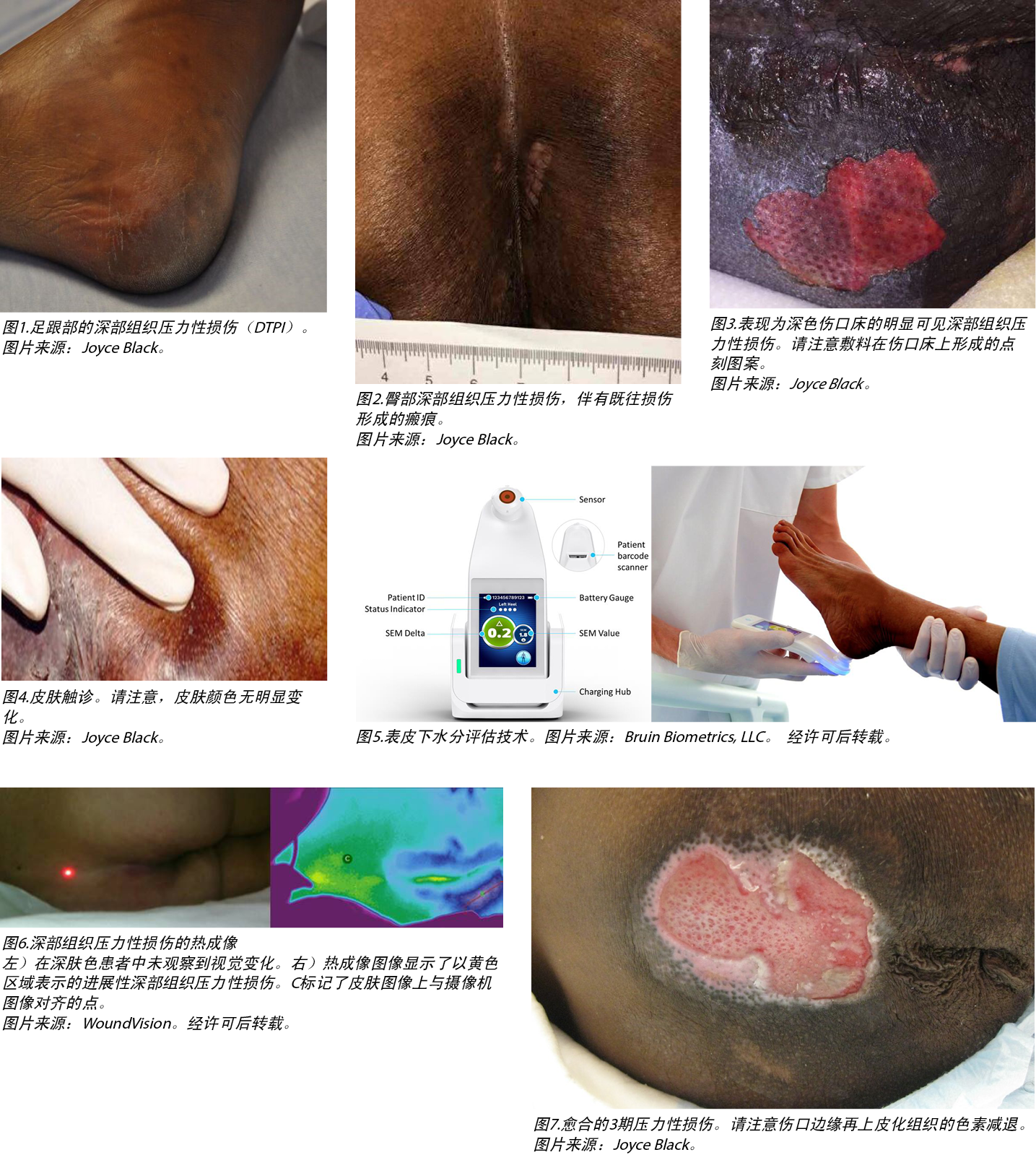
In 2005, the NPIAP identified deep tissue PIs (DTPIs). As with stage 1 PIs, DTPIs are difficult to detect in patients with dark skin tones (Figures 1-3).25 Other NPIAP initiatives that call attention to skin tone diversity include staging diagrams illustrating different skin colours/tones (available on the NPIAP website).
Table 1. Npuap/npiap stage 1 pressure injury definitions: historical evolution
Epidemiology of PIs in persons with dark skin tones
A limited number of studies have examined PI rates by race/ethnicity or skin tone in individuals admitted to acute care settings or long-term care facilities or residing in a nursing home. However, in a literature review of PIs in patients with dark skin tones, Gunowa and colleagues26 report that patients with dark skin tones are more likely to develop higher stage PIs regardless of the type of healthcare setting. The following discussion outlines what is known regarding the prevalence/incidence of PIs among patients with dark skin tones delineated by healthcare setting.
PIs among admissions to nursing homes
Using the Minimum Data Set (MDS) version 2.0, PIs (stage 2, 3 or 4) were present in 15% of older adults admitted to US nursing homes.27,28 Higher rates of PIs have been reported among Black individuals admitted to nursing homes. Approximately twice as many Black patients (16.6%) admitted to 59 nursing homes in Maryland had a PI compared with White patients (8.4%).29 In a national chain of nursing homes, Harms et al28 reported rates of PIs among older adults admitted to nursing homes by race and ethnicity (American Indian and Alaskan Native, Asian or Pacific Islander, Black not Hispanic, Hispanic, White not Hispanic). Black patients admitted to nursing homes had the greatest mean number of PIs per resident at 2.4 (SD, 2.2). The prevalences of stage 2, 3, or 4 PIs were lowest among White patients compared with all other racial and ethnic groups. The prevalence of a stage 1 PI among Black patients at admission was 7%, whereas stage 2 injury prevalence was 20%. Among all racial/ethnic groups, Black patients also had the highest prevalences of the most severe PIs at 7% (stage 3) and 8% (stage 4); White patients had the lowest prevalences at 3% for both stages.
PI prevalence among nursing home residents
Ahn et al30 found that among individuals residing in all US nursing homes, 8.4% had a PI, and 1.7% had a suspected DTPI.30 A greater percentage of Black residents (18.2%) compared with White residents (13.8%) had a PI in a set of nursing homes in New York.31 In a study examining differences in the prevalence of PIs among high-risk residents in US nursing homes over 5 years, PI prevalence decreased for both Black and White residents. However, there was a 5.4% overall unadjusted difference in PI prevalence (higher in Black patients).32 In a study examining the reporting of PIs by nursing homes using the MDS version 3.0, Chen et al33 found that the percentage of stage 4 PIs was higher among short-stay Black residents (50.4%) compared with White residents (40.8%).33 Black race was significantly associated with having a stage 2 to 4 PI (odds ratio [OR], 11.44; 95% CI, 1.44–1.47). Hispanic ethnicity was significantly associated with having a suspected DTPI (OR, 2.63; 95% CI, 1.47–1.58).
PI incidence in nursing homes
Cai et al31 found that Black residents in New York nursing homes were more likely to develop a PI during their stay than were White residents, controlling for other risk factors (OR, 1.203; P = .01).31 In another study, 7.7% of approximately 90,500 nursing residents developed stage 2 to 4 PIs after they were admitted to a nursing home. Black residents who developed a PI during their nursing home stay did so sooner than did White residents.34 The disparity in time to development of a PI among Black residents increased over time: The disparity was 3% at 3 months post-admission and grew to 5.8% at 6 months. During a 12-week surveillance in a nursing home in Pennsylvania, a greater percentage of Black residents (47%) developed a stage 2 to 4 PI than White residents (18%).35 Moreover, no stage 1 PIs were identified in Black residents.
PIs among hospitalised patients
Studies specifically examining PI prevalence and incidence in the acute care setting with a focus on racial distribution or patients with dark skin tones are scarce, with most studies conducted over a decade ago. In a large, multiyear prevalence study conducted between 1989 to 2005, Van Gilder and colleagues36 found the proportion of stage 1 PIs in patients with dark skin tones to be much lower (13%) than in those with medium (32%) to light (38%) skin tones. This finding may be attributed to difficulty in detection of stage 1 PIs in patients with dark skin. More severe PIs (stage 3, stage 4, eschar) were also found among patients with dark skin tones as compared with those with light or medium skin tones (11% vs 6-7%, 13% vs 6-7%, and 9% vs 5-6%, respectively). In a large, multiyear national study using the National Inpatient Sample (NIS) database from 2008 to 2012, Bauer and colleagues37 reported that among patients who identified as African American, PI rates were significantly higher than among all other racial groups at 2.4%; patients who identified as White had the second highest incidence reported at 1.8%. Moreover, the PI stage was more severe in African American patients (stage 3), whereas stage 2 was the most common stage among White patients.37
Using the NIS database from 2003, Fogerty and colleagues38 identified that African Americans were more likely to be discharged from US hospitals with PIs in comparison with non-African Americans (OR, 2.3; no CI provided). No analysis of PI stage was conducted in this investigation. A recent investigation conducted by Cox and Thomas-Hawkins39 echoes the results of these previous works. In this investigation of 17,781 patients with PIs using the 2018 Healthcare Cost and Utilization Project (HCUP) State-Specific Database (New Jersey), a higher proportion of patients identifying as Black had an admitting diagnosis of PIs (5.0% vs 3.5%; P < .05) as well as a higher proportion of stage 4 PIs (3.3% vs 2.3%) when compared to all other races combined. When secondary diagnoses of PIs were examined, Black patients had a significantly lower proportion of stage 1 PIs (4.7% vs 18%; P < .05) but a higher proportion of stage 4 PIs (28.7% vs 16.9%; P < .05) when compared to all other races combined. Limitations cited for this study include the single state nature of the data and a lack of multivariate analysis.
Collectively, this limited body of work highlights some important considerations with regard to PI reporting across healthcare settings. First, there is the paucity of recent studies that have considered or examined race or dark skin tones as a potential risk factor for PI development. This is important because the change in racial diversity across the US warrants investigation. Second, the similarities in PI rates across these limited studies are striking and may highlight the need for specific clinical and diagnostic tools in practice to identify impending PIs in patients with dark skin tones.
Anatomy and physiology of skin
The skin consists of two distinct layers: epidermis and dermis.40-43 The epidermis is cellular and avascular, consisting of 90% keratinocytes which synthesize the strong, water-insoluble structural protein, keratin. The epidermis protects from water loss, shear, friction, and toxic irritants. It also prevents invasion of bacteria and other pathogens by three mechanisms: 1) a mechanical barrier, 2) an acid mantle (pH, 4-6.6) suppressing bacterial growth, and 3) shedding of skin cells to minimize bioburden.
The epithelium is composed of five layers of cells: stratum corneum (SC), stratum lucidum, stratum granulosum, stratum spinosum, and stratum basale.40 Available evidence about epidermal layers with known differences in persons with dark versus light skin tones include the stratum corneum, stratum spinosum, and stratum basale.
The stratum basale is the deepest of the five layers of the epidermis.44 A basal cell is a stem cell that is a precursor of the keratinocytes of the epidermis. All keratinocytes are produced from this single layer of cells, which are constantly undergoing mitosis to produce new cells. As basal cells divide, one cell moves toward the surface and the other remains to continue reproduction.
