Volume 25 Number 1
Topical negative pressure therapy and compression in the management of venous leg ulcers: a pilot study
Edward Wang, Robert Tang, Nicole Walsh, Lucy Stopher, Chrianna Bharat, Stefan Ponosh and Shirley Jansen
Keywords Venous, ulcer, negative, pressure, compression.
Abstract
Objective: Venous leg ulceration (VLU) is a common condition associated with significant patient morbidity. This prospective case series investigated the use of PICO® topical negative pressure therapy (TNPT) in addition to graduated compression therapy for VLU.
Methods: Patients with VLU were recruited from a specialist vascular surgery clinic and treated with the novel combination of TNPT (PICO) underneath graduated compression therapy.
Results: Twelve patients (15 VLUs) were treated over a median of 20 days (7–42). Median surface area reduced from 2.1 cm2 (1.1–5.8) to 0.8 cm2 (0.2–3.2) (p=0.022), and depth from 3.0 mm (1.8–3.3) to 0 mm (0–0) (p=0.005 Wilcoxon Signed-Rank Test). Time to heal was compared to a historical cohort with similar characteristics that was treated with compression therapy alone. The addition of TNPT was found to reduce time to heal from 6.3 to 4.3 weeks (p=0.024).
Conclusion: This pilot study supports the benefit of combining TNPT (PICO) with compression bandaging to accelerate VLU healing.
Introduction
Chronic venous insufficiency with ulceration is common and impacts significantly on patient quality of life. Contemporary treatment costs in Australia are estimated at $2 billion annually1. Once alternative contributing factors have been eliminated, the current standard of care for venous leg ulcers (VLU) is four-layer compression bandaging (CB) and wound bed care2-4. The average time to heal with four-layer CB is 6.3 weeks5, with 74% of ulcers healed at 12 weeks5 if instituted and supervised by trained personnel. Topical negative pressure therapy (TNPT), since its development in the late 1980s6, has been used successfully in the management of chronic wounds of varying aetiology7. The published literature on TNPT in the clinical setting is flawed by significant heterogeneity in wound aetiology. The exudative nature of VLUs lends itself to management with TNPT. V.A.C.® has been used previously on VLUs underneath compression and was found to produce rapid granulation of the VLU wound bed8. The V.A.C. device, however, is bulky and can impair patient mobility. PICO® offers TNPT with a smaller more portable device than V.A.C., which can allow normal patient mobility and community-based treatment9. This study aimed to assess the use of PICO9 TNPT underneath compression in the treatment of VLU and its effect on time to granulation and time to epithelialisation.
Methods
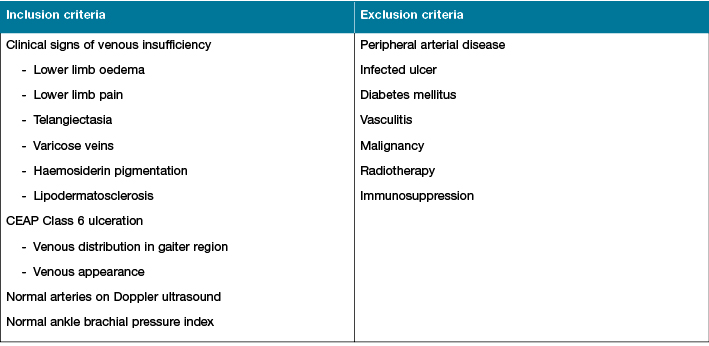
This study was approved by the Human Research Ethics Committee at Sir Charles Gairdner Hospital. Patients were recruited from the Vascular Surgery Specialist Foot and Leg Ulcer Clinic at Sir Charles Gairdner Hospital; a quaternary teaching hospital in Western Australia. Patients were selected if they displayed clinical evidence of chronic venous insufficiency with CEAP class 6 ulceration as detailed in Table 110. VLUs were diagnosed based on the presence of venous ulcer characteristics; location in the gaiter region, ulcer appearance, edges, shape and the presence of other venous disease-associated signs including dependent lower limb oedema, haemosiderin pigmentation and lipodermatosclerosis. Selection also mandated the absence of arterial disease, confirmed by a normal ankle brachial pressure index or peripheral arterial duplex ultrasound examination. Patients with infected ulcers, diabetes mellitus, vasculitis, malignancy, radiotherapy, immunosuppression and peripheral arterial disease were excluded.
