Ahead of Print
Topical phenytoin for wound healing: a narrative review
Mohammad S Qadirifard, Mohammad Qadirifard, Ghazal Tavakoli, Fariba A Mojeni, Seyede Z Mohagheghi, Seyyed K S Rafiei, Yasaman Salimi, Reza Taherinik, Farzad Sheikhzadeh, Neda Pakrou, Mohadeseh Poudineh, Kosar Gholami, Maryam Dianati, Hoda Mehrabi, Mobina Fathi, Niloofar Deravi
Keywords wounds, wound healing, review, phenytoin
For referencing Qadirifard MS et al. Topical phenytoin for wound healing: a narrative review. Wound Practice and Research 2024; 32(1):to be assigned.
DOI
10.33235/wpr.32.1.to be assigned
Submitted 21 April 2023
Accepted 13 December 2023
Abstract
Background In 1937, oral phenytoin medication was introduced as an anti-epileptic drug. For many years, researchers have shown interest in how topical phenytoin may be used to promote wound healing in various wounds.
Purpose This review article aims to summarise and critically appraise the clinical evidence available on the effects of topical phenytoin on wound healing.
Methods By using the keywords phenytoin, injuries, wound, wound care, and wound healing, we extracted articles published up to April 2022 through a search in PubMed, ISI Web of Science, SID, Google Scholar and Scopus.
Result We identified 17 studies performed on human subjects and eight on rats. Phenytoin effectiveness is also compared with other wound care substances, including tretinoin, silver sulfadiazine, EUSOL, honey, dexamethasone and hypericin.
Conclusion This review illustrated the significant effects and rare adverse events of topical phenytoin on wound healing.
Introduction
In 1937, oral phenytoin medication was introduced as an anti-epileptic drug1. Many clients who had been managed in this way presented with gingival hyperplasia2–4. This was discerned in about 20% of patients during chronic treatment with topical phenytoin due to increased manufacture of inflammatory fibroblasts, cytokines, growth factors and genetic susceptibility5. The apparent stimulatory effect of phenytoin on connective tissue has propounded the possibility of using it in wound healing6. Studies have shown that topical phenytoin promotes the healing of traumatic wounds, venous stasis, diabetic ulcers, decubitus ulcers, burns, leg ulcers, pyoderma gangrenosum ulcers, chronic wounds and leprosy trophic ulcers7–10. Different types of phenytoin are usable orally, by injection, and topically11–13. Intravenous phenytoin is often considered the drug of the first choice in benzodiazepine-resistant status in convulsive status epilepticus14. An intervention review by Hao et al15 assessed the effects of topical phenytoin on the rate of healing of pressure ulcers. Their data showed a need for further studies. Nevertheless, the topical form has a significant impact on healing wounds. This study therefore aimed to outline the healing effects of phenytoin on a variety of wounds.
Material and methods
This study aimed to gather and present information on the therapeutic potential of topical phenytoin on wound healing by covering original articles on this subject published up to December 2022. For this purpose, several databases such as Scopus, Google Scholar, Web of Science and PubMed were thoroughly searched. These keywords included phenytoin, injuries, wound, wound care, and wound healing.
A total of 364 studies were found. Of these, 36 articles were excluded since they evaluate gingival hyperplasia, and 72 were duplicates. Out of the 256 remaining articles, 172 were irrelevant. From 84 potentially relevant articles, 52 articles were excluded due to unsuitable study types. Finally, 26 articles were included in the narrative review. Some researchers used phenytoin in their study but did not give details on how phenytoin’s effect was measured on wound healing. Most papers failed to describe treatment allocation and randomisation in the trials (see Figure 1 regarding why these articles were excluded).
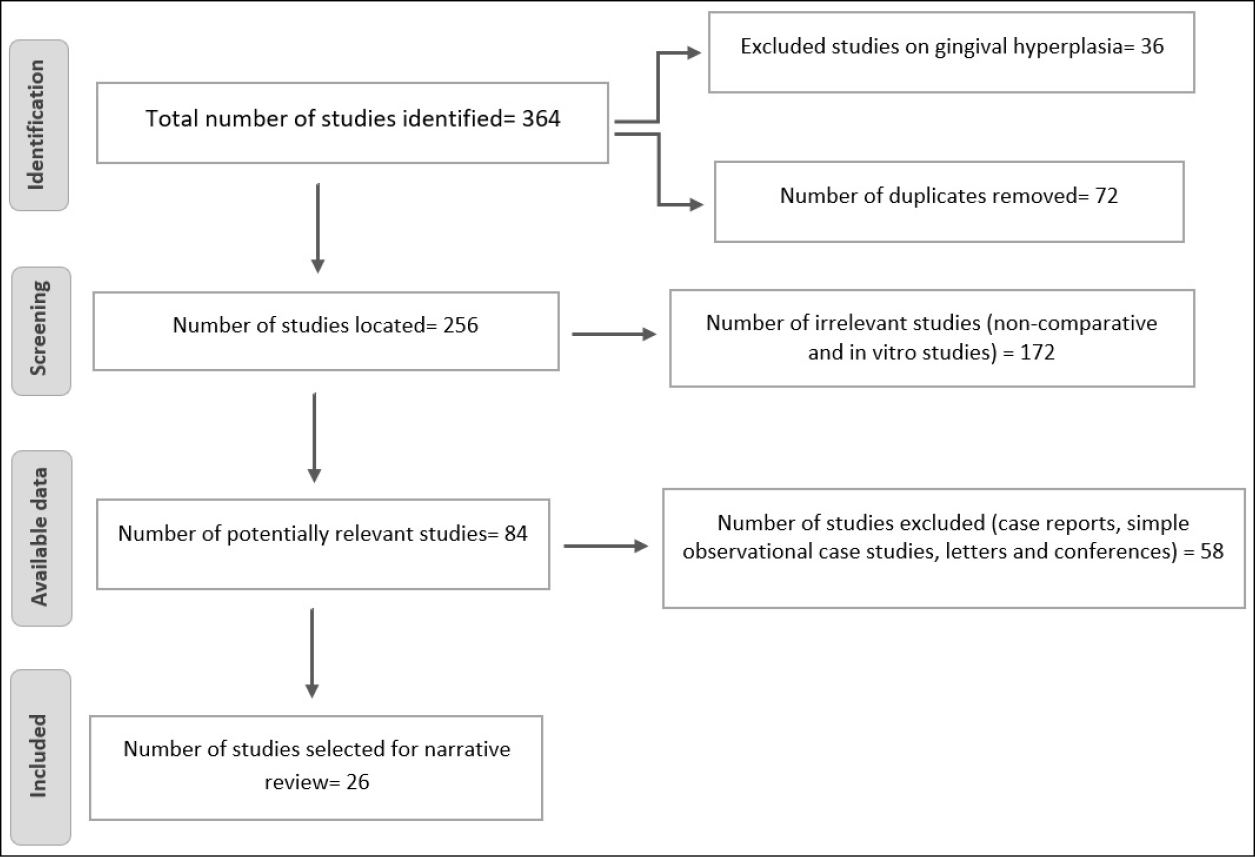
Figure 1. Number of papers located and selected on phenytoin therapy and wound healing
Studies performed on human and rat subjects (Tables 1 & 2) were included in the review. Knowing the effects of drugs on animals can give us a more comprehensive view of the drug mechanism, and it can be used in humans if it is safe for animals. Animal studies are better for finding the mechanism of the drug, and they improve our understanding of the science. Since animal studies have valuable data, we therefore included them in our study. Studies reported the effects of phenytoin in a variety of wound healing. In the mentioned articles, a description of any adverse events was rarely reported. Phenytoin has been compared with other wound care drugs in order to better evaluate its effect on different types of wounds.
The quality of both animal and human studies was assessed using the checklist from https://jbi.global/critical-appraisal-tools. Two authors assessed the quality of the studies using the mentioned checklist and, in the case of the conflicts, a third author made the final decision.