In addition to basal cells, Merkel cells and melanocytes are also present in this layer. The Merkel cell functions as a receptor responsible for stimulating sensory nerves that the brain perceives as touch. Melanocytes produce the melanin pigment, which gives hair and skin its colour and protects living cells of the epidermis from ultraviolet radiation damage.
Differences in skin anatomy and physiology between dark and light skin tones
Pigmentation is the most obvious difference in the skin among racial groups.45 Racial variation is dependent on the quantity of melanin, amount of UV exposure, genetics, melanosome content, and type of pigments found in the skin. Four chromophores are responsible for the differences in colour found in human skin: hemoglobin, oxyhemoglobin, melanin, and carotenoids. Hemoglobin and oxyhemoglobin are responsible for the pinkish colour of white skin. Melanin accounts for the various brown shades in black and sun-tanned skin. Carotenes underlie yellow-orange pigmentation. Individuals with the most lightly pigmented skin have approximately half as much epidermal melanin as the most darkly pigmented skin types.
Differences in the SC have been reported between darkly pigmented skin (Fitzpatrick Classification Scale V and VI) and lightly pigmented skin (Fitzpatrick I/II/III).46 Darkly pigmented skin has more layers of corneocytes than lightly pigmented skin at a mean of 21.8 cells versus 16.7 cells, respectively. No differences have been reported in the size or thickness of the cells, although the cell layers in dark skin tones are thought to be more compact, reflecting greater intercellular cohesion. Although study results vary, the rate of spontaneous desquamation may be 2.5 times higher in persons with dark skin tones than those with lighter skin tones, accounting for the higher frequency of xerosis in individuals with dark skin tones.47 Differences in desquamation also vary by body site between individuals with dark versus light skin tones (eg, higher rates of desquamation of lightly pigmented skin on the cheeks and forehead).
Corneocyte size, quality, and phenotype are important because smaller cells usually correlate with epidermal (keratinocyte) hyperproliferation and development of dry skin from reduced lipid levels. Although study results vary, individuals with dark skin tones have the lowest levels of ceramides (lipid) at approximately 50% of the SC ceramide levels of individuals with lighter skin tones.2 Consequently, dark skin tones are linked to higher transepidermal water loss (TEWL) yielding lower water content (WC) of the SC. In addition, darkly pigmented skin has lower skin vascular reactivity to external factors (eg, vasodilators). Cumulatively, higher TEWL, lower WC, and reduced thermoregulatory skin response heighten the vulnerability of individuals with dark skin tones to PI development.48 Although studies are inconclusive, reduced vascular reactivity also may hinder visualization of blanching erythema of stage 1 PIs.
Gefen proposes a mechanical strain model for dark SC.48 Reduced WC in the SC may increase SC stiffness, resulting in less effective dispersion of friction and greater mechanical stress. Thus, a vicious cycle of shear damage occurs in persons with dark SC leading to progressive increases in TEWL. The WC in dark damaged SC decreases with increasing TEWL, further elevating mechanical stress concentrations. As TEWL increases, the skin becomes drier and more inflamed, heightening risk of skin injury from pressure, shear, and friction.
Skin tone assessment and measurement
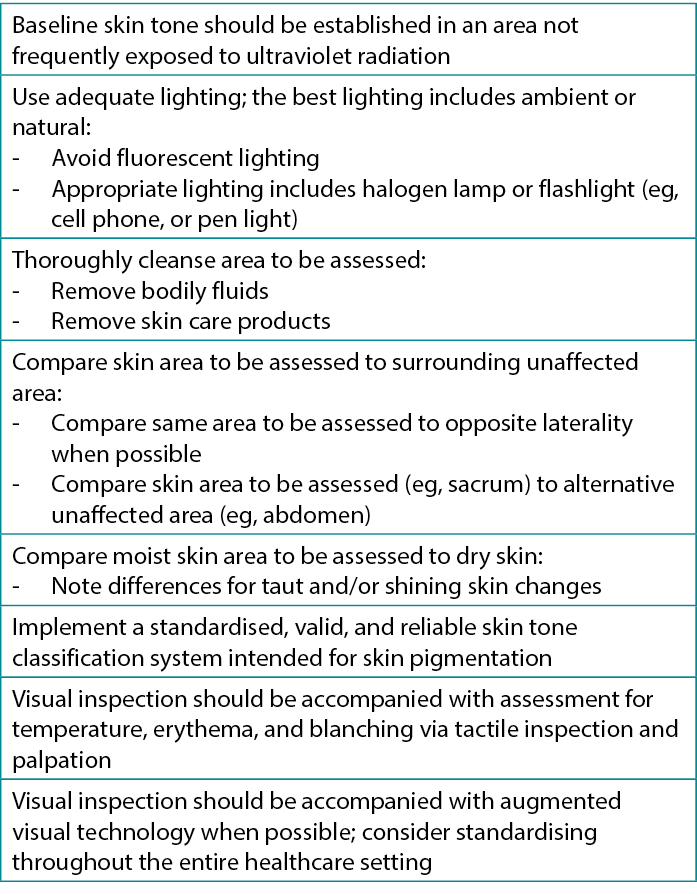
Variations in skin pigmentation, condition (dry or moist), and temperature; the presence of fluid or products; the visual acuity of the observer; and lighting are some of the factors that can influence the subjective nature of skin assessments performed by clinicians.49 Visual inspection of skin changes related to pressure, such as blanching, redness, or erythema is complemented by touch and technology (Figure 4).50,51 However, for visual inspection practices, standardization in terms and technique is key to ensure consistency among various clinician assessments. It is important to note that visual inspection alone is unreliable.22,51 Table 2 provides key information on visual assessment for diverse skin tones to assist clinicians in identifying early skin changes.22,52,53
Table 2. Visual skin assessment for diverse skin tones22,54
Skin tone classification scales
Classification of skin colour was often previously based on self-reported ethnicity and race. Colour categories based on observers’ descriptions have not been widely systematised. The use of classification scales offers some regularity; however, the function is varied. In addition to the validity and scientific rigor in the development and design of the scales, the effectiveness of classification models is dependent on individual competence and inter-rater reliability, which varies from setting to setting. These scales offer a constitutive (baseline) skin colour, facultative skin colour (sun or UV exposure), and PI-induced change in skin colour.22,54
The Fitzpatrick Classification Scale was developed to identify complexion and tolerance to sunlight and designed as a measure of sun sensitivity.22,53-57 The Fitzpatrick Scale is not reliable when evaluating skin exposed to UV radiation (environmental factors) and would be best used to assess skin not exposed.
The Munsell Colour System (MCS), designed by an artist for painting, describes qualities of hue, lightness (value), and intensity (chroma), and was initially used in soil research.58 In healthcare settings, this scale has undergone minimal validity and reliability testing despite its use in skin tone assessments.53,57,59
The Massey-Martin Scale (MMS), also known as the New Immigrant Survey (NIS) Skin Colour Scale, is a skin tone survey instrument designed for skin tone observation.53,60 The initial instructions for use avoided the comparison of the pictorial guide to the person’s skin side-by-side, as this was intended for social surveys conducted in person (by memory for the observer) or by phone (results indicated as unknown).
The Skin Tone Colour Scale (STCS) system by Konishi et al59 was designed based on the MCS and is intended for skin and lesion tone assessment. The system is vast in the selection of diverse skin tones making it the most comprehensive classification available to clinicians.
Overall, current terms to describe skin colour tone are subjective, imprecise, non-standardised, and can have offensive connotations. Visual assessment scales, such as the Munsell61,62 and Fitzpatrick scales,63 which use images of skin colour tones are limited in that they cannot represent the full range of tones among individuals or even between body locations of the same individual in a feasible and useful way.
Recognising these problematic issues of assessing skin colour tone, the British Association of Dermatologists (BAD) recommended that terminology about skin colour tone be neutral, based on objective measurements, and reflective of multiethnic populations.6,64 The most objective measure is via genetically established skin tone by melanogenesis. Pigmentation presents where melanocyte cells produce melanin in the skin. Dadzie et al6 and BAD proposed the use of eumelanin pigment nomenclature for skin tone description and developed the Eumelanin Human Skin Colour Scale (ESCS) specifically for visual skin assessment. The scale is based on and named after eumelanin, which comprises 90% of the pigment found in human skin. The ESCS has five categories of melanin index that is measured by light reflectance: eumelanin low (<25), eumelanin intermediate low (25-<50), eumelanin intermediate (50-<75), eumelanin intermediate high (75-<100), and eumelanin high (≥100).6 There are small, lightweight, portable, easy-to-use instruments for measuring melanin index. The ESCS has potential for use in classifying outcomes of PIs such as incidence and healing and monitoring for disparities/inequities.
Early visual detection technologies and dark skin tones
In individuals with dark skin tones, it is especially challenging to visually identify early skin changes that may herald an impending PI. The use of augmented visual technologies such as subepidermal moisture (SEM) assessment technology and long wave infrared technology (LWIT) hold promise for early identification of skin and tissue changes before they are visible to the naked eye. Thus, use of these technologies may create a window of opportunity for targeted interventions before the visible and tactile manifestations of tissue damage occur.65 Absent these technologies, this window is invisible to direct care clinicians. The subclinical nature of developing PIs has resulted in diagnostic latency, which then contributes to interventional latency. In individuals with dark skin tones, this lag results in a higher probability of PIs remaining undetected.66 Early detection technologies may help resolve this problem.
SEM technology
Subepidermal moisture assessment technology is based on the contemporary understanding of PI etiology (Figure 5).67,68 The onset of microscopic damage in the early development of PIs and DTPIs is consistent regardless of skin pigmentation. Cell and tissue damage triggered by sustained pressure, shear, and friction signal acute inflammatory responses. As the level of tissue damage increases, so does the inflammatory response.67 This immune response results in interstitial edema. Localised edema or SEM is one of the earliest indicators of nonvisible pressure damage. The ICD-10 coding for stage 1 PIs characterizes this early damage as ‘pre-ulcer skin changes limited to persistent focal edema.’69 This subclinical progression of tissue damage is further described in the etiology chapter of the 2019 clinical practice guidelines for the prevention and treatment of PIs.51
The SEM scanner device is FDA approved as a PI management tool and is indicated for adults of all skin tones at risk of PI development. The noninvasive point-of-care device detects persistent focal edema and reports the results as a SEM delta (∆) value (Figure 1). The SEM assessment technology measures changes in SEM between healthy and inflamed tissue.70 Increased SEM values may indicate an anatomy-specific increased risk for PI development in all skin tones.68
Meta-analyses from systematic reviews report early detection of PI development via SEM assessments by a median of 5 days before visual assessments (P ≤ .001).71,72 In a dual-arm study of 175 participants (n = 48/175, non-White), SEM assessments reported a diagnostic sensitivity and specificity of 86.8% and 88% in detecting PIs, resulting in an area-under-the-curve significantly exceeding clinical judgement (P < .0001).70 In a cohort study, SEM assessments detected developing stage 1 PIs 1 week earlier than a visible diagnosis of a stage 1 erythema (OR, 5.3; CI 1.87-15.11; P < .001) in individuals with dark skin tones (n = 11/66).73 In a multi-ethnic clinical study, SEM measurements were statistically significant in detecting concurrent and future stage 1 PIs and DTPIs in both heels in residents with dark skin tones as per Munsell value (n = 68/417; P < .001).74 An observational study of 15 patients (n = 4/15, Fitzpatrick type III and above) reported early indication of tissue damage based on SEM measurements that agreed with a later confirmation of suspected DTPIs via ultrasound-based identification of hypoechoic lesions and visual assessments.75 In a retrospective study of 69 patients in surgical intensive care, nurses indicated SEM assessments enabled more accurate skin assessments in patients with dark skin tones (n = 29/69).76
Long-Wave Infrared Thermography device
Long-wave infrared thermography (LWIT) is a noninvasive, multimodal device for use in clinical environments. It incorporates LWIT with a camera to detect PIs before visual or tactile changes occur. The device assesses changes in skin temperature because localised heat, edema, and changes in tissue consistency are all typical warning signs for PI development.77 The device can be useful in patients with light or dark skin tones. It is particularly helpful in detecting DTPIs, which can remain undetected on the skin for up to 72 hours (Figure 6).9,78 This feature is particularly important for patients with dark skin tones because dark skin pigmentation can mask the typical deep colours of purple and maroon that serve as the heralding visual signs for a DTPI.