Table 1: Inclusion and exclusion criteria
Patients that were recruited for this study were treated with PICO9 TNPT applied by a wound care clinical nurse specialist underneath compression therapy. PICO TNPT dressings were applied directly to the VLU wound bed, without additional foam, in accordance with the product instructions for use. Follow-up dressings were completed by trained community nurses who reapplied the TNPT dressings and compression bandaging two times a week. The TNPT suction canisters were changed once a week, dictated by battery life, and patients were followed up by a wound care nurse specialist every two weeks. TNPT was applied until there was complete ulcer bed coverage with granulation tissue with no residual depth to the VLU tissue defect. Once TNPT treatment was removed, compression therapy was continued until complete wound epithelialisation. Compression therapy varied between four-layer compression and compression hosiery, dictated by patient tolerability, preference and where the patient lived; remote patients who could not access appropriately trained staff for four-layer CB were treated with compression stockings. Compression therapy was applied day and night and changes made two times a week. Remote patients were treated via telehealth.
VLU measurements of length, width and depth were taken using acetate tracing planimetry at follow-up, every two weeks by wound care nurse specialists. Duration of TNPT, time to VLU granulation, time to VLU epithelialisation and adverse events with TNPT were also documented and medical photography taken at these appointments.
Statistical analysis was completed using the R Environment for statistical computing11. Surface area of all ulcers was approximated using the following formula for the area of an ellipse: area = (width x length x π)/412. The non-parametric data from this study was analysed using Wilcoxon-Signed Rank tests.
Results
Twelve patients with 15 VLU met the study inclusion criteria without exclusions and were treated with TNPT and compression from 2014 to 2015. The median age of all participants was 70 years with a range from 47 to 98 years. Of the participants, six were male and six female. Combined TNPT and compression was applied for a median of 20 days (eight–42). Compression therapy was guided by patient tolerability, preference and access to specialist nurses trained in the application of compression. Four patients were treated with four-layer CB, five were treated with three-layer CB and three wore class II compression stockings. Six patients completed TNPT treatment but were lost to follow-up prior to complete epithelialisation. As such, no date of ulcer healing data was available for these patients. There were no adverse events from PICO in this pilot study. Only one ulcer was seen to have increased in size.
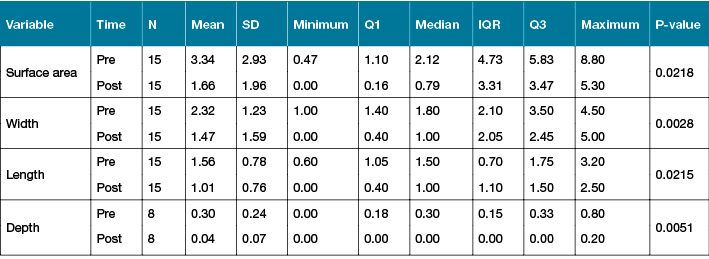
VLU surface area decreased significantly with the combination of TNPT and compression from a median of 2.1 cm2 (1.1–5.8) to 0.8 cm2 (0.2–3.2) by Wilcoxon Signed-Rank Test (p=0.022). Likewise a reduction in ulcer depth was seen with a pre-treatment median of 3.0 mm (1.8–3.3) reducing to 0.0 mm (0.0–0.0) (p=0.005). Similarly, reductions in raw planimetry measurements were seen: length decreased from 1.5 cm (1.1–1.7) to 1.0 cm (0.4–1.5) (p=0.021) whilst width decreased from 1.8 cm (1.4–3.5) to 1.0 cm (0.4–2.4) (p=0.003). Summary statistics including mean, standard deviation (SD), median, interquartile range (IQR), minimum and maximum, are provided for all continuous variables in Table 2.
Table 2: Summary statistics for surface area and ulcer depth pre- and post-treatment
Representative plots of the therapy-related reductions in VLU size are illustrated in Figure 1.
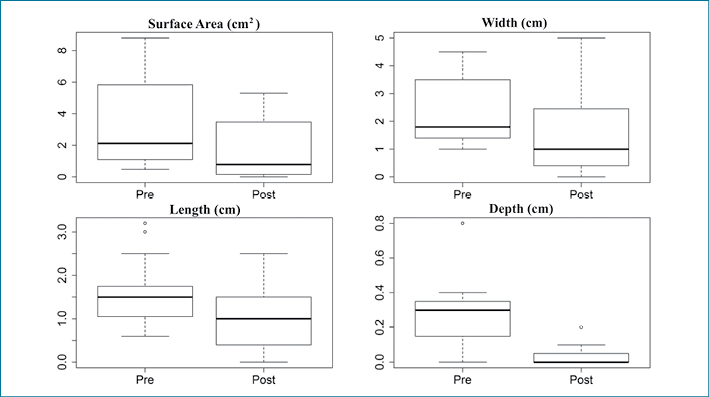
Figure 1: Boxplots of surface area and ulcer depth both pre- and post-treatment
In terms of rate of healing with combined TNPT-compression therapy, mean time to granulation in all ulcers was found to be 2.8 weeks (SD 1.5). The mean linear healing rate was 1.03 cm2/week (SD 1.06) and the percentage of VLUs healed at 12 weeks was 73%. Although time to granulation was recorded in all patients, time to complete epithelialisation could only be recorded in 50% of patients, therefore time to heal for the other patients was extrapolated from linear healing rate and ulcer surface area. Estimated mean time to heal was 4.3 weeks (SD 2.5). Blair et al. reported a historical cohort of VLUs managed with four-layer CB5 with similar patient demographics to our study; selection at a tertiary vascular clinic, comparable mean age at 71, equal gender representation, proven venous leg ulcers (gaiter region with normal ankle brachial pressure index) and treatment with four-layer CB. In comparison to Blair et al. (n=148)5 this study found a shorter time to heal by two weeks: mean time to heal reported in Blair et al. was 6.3 weeks (SD 0.6) compared with 4.3 weeks (SD 2.4) in this study (p = 0.024 Two proportions test)5.