Results
The mechanism of phenytoin’s action
Phenytoin is a drug from the group of hydantoins that is used as an anticonvulsant drug. Because of the chemical effect on sodium and calcium channels in the nerve tissue of the brain, it inhibits them and prevents the generation of action potentials. One of the side effects of phenytoin that was observed after its long-term use was hyperplasia of patients’ gums which became the basis for further studies on the effect of phenytoin on wound healing8,9.
Wound healing has different stages, which include inflammation, re-epithelialisation, formation of granulation tissue, wound contraction and tissue regeneration, respectively, and these stages overlap. The restoration and re-strengthening of the skin and the size of the final scar depend on how these steps are performed. Fibroblasts and keratinocytes play an essential role in healing, but cell matrix, growth factors, cytokines and their receptors are also important30.
Based on the studies, it is stated that the use of phenytoin reduces pain and inflammation, and increases the speed of wound healing, but the mechanisms involved are not known8,30.
In general, phenytoin stimulates the connective tissue by increasing the activity of growth factors, mediators and various cytokines, stimulating the proliferation of fibroblasts and myofibroblasts, increasing the formation of granulation tissue, and stimulating the production of extracellular matrix and proteins such as collagen. As a result, more collagen is deposited, and the strength of the wound increases, thereby accelerating wound healing. On the other hand, phenytoin prevents collagen breakdown by reducing tissue collagenase activity8,30.
The increase in the activity of growth factors is mediated by increasing the gene expression of the platelet-derived growth factor beta chain in macrophages and monocytes. Also, phenytoin reduces oedema and wound secretion. It inhibits glucocorticoid activity and causes neovascularisation. Phenytoin reduces pain by stabilising the membrane. Moreover, with direct and indirect effects on inflammatory cells, it reduces the bacteria in the wound30.
Phenytoin effects on wound in different ways. It activates gp130-JAK-STAT3 pathway which leads to increased vascular endothelial growth factor (VEGF), matrix metalloproteinases (MMPs) and collagen level, and also increased NF-岱B and transforming growth factor beta (TGF-ß1). On balance, it leads to increased granulation tissue and decreased healing time, inflammation and wound and erythema size (Figures 2 & 3)33.
Cytochrome p450 isozyme found in epidermal tissue and skin appendages such as sebaceous glands and hair follicles can break down phenytoin. Absorption of topical phenytoin is related to wound fluid and its pH, and its effect on wound healing is related to its dose8.
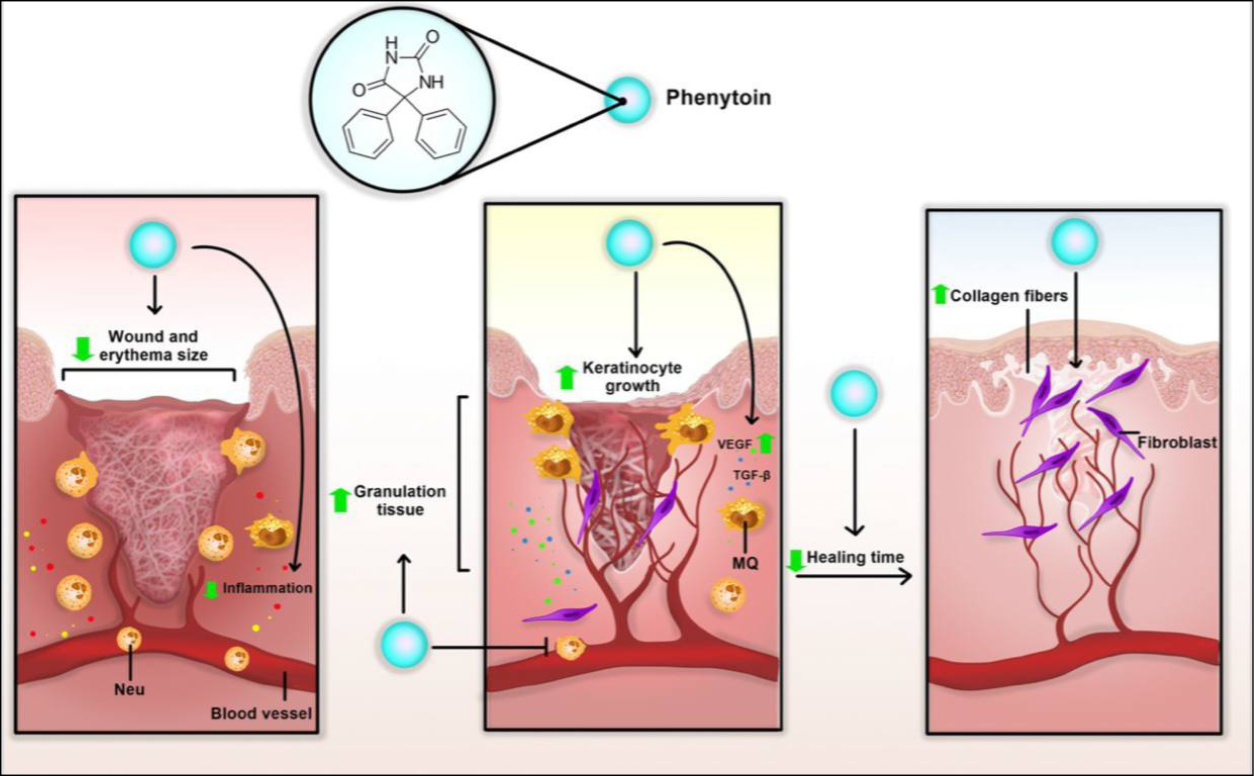
Figure 2. Potential mechanism of topical phenytoin in wound healing. Topical phenytoin can reduce inflammation, wound and erythema size, and healing time. Phenytoin can also induce granulation tissue, keratinocyte growth and collagen fibre, accelerating wound healing procedure
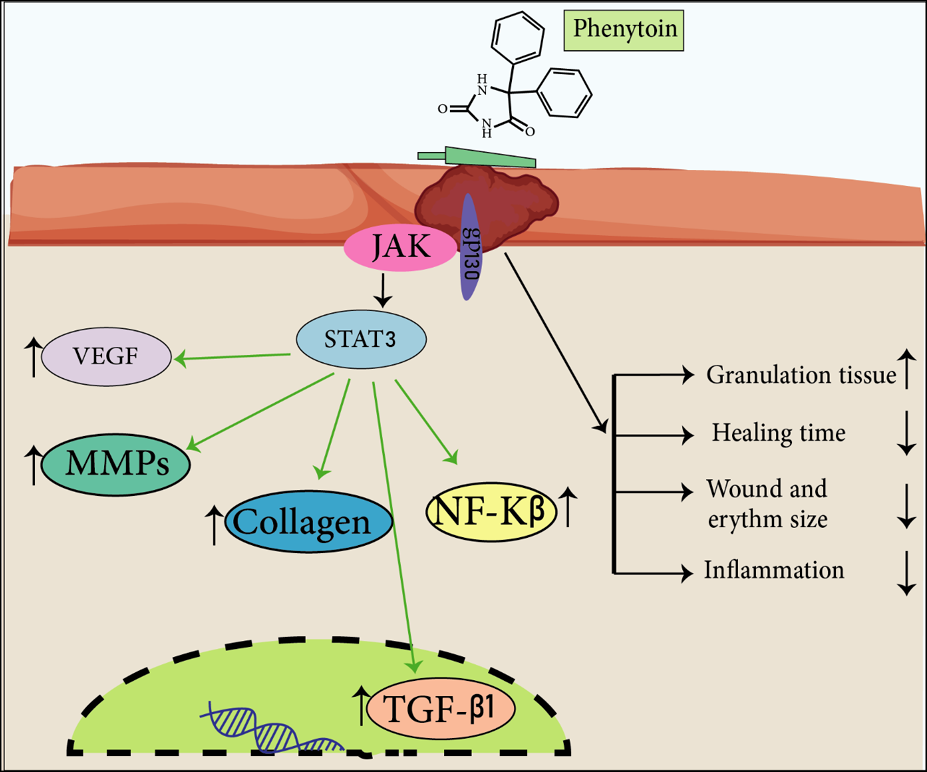
Figure 3. Phenytoin effects wounds in different ways. It activates a gp130-JAK-STAT3 pathway which leads to increase VEGF, MMPs, collagen level and also increases NF-岱B and TGF-ß1. On balance, it leads to an increase in granulation tissue and a decrease in healing time, inflammation and wound and erythema size.