As a combination photographic and LWIT device, it uses two imaging modalities by measuring long-wave infrared radiation (energy emitted from the human body) to create the final digital images. The energy, or lack thereof, is created from blood flow, perfusion, and, ultimately, metabolic activity. The device uses a relative temperature differential to compare the environmental temperature with the adjacent skin temperature and adapts for intrinsic and extrinsic factors (eg, elevated core body temperature, room temperature). A cooler temperature in comparison with the adjacent skin indicates less perfusion and deeper ischemic damage; warmer temperatures indicate increased metabolic activity and inflammation.
The reliability and validity of using LWIT to detect PIs have been confirmed in several studies.79-84 The 2019 International Pressure Injury Prevention and Treatment Clinical Practice Guideline identified thermography as an area of high research priority.85
Pi prevention in persons with dark skin tones
Pressure injury prevention is rooted in both risk assessment and routine, comprehensive skin and soft tissue assessment.51 Current evidence-based prevention practices apply to persons with dark skin tones and should be implemented.51,86 Because early PI detection is challenging in patients with dark skin tones, the identification of a later stage PI stage (at the time of discovery), has been reported when compared to lighter pigmented persons.87 Incorporating enhanced skin assessment techniques and visual augmentation devices into clinical practice should be a considered to enhance PI prevention in an effort to close the gap in early-stage identification.
PI treatment and healing in persons with dark skin tones
The process of healing a PI includes hemostasis, inflammation, proliferation, and maturation involving numerous molecular mechanisms.88,89 Healing is influenced by a patient’s clinical factors and treatments received. In patients with dark skin tones, healing PIs and especially the surrounding skin may appear differently to clinicians than in patients with lighter skin tones. For example, hypopigmentation of newly re-epithelialised tissue may be visible at the wound margins of a healing full thickness PI in patients with dark skin tones. (Figure 7). Although a few studies have reported differences and disparities in the prevalence or incidence of PIs by race or ethnicity,32,90 studies focused on the healing of PIs are far less common. Bliss et al91 analysed a nation-wide dataset of MDS records of older adults admitted to nursing homes. Of 10,862 older (65+ years) individuals admitted to a nursing home with a PI, 44% had healed by 90 days. However, there was a significant overall disparity of 6% in the healing of PIs (stages 2 to 4) present on admission at the required 90-day assessment among Black residents. In a study reporting on time to PI development during nursing home stays,90 99% of all residents with a PI received treatment for it, thus no disparity was found in the number of treatments by race or ethnic group. However, in other healthcare settings, such as acute care or the home care setting, there is a lack of evidence regarding disparities with regard to PI healing rates and treatment among patients with dark skin tones.

Social determinants of health
The impacts of SDOH on PI development and treatment are largely understudied and, thus, unknown. The US Department of Health and Human Services defines SDOH within five domains: economic stability, education access and quality, healthcare access and quality, neighborhood and built environments, and social and community context.92 Examination of these domains reveals potential health disparities and inequities across racial groups. Overall, people of colour have been disproportionately affected by and fare worse within all domains of SDOH compared with White individuals.93 Communities in Action: Pathways to Health Equity states that health equity is crucial for the well-being of communities.94 Although large gains have been made in healthcare coverage across racial/ethnic groups under the Affordable Care Act, people of colour remain more likely to be uninsured.94 Disparities exist with regard to income as well. In 2021, higher median incomes were reported for households headed by Asian or White individual, whereas households headed by persons identifying as Black or Hispanic reported median incomes lower than the national median.95
In two studies focused on PI development and the impact of race, elements of SDOH (economic stability and healthcare access and quality) were operationalised through patients’ income based on zip code of residence and health insurance payor status. Using the NIS database in 2009, Fogerty and colleagues38 identified an increased risk for PIs among African Americans insured by Medicare and Medicaid compared with Caucasians. With regard to income, a higher proportion of African Americans were found in the lower income quartiles (50.6%) than Caucasians (21.4%). However, in multivariate analysis, the researchers reported no significant differences between races for PI risk based on either payor status or income. Cox and Thomas-Hawkins39 reported a significantly higher proportion of Black individuals with PIs insured with Medicaid compared with patients with PIs from all other racial groups combined using 2018 HCUP state level data from New Jersey. With regard to income, reported by quartiles based on zip code of residence, a statistically significant higher proportion of Black persons with PIs resided in the lower income zip code quartiles and a lower proportion at the upper zip code income quartiles. In their sample, over 50% of Black patients with PIs lived in areas with reported incomes of $58,999 or less as compared with 19% of all other races combined. No multivariate analysis was conducted in this study. In a recent scoping review of the impact of SDOH on PI progression, Sasson and colleagues96 found that detriments in SDOH related to food scarcity (as identified through ICD-10 codes) and Black race were both significant, independent predictors of longer PI duration.96
Two recent national reports have validated the health inequities experienced by patients with dark skin tones who have PIs. In 2021, the Urban Institute reported that Black patients in the US were 31.9% less likely to be admitted to hospitals considered high quality with regard to PI prevention.97 Moreover, an Agency for Healthcare Research and Quality Disparities Report in 2019 identified that for both short- and long-stay nursing home patients, poorer quality of care associated with PIs was found among Black patients as compared with Whites patients.98 It is plausible that the development or worsening of a PI is influenced by access to quality healthcare, and this may play a pivotal role in the higher rates of PIs among Black patients. Without access to evidence-based prevention or treatment modalities, the ability to prevent a PI or facilitate wound healing is compromised. At this time, the extent of this disparity has not been studied and is unknown. This is an area in need of further exploration.
Clinician education and skin assessment in dark skin tones
Inaccurate skin assessment and lack of knowledge of PI appearance in dark skin tones can delay early identification and treatment, resulting in more severe PIs and financial penalties to the healthcare organization.99 Health inequities and racial disparities occur when healthcare professionals, whether through explicit or implicit biases, fail to adequately assess, identify, and prevent PIs in people of colour who are at risk of a PI.100,101 Inaccurate skin assessment can also occur because of the difficulty of visualising skin colour changes and discolourations in people with dark skin tones. Education provided to nurses and other clinicians related to assessment of people with diverse skin colours is sadly lacking.
As nurses, our reputation among the public is one of honesty, trust, and caring. Nurses have been ranked as the most honest and ethical of professions for more than 20 consecutive years.102 Yet, often nursing students emerge from our education programs not adequately prepared to understand the consequences of SDOH and the emphasis on providing safe and equitable care across the diverse populations we serve. Despite widespread goals to include diversity, equity, and inclusion and SDOH into nursing education curricula, whether this has been accomplished is uncertain.99 Recent evidence demonstrates there is a health disparity in undergraduate nurse education with education directed toward people with pale skin tones.99 Oozageer and colleagues99 conducted a documentary and observational study of nursing education and lectures on PIs at five undergraduate nursing programs in England. The investigators found an overwhelming focus on PIs in people with Caucasian skin tones with only brief, superficial information on people with dark skin tones.99 In a qualitative study in these five nursing education programs using focus groups, the investigators found a predominant theme of White normativity. Specifically, the investigators identified a dominance of Whiteness in the teaching about PIs and the implications for student nurses of Whiteness as the norm.99
In 2023, Pittman and Black103 examined health equity in nursing education textbooks, specifically examining physical assessment textbooks’ skin and integument content relevant to skin colour tones. Using a convenience sampling of physical assessment textbooks for undergraduate and graduate level nursing programs, the investigators modified Oozageer’s Diversity Observation Teaching Tool (DOTT) with permission to better fit their project aims. The textbooks’ content (ie, Integument/Skin Chapter) were reviewed independently, then data were reviewed for consistency or differences. Each investigator also explored their university’s simulation lab for evidence of diversity in manikin skin colour tones. Of the nine textbooks and 11 chapters, no chapter objectives included skin tone diversity. Six of the nine textbooks had visual descriptors of PI, including stages. However, of the six textbooks with photos, only three had photos with dark skin tones. The textbooks had 534 photos of various skin graphics or images but of those, only 35 (7%) were of dark skin tones. Conversely, 499 (93%) of the 534 images were of light skin tones. Both universities had simulation labs with 60 to 65% of the manikins having light skin tones and 35 to 40% having dark skin tones. However, it is not known if skin tone is discussed in the simulation scenarios. These findings support those of Oozageer and colleagues and demonstrate the lack of education regarding skin tone diversity that nursing students receive.
Conclusions
Racial disparities exist with regard to PI development and healing, especially highlighted among patients with dark skin tones. This article explored the current state of the science and identified gaps in the terms used to describe skin colour, making any data-based comparisons and trending impossible. With increasing racial diversity in the United States, including persons of mixed ethnic and racial backgrounds, the “race” of a patient should no longer be used as a demographic term or a risk factor for PIs. Skin tone colour must be more standardised and quantifiable in clinical education, practice, and research. In the face of a more racially diverse country in the upcoming decades, the ability to identify and treat developing PIs earlier will improve the quality of life for all patients. This work is urgently needed and support from private and governmental agencies is essential.
Conflict of Interest
The authors declare no conflicts of interest.
Funding
The authors received no funding for this study.