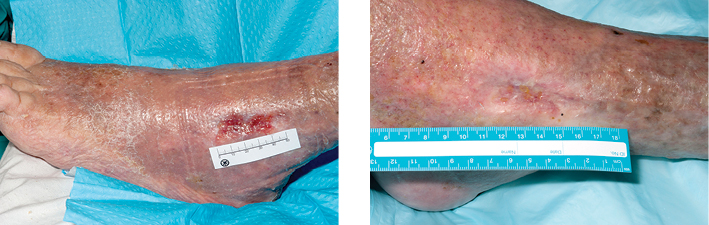
Figure 2: Pre- and post-treatment
Discussion
VLUs are common, costly and debilitating13-15. They affect up to 3% of people over 60 and 5% over 80 years old13. Australians aged over 65 years currently account for 14.2% of the population, but will account for 25% by 205614 and a corresponding rise in VLUs can be anticipated. Therefore, it is important that there is continual research into improving the treatment of VLUs. Reducing VLU time-to-heal will improve patients’ ulcer pain, physical and social functioning and allow quicker return to activity. Decreased VLU healing time will also reduce clinic and nursing time, number of in-patient admissions, length of stay and ultimately health-related cost.
Our case series investigated the novel use of TNPT in conjunction with compression5, using a small, portable TNPT device (PICO)9. Our results suggest that combined PICO TNPT underneath compression reduces surface area and depth of VLU. However, VLU management with compression is accepted practice, and the surface area reduction seen in our study does not demonstrate the specific benefit of combined TNPT-compression therapy. Unfortunately, our study lacked a compression-only control group. Therefore we used a comparable historical cohort treated with four-layer CB alone to determine if an additional advantage was seen with TNPT. In comparing time to VLU healing with this historical cohort, our study found a shorter time to heal by two weeks; mean time to heal reported by Blair et al. was 6.3 weeks (SD 0.6)5 compared with 4.3 weeks (SD 2.4) in our study (p = 0.024 Two proportions test). At 12 weeks Blair et al. found CB had healed 74% of VLU5, whilst our study had a similar healed proportion (73%). This may represent the recognised proportion of VLU that are refractory despite compression treatment. It must be noted that conclusions drawn from the comparison with Blair et al.5 are limited by our incomplete time to heal data. There is also a disparity in VLU size between our study and Blair et al., 2.12 cm2 and 15.4 cm2 respectively5. We also acknowledge the potential for bias in comparing with historical cohorts, selection bias in our recruitment from a tertiary centre, small sample size and that our selected VLU were small, which was limited by the TNPT device dimensions. Nonetheless, these findings suggest that the treatment combination of TNPT and CB shows promise and may accelerate ulcer healing.
In assessing our study’s significance in the context of the available published literature, TNPT with compression therapy for VLU has only been assessed in one case series previously8. Kieser et al.8 was a small case series (n = 7) that used the larger V.A.C. device underneath compression and demonstrated similarly promising results with rapid progression in percentage of granulation from 30.8% (±38.6%) to 100% in the first 48 hours of TNPT8. The reported time to granulation in Kieser et al. was remarkably shorter than the 2.8 weeks in our study. This may be the result of a difference in the definition for time to granulation; our study looked at time to complete wound bed granulation with no residual depth to the VLU defect, whilst Kieser et al. assessed percentage of wound bed granulation, irrespective of depth. Kieser et al. also reported promising early reductions in surface area; however, these did not reach significance8.