Tretinoin versus phenytoin
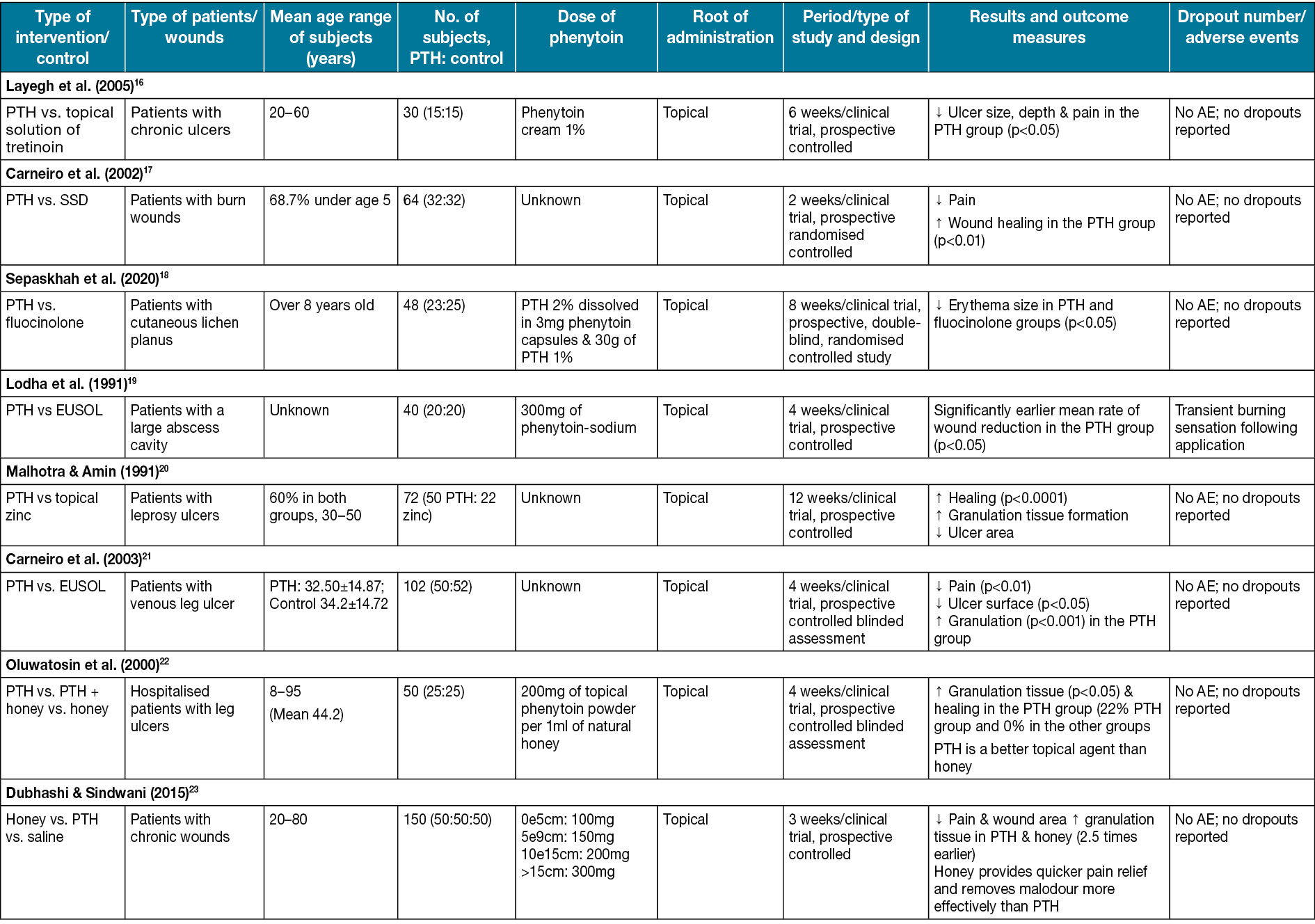
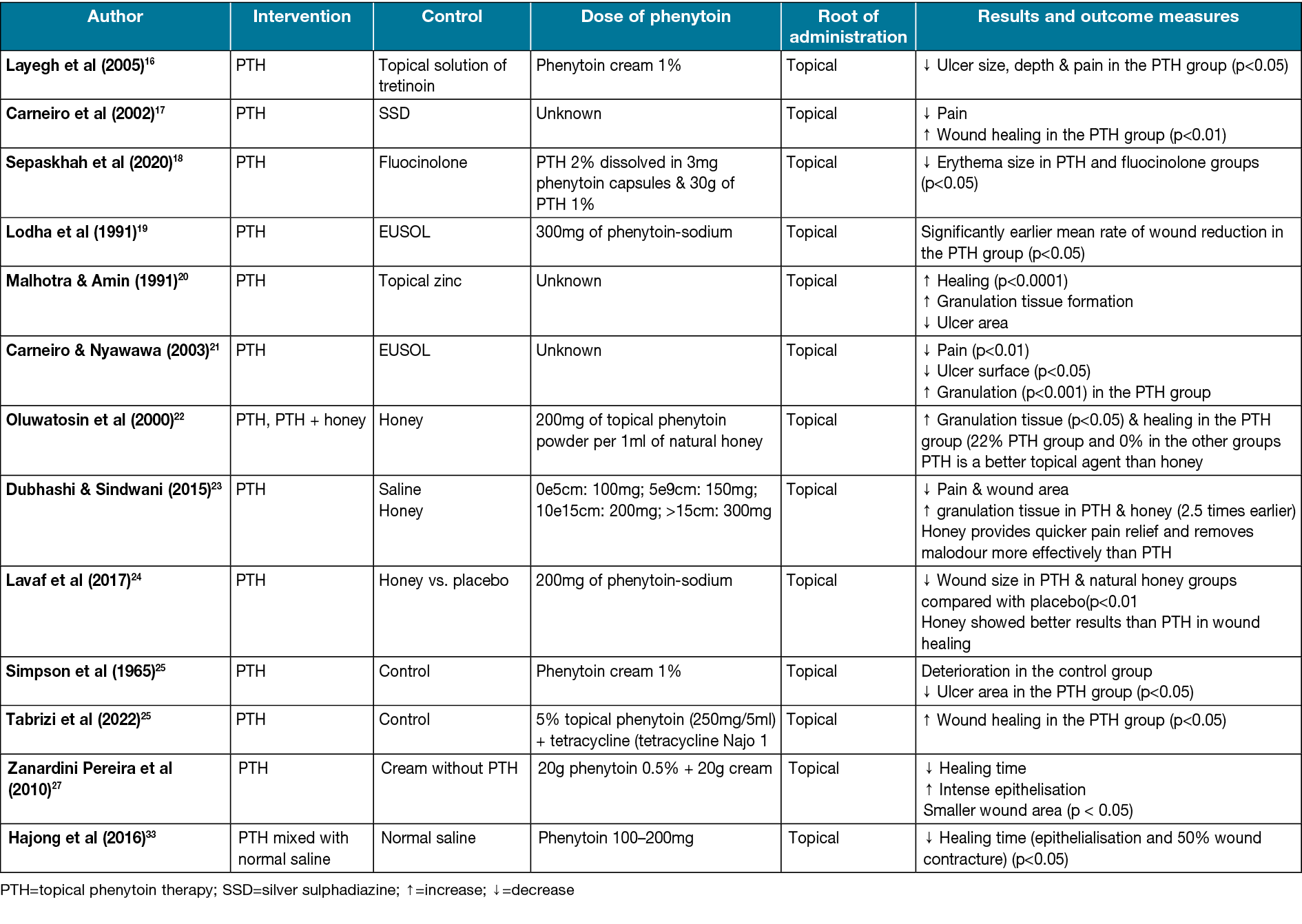
Topical tretinoin has been found to stimulate epithelial cells and improve healing markedly. It also has been used in the improvement of photoaged skin disease for a long time, and using it on wounds has shown significant efficiency36. Studies have shown that tretinoin treatment increases granulation tissue, new collagen formation, and new vascular tissue in 1–4 weeks37. Layegh et al16 compared phenytoin and tretinoin effects on the ulcers of human subjects. Out of a total of 15 patients, 18 ulcers were treated with 1% topical phenytoin and 19 ulcers were treated with 0.05% tretinoin. The phenytoin group represented a decrease in ulcer surface area. Before using phenytoin, 44.4% of ulcers were without pain; however, after introducing phenytoin, 100% of ulcers had no pain. After 6 weeks of treatment with phenytoin, the ulcer size, depth and pain reduction were significant (p<0.05) (Table 1).