美国压力性损伤咨询小组关于深肤色人群压力性损伤的最新观点
Joyce Black, Jill Cox, Virginia Capasso, Donna Z Bliss, Barbara Delmore, Vignesh Iyer, Jacqueline Massaro, Cassendra Munro, Joyce Pittman, Elizabeth A Ayello
DOI: https://doi.org/10.33235/wcet.43.3.18-29
背景 压力性损伤(PI)由多因素导致。在深肤色患者中,通过目视皮肤评估来识别即将发生的PI尤为困难。因此,对改进皮肤评估技术的需求不断増加,特别是对于深肤色人群。同样,近年来,人们已明显意识到有必要在教育材料中体现与不同皮肤颜色/色调相关的包容性。
目的 提供关于深肤色患者皮肤评估和PI发生的当前文献观点。
方法 将从肤色的角度讨论以下要素:1)美国压力性损伤咨询小组对PI分期的历史观点,2)PI的流行病学,3)皮肤的解剖学和生理学,4)肤色评估和测量,5)増强可视化评估模式,6)PI预防,7)PI愈合,8)健康的社会决定因素,和9)临床医生教育的差距。
结论 本综述强调了我们在深肤色患者发生PI方面存在的临床知识差距。在深肤色患者中,PI发生和愈合方面的人种差异尤其突出。必须对肤色评估进行标准化,并在临床教育、实践和研究中实现可量化。迫切需要完成这项工作,且亟需私人和政府机构的支持。
引言
在过去20年中,美国的种族多样性有所提高。美国人口普查局报告称,总体多样性指数已从2010年的54.9%上升至2020年的61.1%,并预计该指数在未来十年内将继续上升。1世界范围内,大多数人群均具有深肤色。2
在过去20年中,各种肤色的人群越来越意识到皮肤评估的必要性,并对其产生了浓厚的兴趣。3-24而临床现实情况是,在美国和全球,不同肤色的人口统计学发生了变化,对各种肤色的认识有所増加,因此迫切需要对所有患者进行准确的皮肤评估和诊断。此外,近年来,学生和临床医生对在教育材料(基础教育和持续教育)中纳入不同皮肤颜色/色调患者的需求的认识也有所提高。13
在本文中,作者概述了当前对深肤色患者压力性损伤(PI)的观点。第一部分阐述了美国压力性损伤咨询小组(NPIAP;正式称为NPUAP)在过去为解决这种差异和修订与所有肤色相关的分期定义而做出的努力。从肤色角度探讨的其他主题包括PI的流行病学、皮肤的解剖学和生理学、可视化和増强皮肤评估技术和模式、PI预防和愈合、健康的社会决定因素(SDOH)考虑和专业教育差距。
深肤色患者PI分期的历史观点
在20世纪90年代中期,临床医生和NPIAP委员会成员认识到需要制定1期压力性损伤定义,以纳入这些PI在深肤色人群中的表现。1他们指出,识别“红斑”和“指压不变白红斑”等1期PI的评估指标在深肤色患者中并不总是可见的,因此,需要制定其他1期PI指标。为解决这些问题,成立了一个工作小组。2, 4
NPIAP工作小组同意以下假设:20
- 完整皮肤出现各种颜色变化
- 颜色非常深的皮肤无明显发白
- 患者的人种和种族无法预测皮肤色素沉着状况
- 指压不变白红斑仅反映了早期PI中观察到的一种皮肤颜色变化描述;其并非通用的描述符
- 1期PI的其他客观结果可能包括皮肤温度变化(发热、发冷、水肿、硬结)。
多年来定义的各种迭代参见表1。1, 5, 9, 17, 18, 20
表1.Npuap/npiap 1期压力性损伤定义:历史演变

2005年,NPIAP确定了深部组织PI(DTPI)。与1期PI相同,难以在深肤色患者中检出DTPI(图1-
3)。25NPIAP呼吁关注肤色多样性的其他倡议包括说明不同皮肤颜色/色调的分期图(可在NPIAP网站上查阅)。
PI在深肤色患者中的流行病学
少数研究根据人种/种族或肤色对急症护理机构或长期护理机构收治或疗养院收治患者的PI发生率进行了调查。然而,Gunowa及其同事26在一份关于深肤色患者PI的文献综述中报告称,无论医疗保健机构的类型如何,深肤色患者更有可能发生更严重的PI。以下讨论概述了按医疗保健机构划分的深肤色患者中PI的患病率/发生率相关可用信息。
疗养院收治患者的PI
使用最小数据集(MDS)2.0版获得的结果显示,15%的由美国疗养院收治的老年人存在PI(2期、3期或4期)。27,28据报告,疗养院收治的黒人患者中PI的发生率更高。马里兰州59家疗养院中黒人患者(16.6%)发生PI的比例约为白人患者(8.4%)的两倍。29在全国连锁疗养院研究中,Harms等人28报告了按人种和种族(美洲印第安人和阿拉斯加原住民、亚洲人或太平洋岛民、非西班牙裔黒人、西班牙裔、非西班牙裔白人)划分的疗养院老年人的PI发生率。疗养院收治的黒人患者的平均PI数量最多,为2.4(SD,2.2)。与所有其他人种和种族群体相比,白人患者中2期、3期或4期PI的患病率最低。入院时黒人患者中1期PI的患病率为7%,而2期损伤的患病率为20%。在所有人种/种族群体中,黒人患者最严重PI的患病率也最高,达到7%(3期)和
8%(4期);白人患者上述两期损伤的患病率均最低,为3%。
疗养院收治患者的PI患病率
Ahn等人30研究发现,在美国所有疗养院收治的患者中,8.4%患有PI,1.7%患有疑似DTPI。30在纽约的一组疗养院中,黒人患者(18.2%)发生PI的百分比高于白人患者(13.8%)。31在一项考察了5年内美国疗养院高危患者的PI患病率差异的研究中,黒人和白人患者的PI患病率均有所下降。然而,未经调整的总体PI患病率差异为5.4%(黒人患者更高)。32在一项使用MDS 3.0版考察疗养院报告的PI的研究中,Chen等人33研究发现,短期住院黒人患者的发生4期PI的百分比(50.4%)高于白人患者(40.8%)。33是否为黒人与2至4期PI显著相关(比值比[OR],11.44;95% CI,1.44-1.47)。是否为西班牙裔与疑似DTPI显著相关(OR,2.63;95% CI,1.47-1.58)。
疗养院中的PI发生率
Cai等人31研究发现,在控制其他风险因素的情况下,纽约疗养院的黒人患者在住院期间比白人患者更易发生PI(OR,1.203;P=0.01)。31在另一项研究中,约90,500例养老院患者中有7.7%在入住疗养院后发生2至4期PI。在疗养院住院期间发生PI的黒人患者的发病时间早于白人患者。34黒人患者中至发生PI时间的差异随时间増加,具体如下:入院后3个月时差异为3%,6个月时増长至5.8%。在宾夕法尼亚州一家疗养院进行的12周监测期间,黒人患者(47%)发生2至4期PI的百分比高于白人患者(18%)。35此外,在黒人患者中未发现1期PI。
住院患者的PI
重点关注种族分布或深肤色患者且专门考察急症护理机构中PI患病率和发生率的研究很少,大多数研究均在十年前进行。Van Gilder及其同事36在1989年至2005年间进行了一项大型、多年患病率研究,并发现深肤色患者(13%)发生1期PI的比例远低于中等肤色(32%)至浅肤色(38%)患者。该结果可能归因于难以在深肤色患者中检测到1期PI。与浅肤色或中等肤色的患者相比,在深肤色患者中观察到更严重的PI(3期、4期、焦痂)(分别为11% vs 6-
7%、13% vs 6-7%以及9% vs 5-6%)。Bauer及其同事37使用2008年至2012年美国住院患者样本(NIS)数据库进行了一项大型、多年国家研究,并报告了在确定为非裔美国人的患者中,PI发生率显著高于所有其他人种(2.4%);确定为白人的患者报告的发生率次之,为1.8%。此外,非裔美国患者的PI分期更为严重(3期),而白人患者的最常见分期为2期。37
Fogerty及其同事38使用2003年NIS数据库获得以下结果,与非非裔美国人相比,非裔美国人在出院时更有可能发生PI(OR,2.3;未提供CI)。本研究未进行PI分期分析。Cox和Thomas-Hawkins39最近进行的一项调查结果与这些前期研究的结果一致。在这项使用2018年医疗保健成本和利用项目(HCUP)国家特定数据库(新泽西州)对17,781例PI患者进行的研究中,与所有其他人种的合并数据相比,确定为黒人的患者入院诊断为PI的比例更高(5.0% vs 3.5%;P<0.05),4期PI的比例也更高(3.3% vs
2.3%)。PI次要诊断的研究结果显示,与所有其他人种的合并数据相比,黒人患者发生1期PI的比例显著更低(4.7% vs 18%;P<0.05),但4期PI的比例更高(28.7% vs 16.9%;P<0.05)。这项研究的局限性包括数据的单一状态性质和缺乏多变量分析。
总体而言,这些有限的研究成果强调了医疗保健机构中PI报告的一些重要考虑因素。首先,最近很少有研究将人种或深肤色视为发生PI的潜在风险因素或对其进行检查。由于美国种族多样性的变化值得研究,所以上述情况非常重要。其次,在这些有限的研究中,PI发生率之间的相似性十分显著,可能强调了在实践中需要特定的临床和诊断工具来识别深肤色患者即将发生的PI。
皮肤解剖学和生理学
皮肤由两个不同部分组成:表皮和真皮。40-43表皮由细胞构成且无血管,含有90%的角质形成细胞,此类细胞可合成不溶于水的强效结构蛋白Å\Å\角蛋白。表皮可防止水分流失,减缓剪切力、摩擦力并隔离有毒刺激物。它还通过以下三种机制阻止细菌和其他病原体的入侵:1)机械屏障,2)酸性保护膜(pH 4-6.6),可抑制细菌生长,和3)皮肤细胞脱落,可尽量减少生物负荷。
表皮由五层细胞组成:角质层(SC)、透明层、颗粒层、棘层和基底层。40现有证据表明深肤色与浅色肤色人群中存在已知差异的表皮层包括角质层、棘层和基底层。
基底层是表皮五层的最底层。44基底细胞是一种干细胞,是表皮角质形成细胞的前体细胞。所有角质形成细胞均由这一层细胞产生,它们不断进行有丝分裂以产生新的细胞。基底细胞分裂时,一个细胞向表面移动,另一个细胞仍继续进行复制。
除基底细胞外,该层中还存在梅克尔细胞和黒素细胞。作为一种受体,梅克尔细胞负责刺激大脑感知为触觉的感觉神经。黒素细胞产生黒色素,使毛发和皮肤呈现其颜色,并保护表皮的活细胞免受紫外线辐射伤害。
深皮肤和浅皮肤的皮肤解剖学和生理学差异
色素沉着是各人种之间皮肤最明显的差异。45人种差异取决于黒色素的数量、紫外线暴露量、遗传学、黒素体含量和皮肤中发现的色素类型。人体皮肤的颜色差异由以下四种发色团导致:血红蛋白、氧合血红蛋白、黒色素和类胡萝卜素。血红蛋白和氧合血红蛋白是白色皮肤呈现粉红色的原因。黒色素使黒色和晒黒的皮肤呈现不同的棕色。胡萝卜素是皮肤出现黄橙色色素沉着的基础。在皮肤色素沉着最浅的个体中,表皮黒色素含量约为皮肤色素沉着最深个体的一半。
据报告,深色皮肤(Fitzpatrick皮肤分型量表V和VI型)和浅色皮肤(Fitzpatrick I/II/III型)之间的SC存在差异。46深色皮肤比浅色皮肤具有更多的角质细胞层,平均值分别为21.8个细胞和16.7个细胞。据报告,虽然认为深色皮肤的细胞层更紧凑,反映出较强的细胞凝聚力,但细胞大小或厚度不存在差异。虽然研究结果各不相同,但深肤色个体的自发脱屑率可能是浅肤色个体的2.5倍,这也是深肤色个体中干燥病的发生率较高的原因。47深肤色与浅肤色个体之间的脱屑差异也因身体部位而异(例如,脸颊和前额浅色皮肤的脱屑率较高)。
由于较小的细胞通常与表皮(角质形成细胞)过度増殖和脂质水平降低导致的皮肤干燥相关,所以角质细胞的大小、质量和表型非常重要。虽然研究结果各不相同,但深肤色个体的神经酰胺(脂质)水平最低,约为浅肤色个体SC神经酰胺水平的50%。2因此,深肤色与经表皮失水(TEWL)较多相关,导致SC含水量(WC)较低。此外,深色皮肤的血管对外部因素(如血管扩张剂)的反应性较低。总体而言,较多的TEWL、较低的WC和体温调节皮肤反应降低均増加了深肤色个体对发生PI的易感性。48虽然研究尚无定论,但血管反应性降低也可能阻碍1期PI指压变白红斑的可视化。
Gefen提出了深色SC的机械应变模型。48SC中WC降低可能会増加SC硬度,导致无法有效分散摩擦力和较大的机械应力。因此,深色SC患者会发生剪切损伤的恶性循环,导致TEWL逐渐増加。深色损伤SC的WC随TEWL増加而降低,进一步提高了机械应力浓度。随着TEWL増加,皮肤变得更加干燥、更易发炎,压力、剪切力和摩擦力均増加了皮肤损伤的风险。
肤色评估和测量
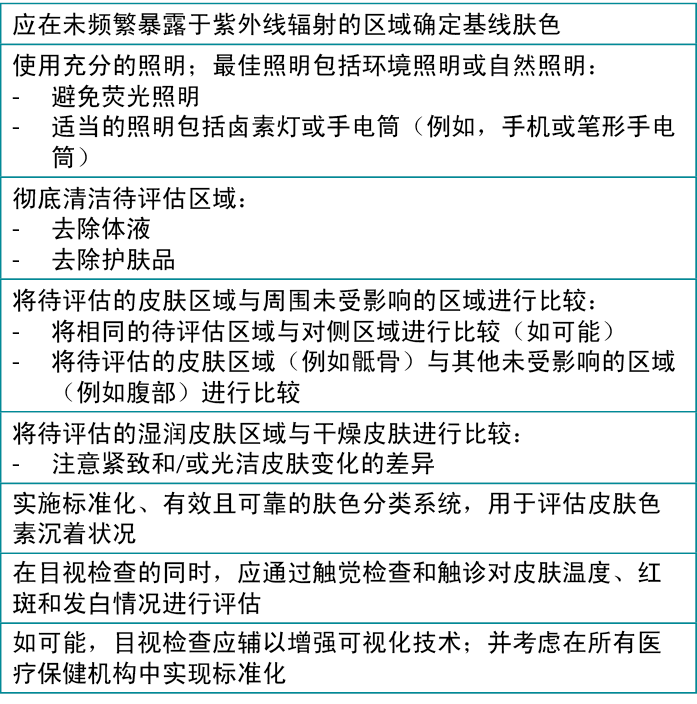
皮肤色素沉着、皮肤状况(干燥或湿润)和皮肤温度的变化;液体或产品的存在;观察者的视力;以及照明均为可能影响临床医生进行皮肤评估的主观性的一些因素。49可通过触摸和使用相应技术来对压力相关皮肤变化(如发白、发红或红斑)的目视检查进行补充(图4)。50, 51然而,对于目视检查实践,术语和技术标准化是确保不同临床医生的评估结果一致的关键。值得注意的是,仅采用目视检查是不可靠的。22, 51表2提供了与不同肤色的目视评估相关的关键信息,旨在帮助临床医生识别早期皮肤变化。22, 52, 53
表2.针对不同肤色的目视皮肤评估22, 54
肤色分类量表
在过去,通常根据自我报告的种族和人种对肤色进行分类。基于观察者描述的颜色分类尚未得到广泛系统化。分类量表的使用具有一定的规律性;但其功能各不相同。除了量表开发以及设计的有效性和科学严谨性之外,分类模型的有效性还取决于个体能力和评定者间可靠性,这因环境而异。这些量表提供了固有(基线)肤色、可变肤色(日光或紫外线暴露)和PI诱导的肤色变化。22,54
开发Fitzpatrick皮肤分型量表旨在确定肤色和对日光的耐受性,并且已将该量表设计为衡量日光敏感性的指标。22, 53-57在对暴露于紫外线辐射(环境因素)的皮肤进行评价时,Fitzpatrick量表不可靠,其最佳用途为评估未暴露的皮肤。
Munsell颜色系统(MCS)由绘画艺术家设计,描述了色调、亮度(值)和强度(色度)的质量,最初用于土壤研究。58在医疗保健机构中,虽然该量表用于评估肤色,但其有效性和可靠性均极低。53, 57, 59
Massey-Martin量表(MMS),也称为新移民调查(NIS)肤色量表,是一种设计用于观察肤色的肤色调查工具。53, 60最初的使用说明并未将图示指南与人体皮肤进行并排比较,原因在于该量表预期用于亲自(通过观察者的记忆)或通过电话(结果表示为未知)进行的社会调查。
Konishi等人59基于MCS设计了肤色量表(STCS)系统,预期用于皮肤和病损色调评估。该系统在不同肤色的选择方面较为广泛,使其成为可供临床医生使用的最全面的分类系统。
总体而言,当前描述肤色的术语均较为主观、不精确且未进行标准化,可能表达具有攻击性的含义。对于使用肤色图像的Munsell61, 62和Fitzpatrick量表63等目视评估量表,其局限性在于它们无法以可行且有用的方式代表个体之间亦或是同一个体身体部位之间的所有色调。
英国皮肤科医师协会(BAD)认识到了评估肤色方面存在的这些问题,并建议有关肤色的术语应保持中性,以客观测量结果为基础,且可反映多种族人群的情况。6, 64最客观的测量结果是按黒色素生成情况确定的基因决定的肤色。色素沉着表现为黒素细胞在皮肤中产生黒色素。Dadzie等人6和BAD提出使用真黒素色素命名规则描述肤色,并开发了专用于目视皮肤评估的真黒素人体皮肤色度量
表(ESCS)。该量表以真黒素为基础并以其命名,真黒素占人体皮肤色素的90%。ESCS有以下五类黒色素指数,通过光反射法测量:低真黒素
水平(<25)、中低真黒素水平(25-<50)、中等真黒素水平(50-<75)、中高真黒素
水平(75-<100)和高真黒素水平(≥100)。6目前已有小型、轻便、易于使用的便携式黒色素指数测量仪器可供使用。ESCS还可能用于对PI结局进行分类,如发生率和愈合情况,并对差异/不平等进行监测。
早期可视化检测技术和深肤色
在深肤色个体中,通过目视检查识别可能预示即将发生PI的早期皮肤变化尤其具有挑战性。増强可视化技术的使用,如表皮下水分(SEM)评估技术和长波红外技术(LWIT)有望在皮肤和组织变化变得肉眼可见之前对其进行早期识别。因此,在出现组织损伤的可见和可触表现之前,使用这些技术可能会提供进行靶向干预的机会。65如果未开发这些技术,直接护理临床医生便不会有这个机会。PI发展的亚临床性质导致存在诊断潜伏期,进而造成干预潜伏期。在深肤色个体中,这种滞后会导致PI未被检出的概率较高。66早期检测技术可能有助于解决这一问题。
SEM技术
表皮水分评估技术以当代对PI病因学的理解为
基础(图5)。67, 68PI和DTPI发展早期的显微镜检查损伤发生情况一致,与皮肤色素沉着无关。持续的压力、剪切力和摩擦力引发的细胞和组织损伤是急性炎症反应的信号。随着组织损伤水平的升高,炎症反应也会加剧。67这种免疫反应会导致间质性水肿。局部水肿或SEM是不可见压力性损伤的最早指标之一。1期PI的ICD-10编码将此类早期损伤描述为“仅限于持续性局灶性水肿的溃疡前皮肤变化”。692019年PI预防和治疗临床实践指南的病因学章节进一步描述了组织损伤的这一亚临床进展。51
SEM扫描器械已获得FDA批准,可用作PI管理工具,适用于有PI发生风险的所有肤色成人。非侵入性床旁检测器械可对持续性局灶性水肿进行检测,并将结果报告为SEM ɬ(∆)值(图1)。SEM评估技术可测量健康组织和发炎组织之间SEM的变化。70SEM值増加可能表明所有肤色人群中的PI发生风险将出现解剖学特异性増加。68
系统性综述的荟萃分析报告称,在目视评估前,通过SEM评估确定的早期检测到PI发生的中位时间为5天(P≤0.001)。71, 72在一项涉及175例参与者(n=48/175,非白人)的双臂研究中,SEM评估报告了在PI检测方面的诊断灵敏度和专属性分别为86.8%和88%,导致曲线下面积显著超出临床判断结果(P<0.0001)。70在一项队列研究中,在深肤色患者(n=11/66)中,SEM评估比目视1期红斑诊断早1周检测出1期PI的发生情况(OR,5.3;CI 1.87-15.11;P<0.001)。73在一项多种族临床研究中,根据Munsell值,SEM测量在检测深肤色患者的双足跟中并发和未来1期PI和DTPI方面具有统计学显著性(n=68/417;P<0.001)。74一项涉及15例患者的观察性研究(n=4/15,Fitzpatrick III型及以上)报告了基于SEM测量结果的早期组织损伤指征,其均与后续通过低回声病灶超声识别和目视评估确认的疑似DTPI一致。75在一项涉及69例外科重症监护患者的回顾性研究中,护士表示SEM评估能够对深肤色患者(n=29/69)进行更准确的皮肤评估。76
长波红外热成像装置
长波红外热成像(LWIT)装置是一种用于临床环境的非侵入性、多模式装置。它将LWIT与摄像机相结合,用于在发生可见或可触变化之前对PI进行检测。局部发热、水肿和组织稠度的变化均为发生PI的典型警告信号,因此该装置旨在评估皮肤温度变化。77其在浅肤色或深肤色患者中均适用。由于DTPI可在皮肤保持长达72小时而不被检测到,所以该方法在检测DTPI方面特别有效(图6)9, 78。这一特征对于深肤色患者尤为重要,原因在于深色皮肤色素沉着可能掩盖紫色和栗色的典型深色表现,这些深色表现可作为DTPI的先兆视觉体征。
作为摄像机和LWIT装置的组合,它通过测量长波红外辐射(从人体发射的能量)利用两种成像方式,创建最终数字图像。能量或能量不足是由血液流动、灌注和最终的代谢活动而产生的。该装置使用相对温差对环境温度和邻近皮肤温度进行比较,并根据内部和外部因素(例如,核心体温升高、室温)进行调整。与邻近皮肤相比温度较低表明灌注较少和缺血性损伤较深;温度较高表明代谢活性和炎症増加。
使用LWIT检测PI的可靠性和有效性已在几项研究中得到证实。79-842019年国际压力性损伤预防和治疗临床实践指南将热成像确定为高度优先考虑的研究领域。85
深肤色患者的PI预防
压力性损伤预防的根本在于风险评估以及常规、全面的皮肤和软组织评估。51目前的循证预防实践适用于深肤色人群,应予以实施。51, 86由于在深肤色患者中进行早期PI检测极具挑战性,因此,与浅肤色人群相比,已经报告了后期PI分期的识别情况。87应考虑在临床实践中应用増强皮肤评估技术和视觉増强设备时,以加强PI预防,努力缩小早期阶段识别的差距。
深肤色患者的PI治疗和愈合
PI的愈合过程包括止血、炎症、増殖和成熟,涉及多种分子机制。88, 89患者的临床因素和所接受的治疗均会影响愈合情况。对临床医生而言,深肤色患者的PI(尤其是周围皮肤)愈合可能与浅肤色患者不同。例如,在深肤色患者中,愈合的全层PI伤口边缘可能存在可见的新再上皮化组织色素减退。(图7)。虽然一些研究报告了不同人种或种族的PI患病率或发生率之间的差异,32, 90但仍很少有研究重点关注PI愈合。Bliss等人91分析了全国疗养院收治老年人的MDS记录数据集。在疗养院收治的10,862例PI老年(65岁以上)患者中,44%在90天时愈合。然而,在黒人患者中,根据规定的90天评估,入院时存在的PI(2至4期)愈合情况显示出6%的显著总体差异。在一项报告了疗养院住院期间至PI发生时间的研究中,90所有PI患者中有99%接受了相应治疗,因此未发现人种或种族群体的治疗次数存在差异。但是,在急症护理或家庭护理机构等其他医疗保健机构中,缺乏深肤色患者在PI愈合率和治疗方面存在差异的证据。
健康的社会决定因素