Table 1. Characteristics of studies on human subjects
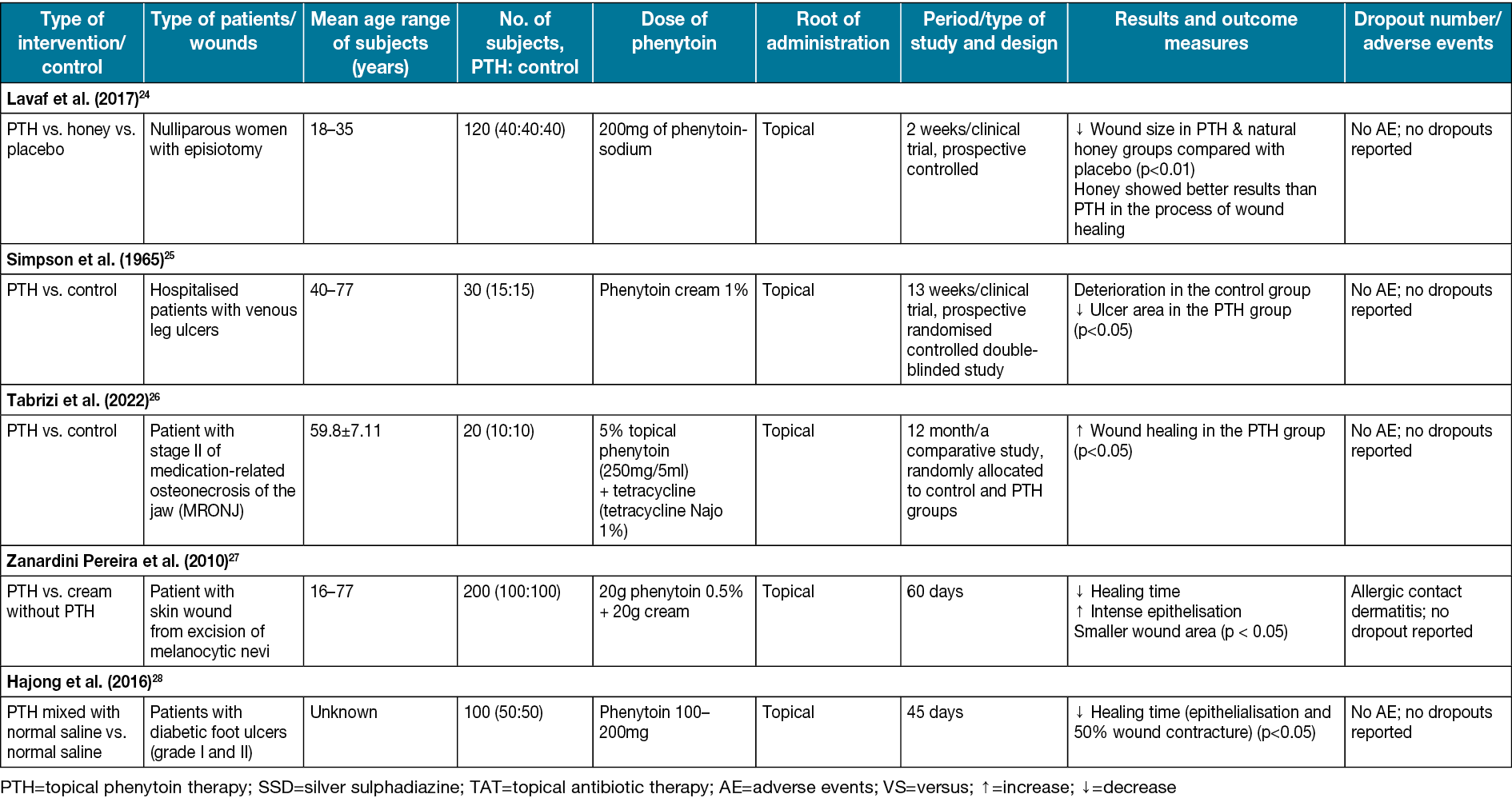
Silver sulfadiazine versus phenytoin
Silver sulfadiazine is a topical anti-bacterial substance that incorporates the anti-bacterial effects of both sulfadiazine and silver39. It helps wound healing, especially burns, through its steady reactions with serum and other body fluids40. Carneiro et al17 compared phenytoin powder and silver sulfadiazine effects on burn wounds in the human body. After the treatment, 78% of patients in the phenytoin group, compared to 47% in the silver sulfadiazine group, had no pain in the wound area. The differences were statically significant (p<0.01). In the trial, 20% of patients in the silver sulfadiazine group (p<0.05) did not heal well and changed their treatment to phenytoin; within 48 hours of hospitalisation the wounds healed (Table 1).
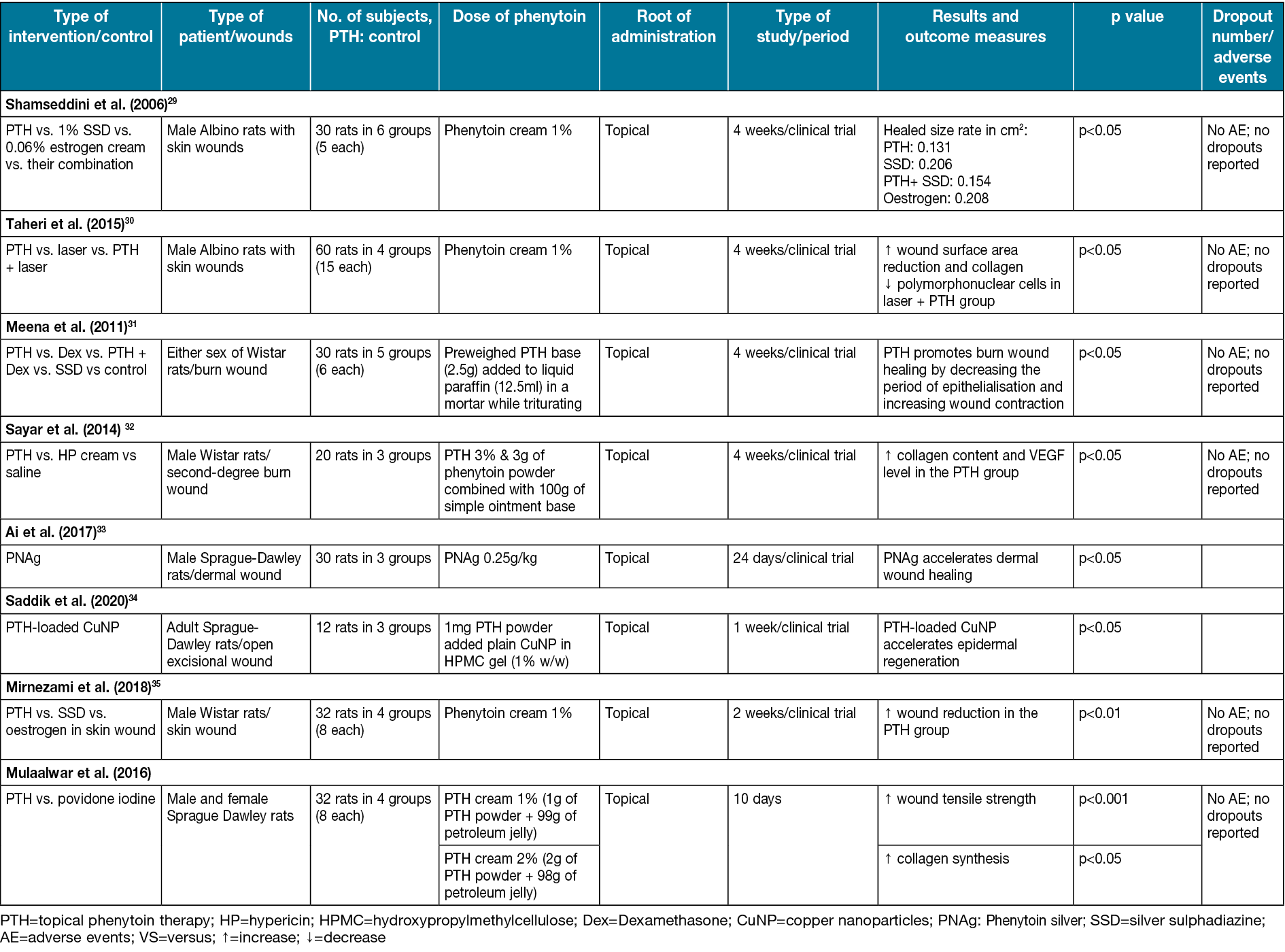
Mirnezami et al35 compared phenytoin cream and silver sulfadiazine on rats with single circular (4mm in diameter) full-thickness skin wounds. The healing duration was 10 days in the phenytoin group and 7.62 days in the silver sulfadiazine group. Wound healing in the phenytoin group was significantly earlier compared to other groups (p<0.01) (Table 2). According to Shamseddini et al29, using phenytoin cream 1% in combination with silver sulfadiazine cream 1% on rat wounds (2cm in diameter) can significantly reduce wound size (p<0.05) (Table 2).
Table 2. Characteristics of studies on rat subjects are included.
EUSOL versus phenytoin
One of the common dressing materials that have been used for decades is EUSOL (Edinburgh University solution of lime), which has the property of generating tissue granulation41. Carneiro & Nyawawa21 compared phenytoin and EUSOL effects on non-malignant chronic leg ulcers. The formation of healthy granulation tissue and clearance of ulcers were significant (0.05%); the mean surface area (cm2) in the EUSOL group (58.7±18.06) and the phenytoin group (66.5±22.01) significantly decreased after 28 days of treatment (p<0.05) (Table 1). A high methodological quality double-blinded trial also on leg ulcers conducted by Simpson et al25 (Table 1) shows notable ulcer area healing in the phenytoin group. Lodha et al19 studied the comparison of topical phenytoin and EUSOL effects in the healing of large abscess cavities. On day 30, the percentage of reduction of the wound in the phenytoin group was 99.7% and, by using phenytoin, the mean rate of reduction was 2.02cm2/day, which was significantly earlier compared to EUSOL with 1.58cm2/day (p<0.05) (Table 1).
Honey versus phenytoin
Natural honey has been used to treat a variety of wounds throughout the ages. It is hygroscopic, and it also can draw moisture out of the environment. This dehydrates bacteria, and its sugar content is also high enough to hinder the growth of microbes. Therefore, it inhibits the growth of different bacterial species that cause wound infections42–47. A study conducted by Lavaf et al24 showed a comparison between using natural honey and phenytoin on the 7th and 14th days of the postpartum period in nulliparous women with episiotomy wounds; 30% of wound reduction in the phenytoin group and 29% in the honey group compared to 8% in the placebo group was observed after 14 days, which was significant (p=0.011). The study’s results represented that although both topical phenytoin 1% and natural honey were effective in episiotomy wound reduction, honey showed better results in the process of wound healing. However, neither of them could reduce the pain (Table 1).
Dubhashi and Sindwani23 studied phenytoin, natural honey and saline effects on chronic wounds. The study revealed that the percentage reduction of wounds using phenytoin 1% is approximately 15.8% which is close to the honey effects (with a 20.6% reduction). However, honey provides quicker pain relief and removes malodour more effectively. Moreover, phenytoin, in comparison to saline effects (with 8.07 reduction), is significantly more practical (p<0.0001) (Table 1). Oluwatosin et al22 compared phenytoin, natural honey, and their mixture effects on chronic leg ulcers of different sizes (166–262mm2) in 4 weeks of treatment duration. In the 4th week, a significant difference was observed (p=0.04). The mixture of honey and phenytoin reduced 85.7mm2 of the ulcer area compared to phenytoin (19.4mm2) and honey (45.1mm2) reduction. Thus, they suggested that phenytoin may be a more effective topical agent than honey in treating chronic ulcers (Table 1).
Dexamethasone versus phenytoin
Dexamethasone is a robust anti-inflammatory glucocorticoid used in skin allografts and organ transplantation48. The dexamethasone treatments strongly interfere with the synthesis of collagen49. Meena et al31 compared using phenytoin, dexamethasone and a combination of them on burn wounds in different groups of rats. The phenytoin group had the highest collagen production. The mean period of epithelisation and wound contraction was approximately 20% better in comparison with other groups (p<0.05) (Table 2).
Hypericin versus phenytoin
Hypericin (HP) is a phytochemical with significant anti-inflammatory activity50. However, its lipophilicity limits its therapeutic applications. The topical form of HP can promote re-epithelialisation in burn wounds and shorten the healing time for superficial burn wounds51. VEGF is a platelet-derived growth factor. Its essential role is to stimulate angiogenesis and endothelial cell migration in wound healing52. Sayar et al32 compared HP with phenytoin effects on burn wounds in rats. Using phenytoin in the treatment significantly promoted VEGF production in the wound regions greater than HP and the control group (p<0.05) (Table 2).
Zinc versus phenytoin
Zinc is an essential element in the human body, and its deficiency can lead to delayed wound healing53,54. According to the study by Malhotra and Amin20, which compared zinc and phenytoin on leprosy ulcers, phenytoin reduced ulcer area and increased granulation tissue formation compared to zinc (p<0.0001) and also demonstrated better results than zinc oxide cream in the healing process of chronic trophic leprosy ulcers.
Phenytoin conjugated with silver and copper nanoparticles
Silver nanoparticles have been used for wound healing due to their antimicrobial and anti-inflammatory effects. In a study by Nasr et al, silver interacts with the bacterial cell wall and blocks its respiratory pathway. Silver’s anti-inflammatory function decreases the level of some inflammatory mediators such as IL-6 mRNA55. In another study, the effects of silver nanoparticles on wound healing in a rabbit model were examined. By evaluating the wound healing process, such as no signs of sepsis in wound closure, longer newly formed epithelium, and thicker granulation, it was concluded that silver nanoparticles could be used in wound healing instead of systemic antibiotics56. By changing fibroblasts into myofibroblasts and improving keratinocyte migration and proliferation, silver nanoparticles accelerate the healing process of diabetic wounds and make them tighter. Silver nanoparticles also avoid severe cellular damage by reducing reactive oxygen species (ROS) through the modulation of cytokine production58.
Ai et al33 studied the pharmacological action of phenytoin silver. They postulated that regulating the expression levels of collagen I, NF-岱B, TGF-ß, MMP-2 and MMP-9 asynchronously promote fibroblast cells (NIH-3T3) and epidermal cells (HaCat) proliferation by regulating Jak/Stat3 pathway; hence phenytoin silver can be used as an effective compound for wound healing. Silver nano compounds promote wound healing by organising fibroblast cells, collagen fibres and blood vessels33. Another beneficial trait of silver nanoparticles being used in wound healing is their low toxicity and low concentration in blood, which was indicated in many studies. However, Nasr et al have made a contrary declaration about silver nanoparticle toxicity. They noted that decreasing mitochondrial function can have toxic effects on human fibroblasts and keratinocytes, which are concentration-dependent55.