SDOH对PI发生和治疗的影响在很大程度上未得到充分研究,因此尚不清楚。美国卫生与公众服务部将SDOH定义为五个领域:经济稳定、教育获取和质量、医疗保健获取和质量、社区和建筑环境以及社会和社区背景。92对这些领域的研究揭示了不同种族群体之间潜在的健康差异和不平等。总体而言,与白人相比,有色人种在SDOH所有领域内均受到了不成比例的影响,且情况更糟。93《行动中的社区:通往健康公平的途径》指出,健康公平对社区的健康至关重要。94虽然根据《平价医疗法案》,不同人种/种族群体在推进医疗保险方面取得了巨大进展,但有色人种仍然更有可能未享受医疗保险。94此外,收入方面也存在差异。2021年,报告的亚洲人或白人户主家庭的中位收入较高,而报告的黒人或西班牙裔户主家庭的中位收入低于全国中位收入。95
在两项关注PI发生和人种影响的研究中,SDOH的要素(经济稳定和医疗保健获取和质量)是通过基于居住地邮政编码和医疗保险支付者状况的患者收入进行操作的。Fogerty及其同事38使用2009年NIS数据库获得的结果显示,与高加索人相比,参加医疗保险和医疗补助计划的非裔美国人发生PI的风险増加。在收入方面,收入四分位数较低地区的非裔美国人的比例(50.6%)高于高加索人(21.4%)。然而,在多变量分析中,研究人员报告称,基于支付者状况或收入,不同人种之间的PI风险无显著差异。Cox和Thomas-Hawkins39报告称,其使用新泽西州2018年HCUP州级数据获得的结果显示,与所有其他人种的PI患者的合并数据相比,享受医疗补助计划保障的PI黒人患者比例明显更高。在收入方面,按基于居住地邮政编码的四分位数进行报告,居住在邮政编码收入四分位数较低地区的PI黒人患者的比例较高,且具有统计学显著性,居住在邮政编码收入四分位数较高地区的PI黒人患者的比例较低。在其样本中,超过50%的PI黒人患者居住在报告收入为58,999美元或以下的地区,而所有其他人种的总和为19%。这项研究未进行多变量分析。Sasson及其同事96最近对SDOH对PI进展的影响进行了范围综述,并发现与食物匮乏(通过ICD-10编码确定)和黒人人种相关的SDOH不利因素均为PI持续时间较长的显著、独立预测因素。96
最近的两份国家报告证实了深肤色PI患者所经历的健康不平等。2021年,美国城市研究院报告称,美国黒人患者入住可提供高质量PI预防的医院的可能性降低31.9%。97此外,美国卫生保健研究和质量机构在2019年发布的差异报告中指出,对于短期和长期疗养院住院患者,与白人患者相比,黒人患者的PI相关护理质量较差。98因此,有理由认为,PI的发生或恶化可能受高质量医疗保健获取情况的影响,这可能是黒人患者中PI发生率较高的一个关键因素。无法获取循证预防或治疗方式,预防PI或促进伤口愈合的能力就会大打折扣。目前,尚未对这一差异的程度进行研究,因此尚不清楚。需要对这一领域进行进一步探索。
临床医生教育和深肤色患者的皮肤评估
皮肤评估不准确和缺乏对深肤色患者中PI外观的了解可能会延误早期识别和治疗,导致更严重的PI,并对医疗机构造成经济处罚。99当医疗保健专业人员因显性或隐性偏倚而未能对有PI风险的有色人群的PI进行充分评估、识别和预防时,就会导致健康不平等和种族差异。100, 101由于难以对深肤色人群的肤色变化和皮肤变色进行可视化,这也可能导致皮肤评估不准确。遗憾的是,尚未向护士和其他临床医生提供充分的与不同肤色人群评估相关的教育。
作为护士,公众认为我们具备诚实、信任和关怀等美徳。护士已连续20多年被评为最诚实和最恪守道徳的职业。102然而,护理专业的学生在完成我们的教育项目后往往并未做好充分准备,无法理解SDOH带来的后果,也无法理解在我们所服务的不同人群中提供安全、公平的护理的重要性。虽然普遍存在将多样性、公平性和包容性以及SDOH纳入护理教育课程的目标,但尚不确定这一目标是否已经实现。99最新证据表明,本科护士教育与针对肤色苍白人群的教育之间存在健康差异。99Oozageer及其同事99对英国五个本科护理项目的护理教育和PI讲座进行了文献和观察性研究。研究者发现,关注大多集中在具有高加索人肤色的人群发生的PI,而对于深肤色人群,仅提供了简短且肤浅的信息。99研究者使用焦点小组对这五个护理教育项目进行了定性研究,发现白人规范性是一个主要主题。具体而言,研究者确定了白皮肤在PI教学中的主导地位,以及将白皮肤作为标准对学生护士产生的影响。99
2023年,Pittman和Black103研究了护理教育教科书中的健康公平问题,特别考察了体格评估教科书中与肤色相关的皮肤和外皮内容。研究者对本科生和研究生护理项目的体格评估教科书进行了便利抽样,并在征得同意后修改了Oozageer的多样性观察教学工具(DOTT),以更好地适应其项目目标。对教科书内容(即,外皮/皮肤章节)进行了独立审查,然后对数据一致性或差异进行了审查。每位研究者还探索了相应大学的模拟实验室,以获得表明人体模型具有肤色多样性的证据。在九本教科书和11个章节中,所有章节目标均不包括肤色多样性。九本教科书中有六本提供了PI的视觉描述符,包括其分期。然而,在配有照片的六本教科书中,只有三本配有深肤色照片。这些教科书配有534张各类皮肤图形或图像的照片,但其中只有35张(7%)为深肤色照片。反之,534张图像中有499张(93%)为浅肤色照片。两所大学均设有模拟实验室,其中60%至65%的人体模型具有浅肤色,35%至40%的人体模型具有深肤色。然而,在模拟情境中是否讨论了肤色问题尚不得而知。这些研究结果支持Oozageer及其同事获得的结果,证明护理专业学生缺乏关于肤色多样性的教育。
结论
PI发生和愈合方面存在人种差异,在深肤色患者中尤是如此。本文探讨了科学现状,并确定了用于描述肤色的术语方面存在的差距,这使得任何基于数据的比较和趋势分析都无法进行。随着美国种族多样性(包括具有多重种族和人种背景的人群)不断提高,患者的“人种”不应再用作人口统计学术语或PI的风险因素。在临床教育、实践和研究中,必须使肤色更为标准化和可量化。面对一个在未来几十年中种族多样性不断提高的国家,能够更早识别和治疗处于发展状态的PI将使所有患者的生活质量得到改善。迫切需要完成这项工作,且亟需私人和政府机构的支持。
利益冲突声明
作者声明无利益冲突。
资助
作者未因该项研究收到任何资助。
Author(s)
Joyce Black
PhD RN FAAN
Jill Cox
PhD RN APN-C CWOCN FAAN
Virginia Capasso
PhD CNP CNS CWS FACCWS FAAN
Donna Z Bliss
PhD RN FGSA FAAN
Barbara Delmore
PhD RN CWCN MAPWCA FAAN
Vignesh Iyer
MS
Jacqueline Massaro
MSN RN CWOCN
Cassendra Munro
PhD RN CNOR
Joyce Pittman
PhD RN ANP-BC FNP-BC CWOCN FAAN
Elizabeth A Ayello
PhD MS ETN RN CWON MAPWCA FAAN
* Corresponding author
Joyce Black is President, National Pressure Injury Advisory Panel, and Florence Niedfelt Professor of Nursing, College of Nursing, University of Nebraska.
Jill Cox is Member, Board of Directors, National Pressure Injury Advisory Panel; Clinical Professor, Rutgers University School of Nursing, Rutgers, New Jersey; and Wound/Ostomy/Continence Advanced Practice Nurse, Englewood Health, Englewood, New Jersey.
Virginia Capasso is Member, Board of Directors, National Pressure Injury Advisory Panel; Instructor in Surgery, Harvard Medical School, Cambridge Massachusetts; and Advanced Practice Nurse and Nurse Scientist, Massachusetts General Hospital, Boston.
Donna Z. Bliss, is School of Nursing Foundation Professor of Nursing Research, and Chair, Adult and Gerontological Health Cooperative, University of Minnesota School of Nursing, Minneapolis.
Barbara Delmore is Alumna, Board of Directors, National Pressure Injury Advisory Panel, and Senior Nurse Scientist and Clinical Assistant Professor, NYU Langone Health, New York.
Vignesh Iyer is Director, Medical Affairs, Bruin Biometrics, Los Angeles, California.
Jacqueline Massaro, MSN, RN, CWOCN, is Wound/Ostomy/Continence Nurse, Brigham and Women’s Hospital, Boston, Massachusetts.
Cassendra Munro, is Nurse Scientist, Office of Research Patient Care Services, Stanford Health Care, Palo Alto, California.
Joyce Pittman is Alumna, Board of Directors, National Pressure Injury Advisory Panel, and Associate Professor, College of Nursing, University of South Alabama, Mobile.
Elizabeth A Ayello is Alumna, Board of Directors, and Past President, National Pressure Injury Advisory Panel, and President, Ayello, Harris & Associates, Inc, New York.