Another nano compound used for wound healing is copper nanoparticles. Due to copper nanoparticles’ trait, an antimicrobial, anti-inflammatory and angiogenesis-enhancing function, it seems to be a good choice for wound healing. By co-factoring enzymes involved in antioxidant processes such as superoxide dismutase and cytochrome oxidase, copper nanoparticles showed antioxidant function. It also has immune boosting activity by stimulating the production of IL-2 and, through induction of VEGF, enhances angiogenesis55. Copper metabolism in phenytoin therapy was tested by Palm and Hallmans58. They found that the level of serum copper was increased by phenytoin therapy, so they claimed that phenytoin might affect the absorption and accumulation of copper, which causes a high level of copper in the blood58. Phenytoin-loaded copper increases the expression of dermal procollagen type 1 and decreases the expression of the inflammatory Jak3, which causes accelerated epidermal regeneration and stimulates tissue formation34.
Laser versus phenytoin
In a study on the treatment of excisional wounds made on the body of rats, Taheri et al30 compared using a low-level diode laser, topical phenytoin, and a combination of them (Table 1). The results indicated no significant difference between laser and phenytoin effects in composing vessels and dense infiltration of lymphocytes. However, more collagen and faster reduction in polymorphonuclear cells in phenytoin and simultaneous laser use were observed, which was statistically significant (p<0.05).
Cream versus phenytoin
A study was conducted by Pereira et al; its purpose was to investigate the effect of phenytoin 0.5 versus cream and the possibility of its better therapeutic and cosmetic results in wound healing caused by the removal of the melanocytic mole. In the obtained results, faster recovery and more intense epithelialisation were observed in the treatment with phenytoin, and the final scar was often smaller, round and flat27.
Povidone iodine versus phenytoin
In the results of Malkalwar et al’s animal study comparing the effect of phenytoin and povidone-iodine, it was found that the measured tensile strength of the wound in patients treated with phenytoin is comparatively higher than the group treated with povidone-iodine. Moreover, as a result, topical phenytoin accelerates the wound healing process in the incision wound model59.
Phenytoin effect on diabetic foot ulcers
A study by Hajong et al28 was conducted with the aim to investigate the effect of topical phenytoin on the healing of grade I and II diabetic foot ulcers. In this study, it was found that the average wound epithelialisation time and, as a result, the wound healing time, is less when taking phenytoin. This action takes place with the following mechanisms – increased proliferation of fibroblasts, increased granulation, increased collagen deposition, decreased collagenase activity, neo-angiogenesis, and decreased bacterial contamination of the wound28.
Phenytoin effect on decubitus wound
A study on the efficacy of topical phenytoin on decubitus wound healing in the sacral region of a motionless patient with a stroke was conducted by Pitiakoudis et al60. It was found that phenytoin increases wound healing by stimulating lymphocyte chemotaxis and increasing the regulation of angiogenesis. Their study showed that phenytoin benefits as decubitus wound healing and helps the healing process on several levels60.
Hypothesis about phenytoin in wound healing
In a hypothesis, Namazi states the possible effect of phenytoin on wound healing61. According to this hypothesis, due to the inhibitory effect of phenytoin on norepinephrine release, cell mediated immunity and monoamine oxidase activity, as well as its potential capability to fixate the melanosome membrane and motivate melanocytes, phenytoin may be effective against vitiligo. Due to the facilitation of collagen deposition and the inhibition of collagenase activity by phenytoin, its simultaneous topical use with steroids may prevent steroid-induced skin atrophy and, at the same time, enhance the anti-vitiligo effect of these agents61.
Finally, Table 3, summarises the different topical forms of phenytoin used versus the comparison product.
Table 3. Comparison of phenytoin usage and outcomes with control substances
Discussion
This review sought current evidence on phenytoin and its effectiveness in wound healing. The results showed that the availability of methodological study designs made it nearly possible to analyse the data or extract conclusions about the effectiveness of topical phenytoin in the healing of wounds.
Studies have demonstrated that phenytoin is a non-sedative anticonvulsant with local pain relief and anti-inflammatory function due to its membrane-stabilising action62. It can depress repetitive neuronal activities and synaptic transmission selectively21. Kumar et al63 reported that phenytoin elevates the expression of platelet-derived growth factor-ß in macrophages and monocytes, which leads to increased neovascularisation. In many studies, it has been concluded that phenytoin increases epidermal growth factor (EGF), a VEGF that elevates the number of blood vessels, and TGF-ß1, which stimulates the deposition of collagen and fibrosis development and causes re-epithelialisation. Increasing the number of follicles and shortening the telogen phase can help improve hair growth in alopecia. By suppressing voltage-gated sodium channels, phenytoin can control seizures12.
A large number of studies noted that phenytoin fortifies the arrangement of granulation tissue, fibroblast multiplication (which clarifies the change in granulation tissue arrangement), tolerance of tissue versatility, restraint of glucocorticoid generation, new collagen arrangement, increment in blood vessels, reduction of collagenase movement, and diminishing tissue oedema38,64. Phenytoin also is known to have an anti-bacterial effect, especially against Staphylococcus aureus, Escherichia coli, Klebsiella and Pseudomonas species32,63. The anti-bacterial action of phenytoin depends on local changes in pH and the improvement of the local circulation19,21. Wounds treated with phenytoin had more negative cultures than the other substances used for wound healing63. Phenytoin also reduces pain caused by superficial burns and chronic leg ulcers. It also can reduce neuropathic pain and heal leprosy ulcers through faster ulcer volume reduction.
Phenytoin can treat abscess cavities by reducing oedema and inflammation and earlier separation of the slough. It also can inhibit swelling through decreasing oedema, and burn wounds through increasing VEGF and TGF-ß expression. It can heal nasal wounds through increasing EGF and proliferating cell nuclear antigen (PCNA) and CD3111,19,31,64,65. EGF is a mitogenic dose-dependent agent that affects increased epithelial cell proliferation and reduced healing time. CD31 is involved in increased angiogenesis65.
The results of this study showed that debridement, along with local administration of phenytoin and tetracycline, improved the healing process and the recurrence rate after treatment in medication‑related osteonecrosis of the jaw (MRONJ) patients26. Considering that the wounds of diabetics have a prolonged inflammatory phase, phenytoin is beneficial for people with diabetes due to its anti-inflammatory action. It is also suggested that phenytoin caused increased granulation tissue, decreased wound exudate, and lowered bacterial load within the diabetic wound bed8,63. Moreover, phenytoin has been influential in treating leprosy wounds, including a reduction in ulcer area and an increase in healing rate8,10.
Pressure ulcers are localised injuries in the underlying tissue, skin or both. It is usually the result of pressure on a bony prominence or accompanied by a shear. Pressure ulcers are a costly, painful and widespread healthcare problem. Some studies show that they are unsure whether topical phenytoin can improve ulcer healing in people with grade I and II pressure ulcers. Phenytoin has been shown to reduce the use of analgesics due to the alleviation of pain and decreases hospitalisation period and hospital admission costs17,21.
Despite phenytoin’s benefit, it has some side effects like gingival hyperplasia and hypertrophic granulation tissue, which can be avoided by stopping the treatment, hypertrichosis in long-term phenytoin therapy, and inhibition of potassium channels and initial burning sensation that can be omitted by using sodium-free phenytoin12,21,63,66.
While most studies and research suggest enhanced and accelerated wound healing using topical phenytoin, there are a few contrary reports. One study showed no significant differences in wound contraction between the control and phenytoin groups68. Another study declared that phenytoin did not improve wound healing, and there was slower epithelialisation and even fewer fibroblasts in the phenytoin-treated group. Taheri et al30 recommend that a combination of phenytoin and laser would have better results and make more collagen fibres. Given phenytoin’s pain-relieving and anti-bacterial properties in moving forward wound mending, as well as its accessibility, low cost, ease of utilising and precise security properties, it can be utilised as a treatment methodology for diverse sorts of wounds63.
Conclusion
The present review summarised and critically appraised the clinical evidence available on the effects of topical phenytoin on wound healing. Through assessment of the comparative trials in this area, an estimation of the improvement level between the phenytoin-treated groups compared with controls was mentioned. Based on the positive growing evidence, phenytoin had a better effect on wound healing than tretinoin, silver sulfadiazine, EUSOL, dexamethasone, HP, saline, zinc, common dressings and cold cream. However, further studies are needed to compare the effects of phenytoin versus honey, fluocinonide and laser on wound healing.
Acknowledgements
We appreciate all included papers’ authors.
Conflict of interest
The authors declare no conflicts of interest.
Ethics statement
An ethics statement is not applicable.
Funding
The authors received no funding for this study.
Author contribution
Study concept and design: ND; acquisition of data: MSQ, MQ, GT, FA, SZM, SKR; drafting of the manuscript: MSQ, MQ, GT, FA, SZM, SKR, YS, RT, FS, NP, MP, KG, MD, HM, MF; critical revision of the manuscript for important intellectual content: ND, MF; study supervision: ND. All authors read and approved the final manuscript.
Author(s)
Mohammad S Qadirifard1,2, Mohammad Qadirifard3*, Ghazal Tavakoli4, Fariba A Mojeni5, Seyede Z Mohagheghi6, Seyyed K S Rafiei7, Yasaman Salimi8, Reza Taherinik4, Farzad Sheikhzadeh4, Neda Pakrou9, Mohadeseh Poudineh10, Kosar Gholami11, Maryam Dianati12, Hoda Mehrabi13, Mobina Fathi7, Niloofar Deravi7*
1Department of Nursing and Midwifery, Islamic Azad University, Tehran, Iran
2Department of Nursing, Garmsar Branch, Islamic Azad University, Garmsar, Iran
3Postgraduate student, Department of Prosthodontics, Faculty of Dentistry, Shahed University, Tehran, Iran
4Student Research Committee, School of Medicine, Iran University of Medical Sciences, Tehran, Iran
5Student Research Committee, School of Medicine, Mazandaran University of Medical Sciences, Sari, Iran
6School of Medicine, Iran University of Medical Sciences, Tehran, Iran
7Student Research Committee, School of Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran
8Student Research Committee, Kermanshah University of Medical Sciences, Kermanshah, Iran
9School of Medicine, Urmia University of Medical Sciences, Tehran, Iran
10School of Medicine, Zanjan University of Medical Sciences, Zanjan, Iran
11Student Research Committee, Semnan University of Medical Sciences, Semnan, Iran
12Student Research Committee, Rafsanjan University of Medical Sciences, Rafsanjan, Iran
13Student Research Committee, School of Medicine, Arak University of Medical Sciences, Arak, Iran
*Corresponding author email mohammad.qadirifard@shahed.ac.ir and niloofarderavi@sbmu.ac.ir
References
Merritt HH, Putnam TJ. Sodium diphenyl hydantoinate in the treatment of convulsive disorders. J Am Med Assoc 1938;111(12):1068–73.
Talas G, Brown RA, McGrouther DA. Role of phenytoin in wound healing – a wound pharmacology perspective. Biochem Pharmaco 1999;57(10):1085–94.
Brown RS, Beaver WT, Bottomley WK. On the mechanism of drug-induced gingival hyperplasia. J Oral Pathol Med 1991;20(5):201–9.
Bonnaure-Mallet M, Tricot-Doleux S, Godeau G. Changes in extracellular matrix macromolecules in human gingiva after treatment with drugs inducing gingival overgrowth. Arch Oral Biol 1995;40(5):393–400.
Arya R, Gulati S. Phenytoin-induced gingival overgrowth. Acta Neurolog Scand 2012;125(3):149–55.
Vyhlídalová D, Kozáková R, Zeleníková R. Management of non-healing wounds with honey dressings: a literature review. Central Eur J Nurs Midwif 2018;9(3):880–8.
Bhatia A, Prakash S. Topical phenytoin for wound healing. Dermatol Online J 2004;10(1).
Shaw J, Hughes CM, Lagan KM, Bell PM. The clinical effect of topical phenytoin on wound healing: a systematic review. Br J Dermatol 2007;157(5):997–1004.
Firmino F, Pereira de Almeida AM, de Jesus Grijó e Silva R, da Silva Alves G, da Silva Grandeiro D, Garcia Penna LH. Scientific production on the applicability of phenytoin in wound healing. Rev Esc Enferm USP 2014;48(1):166–73.
Bhatia A, Nanda S, Gupta U, Gupta S, Reddy BS. Topical phenytoin suspension and normal saline in the treatment of leprosy trophic ulcers: a randomized, double-blind, comparative study. J Dermatolog Treat 2004;15(5):321–7.
Kopsky DJ, Hesselink JMK. Topical phenytoin for the treatment of neuropathic pain. J Pain Res 2017;10:469.
Onaolapo A, Adebayo A, Onaolapo O. Oral phenytoin protects against experimental cyclophosphamide-chemotherapy induced hair loss. Pathophysiol 2018;25(1):31–9.
Tate R, Rubin LM, Krajewski KC. Treatment of refractory trigeminal neuralgia with intravenous phenytoin. Am J Health-System Pharm 2011;68(21):2059–61.
Yasiry Z, Shorvon SD. The relative effectiveness of five antiepileptic drugs in treatment of benzodiazepine-resistant convulsive status epilepticus: a meta-analysis of published studies. Seizure 2014;23(3):167–74.
Hao XY, Li HL, Su H, Cai H, Guo TK, Liu R, et al. Topical phenytoin for treating pressure ulcers. Cochrane Database System Rev 2017(2).
Layegh P, Yazdanpanah MJ, Amouzgar M, Sarraf F. Efficacy of 0.05% topical solution of tretinoin on healing of chronic ulcers and comparison of it with 1% topical cream of phenytoin. Med J Mashad Uni Med Sci 2005;48(89):321–328.
Carneiro P, Rwanyuma L, Mkony C. A comparison of topical phenytoin with silverex in the treatment of superficial dermal burn wounds. Cent Afr J Med 2002 Sep–Oct;48(9–10):105–8.
Sepaskhah M, Boroujeni NH, Javaheri M, Bagheri Z. Comparison of therapeutic efficacy of topical treatment with phenytoin and fluocinolone on cutaneous lichen planus: a randomized, double-blind trial. Dermatolog Therapy 2020;33(4):e13578.
Lodha S, Lohiya M, Vyas M, Bhandari S, Goyal R, Harsh M. Role of phenytoin in healing of large abscess cavities. J Br Surg 1991;78(1):105–8.
Malhotra Y, Amin S. Role of topical phenytoin in trophic ulcers of leprosy in India. Int J Leprosy Other Mycobact Dis 1991;59(2):337–8.
Carneiro PM, Nyawawa ET. Topical phenytoin versus EUSOL in the treatment of non-malignant chronic leg ulcers. East Afr Med J 2003;80(3):124–9.
Oluwatosin O, Olabanji J, Oluwatosin O, Tijani L, Onyechi H. A comparison of topical honey and phenytoin in the treatment of chronic leg ulcers. Afr J Med Med Sci 2000;29(1):31–4.
Dubhashi SP, Sindwani RD. A comparative study of honey and phenytoin dressings for chronic wounds. Indian J Surg 2015;77(3):1209–13.
Lavaf M, Simbar M, Mojab F, Majd HA, Samimi M. Comparison of honey and phenytoin (PHT) cream effects on intensity of pain and episiotomy wound healing in nulliparous women. J Complement Integrative Med 2018;15(1).
Simpson G, Kunz E, Slafta J. Use of sodium diphenylhydantoin in treatment of leg ulcers. New York State J Med 1965;65:886–8.