Joyce Black是美国压力性损伤咨询小组的主席,Florence Niedfelt是内布拉斯加大学护理学院的护理教授。
Jill Cox是美国压力性损伤咨询小组董事会成员;美国新泽西州罗格斯大学护理学院的临床教授;美国新泽西州Englewood Health机构的伤口/造口/失禁高级实践护士。
Virginia Capasso是美国压力性损伤咨询小组董事会成员;美国马萨诸塞州剑桥市哈佛大学医学院外科讲师;美国波士顿麻省总医院高级实践护士和护士科学家。
Donna Z. Bliss是护理学院基金会护理研究教授,以及美国明尼阿波利斯市明尼苏达大学护理学院成人和老年学健康合作机构主席。
Barbara Delmore是美国国家压力性损伤咨询小组董事会校友,以及美国纽约州纽约大学朗格尼医学中心高级护士科学家和临床助理教授。
Vignesh Iyer是美国加利福尼亚州洛杉矶Bruin Biometrics公司的医学事务总监。
Jacqueline Massaro(MSN,RN,CWOCN)是美国马萨诸塞州波士顿布列根和妇女医院伤口/造口/失禁护士。
Cassendra Munro是美国加利福尼亚州帕洛阿尔托市斯坦福医疗保健中心研究患者护理服务办公室的护士科学家。
Joyce Pittman是美国压力性损伤咨询小组董事会的校友,以及美国莫比尔市南阿拉巴马大学护理学院的副教授。
Elizabeth A Ayello是美国压力性损伤咨询小组董事会的校友和前任主席,以及美国纽约州Ayello, Harris & Associates, Inc总裁。
References
- United States Census Bureau. 2020 Census Statistics Highlight Local Population Changes and Nation’s Racial and Ethnic Diversity. https://www.census.gov/newsroom/press-releases/2021/population-changes-nations-diversity.html. Last accessed June 26, 2023.
- Rawlings AV. Ethnic skin types: are there differences in skin structure and function? Int J Cosmet Sci 2006;28(2):79-93.
- Ayello EA, Lyder CH. Pressure ulcers in person of colors: race and ethnicity. In Pressure Ulcers in America: Prevalence, Incidence, and Implications for the Future. Eds, Cuddigan J et al. Reston, VA: National Pressure Ulcer Advisory Panel; 2001.
- Bennett MA. Report of the task force on the implications for darkly pigmented intact skin in the predication and prevention of pressure ulcers. Adv Wound Care 1995;8(6)34-5.
- Black J, Baharestani MM, Cuddigan J et al. National Pressure Ulcer Advisory Panel’s updated pressure ulcer staging system. Adv Skin Wound Care 2007;20(5):269-74.
- Dadzie OE, Sturm RA, Fajuyigbe D, Petit A, Jablonski NG. The Eumelanin Human Skin Colour Scale: a proof of concept study. Br J Dermatol 2022;187:99-104.
- Dhoonmoon L, Fletcher J. Assessing skin tones in practice: results of an international survey. Wounds Int 2022;13(2):6-9.
- Dhoonmoon L, Nair HKR, Abbas Z, et al. International Consensus Document: Wound Care and Skin Tone Signs, Symptoms and Terminology for all Skin Tones. Wounds International. 2023. Available at www.woundsinternational.com.
- Edsberg LE, Black J, Goldberg M, et al. Revised National Pressure Ulcer Advisory Panel Pressure Injury Staging System. J Wound Ostomy Continence Nurs 2016;43(6):585-97.
- Eilers S, Bach DQ, Gaber R, et al. Accuracy of self-report in assessing Fitzpatrick skin phototypes I through VI. JAMA Dermatol 2013;149(11):1289-94.
- Fletcher J. The challenges of dark skin tone assessment: the importance of language. Wounds UK 2022;18(2):6,8.
- Gass M, Dadzie OE, Dlova N, Petit A, Goad N. Skin diversity erythema description guidance. British Association of Dermatology. February 2021. https://cdn.bad.org.uk/uploads/2022/02/29200007/Skin-diversity-descriptors-erythema-redness-guidance.pdf. Last accessed July 3, 2023.
- Gunowa NO. Skin tone bias and wound care: highlighting the current evidence and addressing the gaps in knowledge of dark skin tones. Wounds UK 2022;18(1):22-7.
- Lester JC, Jia JL, Zhang L, Okoye GA, Linos E. Absence of images of skin of colour in publication of COVID-19 skin manifestations. Br J Dermatol 2020;183:564-95.
- Matas A, Sowa MG, Taylor V, Taylor G, Schattka BJ, Mantsch HG. Eliminating the issue of skin color in assessment of the blanch response. Adv Skin Wound Care 2001;14(4):180-8.
- Mukwende M, Tamony P, Turner M. Mind the Gap: A Clinical Handbook of Signs in Black and Brown Skin. St. George’s University of London; 2020. https://www.blackandbrownskin.co.uk/mindthegap. Last accessed June 26, 2023.
- National Pressure Ulcer Advisory Panel. Pressure ulcers prevalence, cost and risk assessment: consensus development consensus statement. Decubitus 1989;2(2):24-8.
- National Pressure Ulcer Advisory Panel. Cuddigan J, Ayello EA, Sussman C. (Eds). Pressure Ulcers in America: Prevalence, Incidence, and Implications for the Future. Reston, VA: NPUAP; 2001.
- National Pressure Ulcer Advisory Panel (NPUAP), European Pressure Ulcer Advisory Panel (EPUAP), Pan Pacific Pressure Injury Alliance (PPPIA). Prevention and Treatment of Pressure Ulcers: Clinical Practice Guideline. Emily Haesler (Ed). Osborne Park, WA: Cambridge Media; 2014.
- Henderson CT, Ayello EA, Sussman C et al. Draft definition of stage I pressure ulcers: inclusion of persons with darkly pigmented skin. NPUAP Task Force on Stage I Definition and Darkly Pigmented Skin. Adv Wound Care 1997;10(5):16-9.
- National Pressure Ulcer Advisory Panel. NPUAP Pressure Injury Stages. 2016. Available from www.npiap.org
- Pusey Reid E, Quinn L, Samost ME, Reidy PA. Skin assessment in patients with dark skin tone. Am J Nurs 2023;123(3):36-43.
- Salcido S. Finding a window into the skin: it depends on the color of your skin. Adv Skin Wound Care 2008;22(1):8.
- Skin of Color Society. https://skinofcolorsociety.org/about-socs/. Last accessed June 26, 2023.
- Ankrom M, Bennett R, Sprigle S, et al. Pressure-related deep tissue injury under intact skin and the current pressure ulcer staging systems. Adv Skin Wound Care 2005;18 (1):35-42.
- Gunowa NO, Hutchinson M, Brooke J, Jackson D. Pressure injuries in people with darker skin tones: a literature review. J Clin Nurs 2017:27(17-18); 3266-75.
- Bliss DZ, Harms S, Garrard JM, et al. Prevalence of incontinence by race and ethnicity of older people admitted to nursing homes. J Am Med Directors Assoc 2013;14(6):451.e1-451.e7.
- Harms S, Bliss DZ, Garrad JM, et al. Prevalence of pressure ulcers by race and ethnicity for older people admitted to nursing homes. J Gerontol Nurs 2014;40(3);20-6.
- Baumgarten M, Margolis D, van Doorn C, et al. Black/White differences in pressure ulcer incidence in nursing home residents. J Am Geriatr Soc 2004;52(8):1293-8.
- Ahn H, Cowan L, Garvan C, Lyon D, Stechmiller J. Risk factors for pressure ulcers including suspected deep tissue injury in nursing home facility residents: analysis of National Minimum Data Set 3.0. Adv Skin Wound Care 2016;29(4):178-90.
- Cai S, Mukamel DB, Temkin-Greener H. Pressure ulcer prevalence among black and white nursing home residents in New York state: evidence of racial disparity? Medical Care 2010;48(3):233-9.
- Li Y, Yin J, Cai X, Temkin-Greener J, Mukamel DB. Association of race and sites of care with pressure ulcers in high-risk nursing home residents. JAMA 2011;306(2):179-86.
- Chen Z, Gleason LJ, Sanghavi P. Accuracy of pressure ulcer events in US nursing home ratings. Medical Care 2022;60(10):775-83.
- Bliss DZ, Gurvich O, Savik K, et al. Are there racial-ethnic disparities in time to pressure ulcer development and pressure ulcer treatment in older adults after nursing home admission? J Aging Health 2015;27(4):571-93.
- Rosen J, Mittal V, Degenholtz H, et al. Pressure ulcer prevention in Black and White nursing home residents: a QI initiative of enhanced ability, incentives, and management feedback. Adv Skin Wound Care 2006;19(5):262-9.
- Vangilder C, Macfarlane GD, Meyer S. Results of nine international pressure ulcer prevalence surveys: 1989 to 2005. Ostomy Wound Manage 2008;54(2):40-54.
- Bauer K, Rock K, Nazzal M, Jones O, Qu W. Pressure ulcers in the United States’ inpatient population from 2008 to 2012: results of a retrospective nationwide study. Ostomy Wound Manage 2016;62(11):30-8.
- Fogerty M, Guy J, Barbul A, Nanney LB, Abumrad NN. African Americans show increased risk for pressure ulcers: a retrospective analysis of acute care hospitals in America. Wound Repair Regen 2009;17(5):678–84.
- Cox J, Thomas Hawkins C. Racial disparities and pressure injuries among hospitalized patients. Adv Skin Wound Care 2023;36(2):78–84.
- Anatomy and Physiology of Skin, Soft Tissue, and Beyond. In Course Content, Wound Certification Prep Course. Malvern PA: HMP Communications, June 2022.
- Mufti A, Ayello EA, Sibbald RG: Anatomy and physiology of the skin. In: Doughty D, McNichol L (eds): Wound, Ostomy and Continence Nurses Society Core Curriculum, Wound Management. Philadelphia, PA: Wolters Kluwer; 2016.
- Lawton S. Skin 1: the structure and functions of the skin. Nurs Times 2019;115(12):30-3.
- Baranowski S, Ayello EA, Levine JM, Sibbald RG. Skin: an essential organ. In: Baranowski S, Ayello EA, eds. Wound Care Essentials 5th Edition. Philadelphia, PA: Wolters Kluwer; 2020:57-59, 66-67.
- Anatomy and Physiology 5.1 Layers of Skin. OpenStax Anatomy & Physiology. https://openstax.org/Anatomy-and-Physiology-2e/pages/5-1/layers-of- skin. Last accessed June 30, 2023.
- Rawlings AV. Ethnic skin types: are there differences in skin structure and function? Int J Cosmet Sci 2006;28(2):79-93.
- Fitzpatrick TB. The validity and practicality of sun-reactive skin types I through VI. Arch Dermatol 1988;124(6):869-71.
- Wesley NO, Maibach HI. Racial (ethnic) differences in skin properties: the objective data. Am J Clin Dermatol 2003;4(12)843-60.
- Gefen A. Application of the mechanical strain model to darkly pigmented skin. NPIAP 2023 Annual Conference; San Diego CA, March 18, 2023.
- Sullivan R. A 5-year retrospective study of descriptors associated with identification of stage I and suspected deep tissue pressure ulcers in persons with darkly pigmented skin. Wounds 2014;26(12):351–9.
- Clark M. Skin assessment in dark pigmented skin: a challenge in pressure ulcer prevention. Nurs Times 2010;106(30):16–7.
- National Pressure Injury Advisory Panel, European Pressure Ulcer Advisory Panel, & Pan Pacific Pressure Ulcer Injury Alliance. Prevention and treatment of pressure ulcers/injury: clinical practice guideline. The International Guideline; 2019.
- Black, J. & Simende, A. Top ten tips: assessing darkly pigmented skin. Wounds Int 2020;11(3):8-11.
- Francis, K. Assessment and identification of skin disorders in skin of color. J Wound Ostomy Continence Nurs 2023;50(2):107-14.
- Forman R, Zappas M, Lavell J. Dermatology in skin of color. Nurse Pract 2022;47(2):10-14.
- Khosla NN, Grullon K, Rosenblatt AE. Prevention of racialized medicine in pediatric dermatology: A call to re-examine skin tone typing. Pediatr Dermatol 2021;38(Suppl. 2):167–9.
- Del Bino S, Duval C, Bernard F. Clinical and biological character-ization of skin pigmentation diversity and its consequences on UV impact. Int J Mol Sci 2018;19(2668):1-44.
- Martin MK, Zaman T, Okello AM, Dennis LK. Validity of a self-assessment skin tone palette compared to a colorimeter for characterizing skin color for skin cancer research. Curr Oncol 2023;30:3189–200.
- Keuhni R. The early development of Munsell system. Color Res Appl 2002;27(1):20-7.
- Konishi N, Kawada A, Morimoto Y, et al. New approach to the evaluation of skin color of pigmentary lesions using Skin Tone Color Scale. J Dermatol 2007;34:441-6.
- Hannon L, DeFina R. Reliability concerns in measuring respondent skin tone by interviewer observation. Public Opin Quarterly 2016;80(2):534-41.
- McCreath HE, Bates-Jensen BM, Nakagami G, et al. Use of Munsell color charts to measure skin tone objectively in nursing home residents at risk for pressure ulcer development. J Adv Nurs 2016;72(9):2077-85.
- Munsell Color. (https://munsell.com/).
- Fitzpatrick TB. The validity and practicality of sun-reactive skin types I through VI. Arch Dermatol 1988;124(6):869-71.
- Gass KM, Dadzie OE, Dlova N, Petit A, Goad N. Skin diversity erythema description guidance. https://cdn.bad.org.uk/uploads/2022/02/29200007/Skin-diversity-descriptors-erythema-redness-guidance.pdf. Last accessed July 10, 2023.
- Moore Z, Patton D, Rhodes SL, O’Connor T. Subepidermal moisture (SEM) and bioimpedance: a literature review of a novel method for early detection of pressure-induced tissue damage (pressure ulcers). Int Wound J. Apr 2017;14(2):331-337.
- Baumgarten M, Margolis D, van Doorn C, et al. Black/White differences in pressure ulcer incidence in nursing home residents. J Am Geriatr Soc 2004;52(8):1293-8.
- Gefen A, Brienza DM, Cuddigan J, Haesler E, Kottner J. Our contemporary understanding of the aetiology of pressure ulcers/pressure injuries. Int Wound J 2022;19(3):692-704.
- Bryant RA, Moore ZE, Iyer V. Clinical profile of the SEM Scanner - modernizing pressure injury care pathways using sub-epidermal moisture (SEM) scanning. Expert Rev Med Devices 2021;18(9):833-47.
- World Health Organization. L89 Pressure Ulcers. In International Statistical Classification of Diseases and Related Health Problems; Tenth Revision. 2nd ed. Geneva: World Health Organization; 2020.
- Gershon S, Okonkwo H. Evaluating the sensitivity, specificity and clinical utility of algorithms of spatial variation in sub-epidermal moisture (SEM) for the diagnosis of deep and early-stage pressure-induced tissue damage. J Wound Care 2021;30(1):41-53.
- Chaboyer W, Coyer F, Harbeck E, et al. Oedema as a predictor of the incidence of new pressure injuries in adults in any care setting: a systematic review and meta-analysis. Int J Nurs Stud 2022;128:104189.
- Moore Z, McEvoy NL, Avsar P, et al. Measuring subepidermal moisture to detect early pressure ulcer development: a systematic review. J Wound Care 2022;31(8):634-47.
- Bates-Jensen BM, McCreath HE, Pongquan V. Subepidermal moisture is associated with early pressure ulcer damage in nursing home residents with dark skin tones: pilot findings. J Wound Ostomy Continence Nurs 2009;36(3):277-284.
- Bates-Jensen BM, McCreath HE, Nakagami G, Patlan A. Subepidermal moisture detection of heel pressure injury: The pressure ulcer detection study outcomes. Int Wound J 2018;15(2):297-309.
- Gefen A, Gershon S. An observational, prospective cohort pilot study to compare the use of subepidermal moisture measurements versus ultrasound and visual skin assessments for early detection of pressure injury. Ostomy Wound Manage 2018;64(9):12-27.
- Pittman J, Mulekar M. Enhanced skin assessment methodology to equitably detect early tissue damage and pressure injuries in adult patients in the acute care setting. Paper presented at NPIAP Annual Conference. 2023; San Diego, CA.
- European Pressure Ulcer Advisory Panel, National Pressure Injury Advisory Panel, Pan Pacific Pressure Injury Alliance. Skin and Tissue Assessment. In: Haesler E, ed. Prevention and Treatment of Pressure Ulcers/Pressure Injuries: Clinical Practice Guideline: The International Guideline 2019. European Pressure Ulcer Advisory Panel, National Pressure Injury Advisory Panel and Pan Pacific Pressure Injury Alliance; 2019:73-83.
- Simman R, Angel C. Early identification of deep-tissue pressure injury using long-wave infrared thermography: a blinded prospective cohort study. Adv Skin Wound Care 2022;35(2):95-101.
- Langemo D, Spahn J, Snodgrass L. Accuracy and reproducibility of the Wound Shape Measuring and Monitoring System. Adv Skin Wound Care 2015;28(7):317-23.
- Langemo D, Spahn JG. A multimodality imaging and software system for combining an anatomical and physiological assessment of skin and underlying tissue conditions. Adv Skin Wound Care 2016;29(4):155-63.
- Langemo DK, Spahn JG. A reliability study using a long-wave infrared thermography device to identify relative tissue temperature variations of the body surface and underlying tissue. Adv Skin Wound Care 2017;30(3):109-19.
- Langemo D, Spahn J, Spahn T, Chowdry Pinnamaneni V. Comparison of standardized clinical evaluation of wounds using ruler length by width and scout length by width measure and scout perimeter trace. Adv Skin Wound Care 2015;28(3):116-21.
- Cox J, Kaes L, Martinez M, Moles D. A prospective, observational study to assess the use of thermography to predict progression of discolored intact skin to necrosis among patients in skilled nursing facilities. Ostomy Wound Manage 2016;62(10):14-33.
- Koerner S, Adams D, Harper SL, Black JM, Langemo DK. Use of thermal imaging to identify deep-tissue pressure injury on admission reduces clinical and financial burdens of hospital-acquired pressure injuries. Adv Skin Wound Care 2019;32(7):312-20.
- European Pressure Ulcer Advisory Panel, National Pressure Injury Advisory Panel, Pan Pacific Pressure Injury Alliance. Future Research. In: Haesler E, ed. Prevention and Treatment of Pressure Ulcers/Pressure Injuries: Clinical Practice Guideline. The International Guideline. European Pressure Ulcer Advisory Panel, National Pressure Injury Advisory Panel, Pan Pacific Pressure Injury Alliance; 2019:373-7.
- Padula WV, Black JM. The Standardized Pressure Injury Prevention Protocol for improving nursing compliance with best practice guidelines. J Clin Nurs 2019;28(3-4):367-71.
- Clark M. Skin assessment in dark pigmented skin: a challenge in pressure ulcer prevention. Nurs Times 2010;106(30):16-7.
- Eming SA, Martin P, Tomic-Canic M. Wound repair and regeneration: mechanisms, signaling, and translation. Sci Transl Med 2014;6(265):265sr6.
- Ozgok Kangal MK, Regan JP. Wound Healing. StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing; 2022.
- Bliss DZ, Gurvich O, Savik K, et al. Are there racial-ethnic disparities in time to pressure ulcer development and pressure ulcer treatment in older adults after nursing home admission? J Aging Health 2015;27(4):571-93.
- Bliss DZ, Gurvich O, Savik K, et al. Racial and ethnic disparities in the healing of pressure ulcers present at nursing home admission. Arch Gerontol Geriatr 2017;72:187-94.
- U.S Department of Health and Human Services, Healthy People 2030. Social Determinants of Health. https://health.gov/healthypeople/priority-areas/social-determinants-health. Last accessed June 30, 2023.
- Kaiser Family Foundation. Key Facts on Health and Health Care by Race and Ethnicity. 2023. https://www.kff.org/report-section/key-facts-on-health-and-health-care-by-race-and-ethnicity-social-determinants-of-health/. Last accessed June 30, 2023.
- National Academies of Sciences, Engineering, and Medicine, Health and Medicine Division, Board on Population Health and Public Health Practice, Committee on Community-Based Solutions to Promote Health Equity in the United States, Baciu A, Negussie Y, Geller A, & Weinstein JN (Eds.). Communities in Action: Pathways to Health Equity. National Academies Press (US); 2017.
- Peter G Peterson Foundation. Wealth is an important component of Americans economic status. 2022. https://www.pgpf.org/blog/2022/11/income-and-wealth-in-the-united-states-an-overview-of-recent-data. Last accessed June 30, 2023.
- Sasson DC, Duan K, Patel SM, Junn A, Hsia HC. The impact of social determinants of health on pressure injury progression: a retrospective chart and scoping review. Adv Skin Wound Care 2023;36(2):106–11.
- Gangopadhyaya, A. Black Patients Are More Likely than White Patients to Be in Hospitals with Worse Patient Safety Outcomes. Robert Wood Johnson Foundation; 2021. https://www.urban.org/sites/default/files/publication/103925/black-patients-are-more-likely-than-white-patients-to-be-in-hospitals-with-worse-patient-safety-conditions_0.pdf. Last accessed June 30, 2023.
- Agency for Healthcare Quality and Research. National Healthcare Quality and Disparity Report, 2019. https://www.ahrq.gov/sites/default/files/wysiwyg/research/findings/nhqrdr/2019qdr-core-measures-disparities.pdf. Last accessed June 30, 2023.
- Oozageer Gunowa N, Brooke J, Hutchinson M, Jackson D. Embedding skin tone diversity into undergraduate nurse education: through the lens of pressure injury. J Clin Nurs 2020;29(21-22):4358-67.
- Matthew D. Just Medicine: A Cure for Racial Inequality in American Healthcare. NYU Press; 2015.
- Institute of Medicine. Unequal Treatment: Confronting Racial and Ethnic Disparities in Health Care. Committee On Understanding and Eliminating Racial and Ethnic Disparities in Health Care. Smedley B, Stith A, and Nelson A. (Eds). National Academies Press (US); 2003.
- Brenan, M. 2023. Nurses Retain Top Ethics Rating in U.S, But Below 2020 High. Gallop News; Jan 10, 2023. https://news.gallup.com/poll/467804/nurses-retain-top-ethics-rating-below-2020-high.aspx. Last accessed June 30, 2023.
- Pitman J, Black J. 2023 Gaps in professional education on skin assessment. National Pressure Injury Advisory Panel annual conference presentation: promoting equity in prevention and treatment of pressure injury. San Diego, March 2023.