Tabrizi R, Khiabani K, Shafiei S, Nosrati G, Moslemi H. Can topical phenytoin combined with tetracycline enhance the healing process in medication-related osteonecrosis of jaw? A comparative study. Nat J Maxillofacial Surg 2022;13(2):195.
Zanardini Pereira CA, de Oliveira de A Alchorne A. Assessment of the effect of phenytoin on cutaneous healing from excision of melanocytic nevi on the face and on the back. BMC Dermatol 2010;10(1):1–7.
Hajong R, Naku N, Hajong D, Anand M, Lenish K, Singh NM. Effect of topical phenytoin on wound healing. Group 2016;1(50):17.36.
Shamseddini S, Yavar Zadeh M, Shamseddini A. Comparison of the healing effects of topical phenytoin, estrogen and silver sulfadiazine on skin wounds in male rats. Iran J Dermatol 2006;8(6):482–8.
Taheri JB, Bagheri F, Mojahedi M, Shamloo N, Nakhostin MR, Azimi S, et al. Comparison of the effect of low-level laser and phenytoin therapy on skin wound healing in rats. J Lasers Med Sci 2015;6(3):124.
Meena K, Mohan A, Sharath B, Somayaji S, Bairy K. Effect of topical phenytoin on burn wound healing in rats. Indian J Exp Biol 2011 Jan;49(1):56–9.
Sayar H, Gergerlioglu N, Seringec N, Ozturk P, Bulbuloglu E, Karabay G. Comparison of efficacy of topical phenytoin with hypericin in second-degree burn wound healing: an experimental study in rats. Med Sci Monitor Basic Res 2014;20:36.
Ai X-y, Liu H-j, Lu C, Liang C-l, Sun Y, Chen S, et al. Phenytoin silver: a new nanocompound for promoting dermal wound healing via comprehensive pharmacological action. Theranostic 2017;7(2):425.
Saddik MS, Alsharif FM, El-Mokhtar MA, Al-Hakkani MF, El-Mahdy MM, Farghaly HS, et al. Biosynthesis, characterization, and wound-healing activity of phenytoin-loaded copper nanoparticles. AAPS PharmSciTech 2020;21(5):1–12.
Mirnezami M, Rahimi H, Fakhar HE, Rezaei K. The role of topical estrogen, phenytoin, and silver sulfadiazine in time to wound healing in rats. Ostomy/Wound Manage 2018;64(8):30–4.
Liu YS, Li TS, Sun CK, Wei KC, Liu CJ. The application of phenytoin in the treatment of diabetic ulcers. Int Wound J 2016;13(5):1077.
Cucé LC, Bertino MC, Scattone L, Birkenhauer MC. Tretinoin peeling. Dermat Surg 2001;27(1):12–4.
Paquette D, Badiavas E, Falanga V. Short-contact topical tretinoin therapy to stimulate granulation tissue in chronic wounds. J Am Acad Dermatol 2001;45(3):382–6.
Fisher NM, Marsh E, Lazova R. Scar-localized argyria secondary to silver sulfadiazine cream. J Am Acad Dermatol 2003;49(4):730–2.
Fox Jr CL, Modak SM. Mechanism of silver sulfadiazine action on burn wound infections. Antimicrob Agents Chemother 1974;5(6):582–8.
Ugane SP, Kalbagwar SK, Kurane SB. A clinical study of Fournier’s gangrene and use of honey dressing in treatment. Int J Res Med Sci 2018;6(3):932–6.
Molan P, Betts J. Clinical usage of honey as a wound dressing: an update. J Wound Care 2004;13(9):353–6.
Blair S, Carter D. The potential for honey in the management of wounds and infection. Aust Infect Cont 2005;10(1):24–31.
Molan PC. Potential of honey in the treatment of wounds and burns. Am J Clin Dermatol 2001;2(1):13–9.
Molan PC. The evidence supporting the use of honey as a wound dressing. Int J Lower Extrem Wounds 2006;5(1):40–54.
Molan PC. The role of honey in the management of wounds. J Wound Care 1999;8(8):415–8.
Molan PC. Re-introducing honey in the management of wounds and ulcers-theory and practice. Ostomy Wound Manage 2002 Nov;48(11):28–40.
Tripathi K. Corticosteroids in essentials of medical pharmacology. New Delhi, India: Jaypee; 1999.
Oishi Y, Fu Z, Ohnuki Y, Kato H, Noguchi T. Molecular basis of the alteration in skin collagen metabolism in response to in vivo dexamethasone treatment: effects on the synthesis of collagen type I and III, collagenase, and tissue inhibitors of metalloproteinases. Br J Dermatol 2002;147(5):859–68.
Wölfle U, Seelinger G, Schempp CM. Topical application of St. John’s wort (Hypericum perforatum). Planta Medica 2014;80(02/03):109–20.
Nafee N, Youssef A, El-Gowelli H, Asem H, Kandil S. Antibiotic-free nanotherapeutics: hypericin nanoparticles thereof for improved in vitro and in vivo antimicrobial photodynamic therapy and wound healing. Int J Pharmaceut 2013;454(1):249–58.
Bao P, Kodra A, Tomic-Canic M, Golinko MS, Ehrlich HP, Brem H. The role of vascular endothelial growth factor in wound healing. J Surg Res 2009;153(2):347–58.
Prasad AS. Zinc: an overview. Nutrition 1995;11(1 Suppl):93–9.
Jones PW, Williams DR. The use and role of zinc and its compounds in wound healing. Metal Ions Biolog Syst 2004;41:139–84.
Nasr M, El-Gogary RI, Abd-Allah H, Abdel-Mottaleb M. Chapter 4: Nanoparticulate systems for wound healing. In: Shegokar R, editor. Nanopharmaceuticals. Elsevier; 2020. p. 73–90.
Amer SA, Nouh SR, Elkammar MH, Shalaby TI, Korittum AS. Silver nanoparticles preparation and their effect on full-thickness skin wound healing in rabbit model. Alexandria J Vet Sci 2018;57(2).
Sabarees G, Velmurugan V, Tamilarasi GP, Alagarsamy V, Raja Solomon V. Recent advances in silver nanoparticles containing nanofibers for chronic wound management. Polymers 2022;14(19):3994.
Palm R, Hallmans G. Zinc and copper metabolism in phenytoin therapy. Epilepsia 1982;23(5):453–61.
Mulkalwar S, Behera L, Golande P, Shah A. Evaluation of wound healing activity of topical phenytoin in an incision wound model in rats. Indian J Pharm Pharmacol 2016;3(2):75–78.
Pitiakoudis M, Giatromanolaki A, Iliopoulos I, Tsaroucha A, Simopoulos C, Piperidou C. Phenytoin-induced lymphocytic chemotaxis, angiogenesis and accelerated healing of decubitus ulcer in a patient with stroke. J Int Med Res 2004;32(2):201–5.
Namazi M. Phenytoin as a novel anti-vitiligo weapon. J Autoimmune Dis 2005;2(1):1–4.
Hasamnis AA, Mohanty BK, Patil S. Evaluation of wound healing effect of topical phenytoin on excisional wound in albino rats. J Young Pharmacist 2010;2(1):59–62.
Kumar CS, Vasudeva N, Rao DV, Naidu CRA. Outcomes of topical phenytoin in the management of traumatic wounds. J Clin Orthopaed Trauma 2021;13:116–21.
Bansal NK. Comparison of topical phenytoin with normal saline in the treatment of chronic trophic ulcers in leprosy. Int J Dermatol 1993;32(3):210–3.
Şimşek G, Ciftci O, Karadag N, Karatas E, Kizilay A. Effects of topical phenytoin on nasal wound healing after mechanical trauma: an experimental study. Laryngoscope 2014;124(12):E449–E54.
Brown RS, Arany PR. Mechanism of drug-induced gingival overgrowth revisited: a unifying hypothesis. Oral Dis 2015;21(1):e51–61.
Pai M, Sitaraman N, Kotian M. Topical phenytoin in diabetic ulcers: a double blind controlled trial. Ind J Med Sci 2001;55(11):593–9.