There is little scientific understanding of how negative pressure therapy affects a wound other than by removal of exudate16,17. Current theories on the molecular and cellular effects of TNPT involve fibroblast mechanoreceptor and chemoreceptor mediated changes16. These cellular signalling processes trigger angiogenesis, extracellular matrix remodelling and deposition of granulation tissue16, but further study is required to elucidate the specific mechanisms involved. It could be hypothesised that these local wound effects, triggered by TNPT, act synergistically with the correction of the abnormal venous haemodynamics by compression to accelerate healing16-19. Furthermore, by using the smaller, portable device, PICO8, patients can be more mobile and stimulate calf venous muscle pump function, further contributing to the apparent success of this treatment combination.
A randomised controlled trial is under way comparing TNPT and compression to compression alone, using sophisticated laser ulcer surface area and depth photographic mapping equipment (Silhouette®, Aranz Medical). This trial will measure health-related quality of life, healing time and cost, to further investigate the role that combination TNPT-compression should play in venous ulcer treatment.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
Declarations
None.
Acknowledgements
Mr David Lewis, Consultant Vascular and Endovascular Surgeon, Department of Vascular and Endovascular Surgery, Royal Infirmary of Edinburgh
Author(s)
Edward Wang*
Department of Vascular and Endovascular Surgery, Sir Charles Gairdner Hospital, Hospital Avenue, Nedlands, 6009 WA, Australia.
Email Edward.Wang@health.wa.gov.au
Robert Tang
Vascular and Endovascular Surgery Department,
Sir Charles Gairdner Hospital, Perth, WA, Australia
Nicole Walsh
Vascular and Endovascular Surgery Department,
Sir Charles Gairdner Hospital, Perth, WA, Australia
Lucy Stopher
Vascular and Endovascular Surgery Department,
Sir Charles Gairdner Hospital, Perth, WA, Australia
Chrianna Bharat
University of Western Australia, Perth, WA, Australia
Stefan Ponosh
Vascular and Endovascular Surgery Department,
Sir Charles Gairdner Hospital, Perth, WA, Australia
Shirley Jansen
Vascular and Endovascular Surgery Department,
Sir Charles Gairdner Hospital, Perth, WA, Australia
University of Western Australia, Perth, WA, Australia
School of Population Health, Curtin University, Perth, WA, Australia
* Corresponding author
References
- Australian Wound Management Association, New Zealand Wound Care Society. Australian and New Zealand clinical practice guideline for prevention and management of venous leg ulcers. Osborne Park: Cambridge Publishing; 2011. Available at www.awma.com.au/publications/2011_awma_vlug.pdf [Accessed 1 Jul 2016].
- O’Meara S, Cullum N, Nelson EA, Dumville JC. Compression for venous leg ulcers. Cochrane Database Syst Rev [internet] 2012 [cited 2016 Jul 1]. Available from: http://onlinelibrary.wiley.com.qelibresources.health.wa.gov.au/doi/10.1002/14651858.CD000265.pub3/full.
- Barwell JR, Davies CE, Deacon J, Harvey K, Minor J et al. Comparison of surgery and compression with compression alone in chronic venous ulceration (ESCHAR study): randomised controlled trial. Lancet 2004;363(9424):1854–9.
- O’Brien JF, Grace PA, Perry IJ, Hannigan A, Clarke Moloney M et al. Randomized clinical trial and economic analysis of four-layer compression bandaging for venous ulcers. Brit J Surg 2003;90(7):794–8.
- Blair SD, Wright DDI, Backhouse CM, Riddle E, McCollum CN. Sustained compression and healing of chronic venous ulcers. Brit Med J 1988;297(6657):1159–61.
- Argenta LC, Morykwas MJ. Vacuum-assisted closure — a new method for wound control and treatment: clinical experience. Ann Plas Surg 1997;38(6):563–76.
- Ubbink DT, Westerbos SJ, Evans D, Land L, Vermeulen H. Topical negative pressure for treating chronic wounds. Cochrane Database Syst Rev [internet] 2008 [cited 2015 Jul 1]. Available from: http://onlinelibrary.wiley.com.qelibresources.health.wa.gov.au/doi/10.1002/14651858.CD001898.pub2/full.
- Kieser DC, Roake JA, Hammond C, Lewis DR. Negative pressure wound therapy as an adjunct to compression for healing chronic venous ulcers. J Wound Care 2011;20(1):35–7.
- Dowsett C, Grothier L, Henderson V, Leak K, Milne J et al. Venous leg ulcer management: single use negative pressure wound therapy. Br J Community Nurs 2013;S6:8–10.
- Eklof B, Rutherford RB, Bergan JJ, Carpentier PH, Gloviczki P et al. Revision of the CEAP classification for chronic venous disorders: consensus statement. J Vasc Surg 2004;40(6):1248–52.
- R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria; 2015. URL http://ww/R-project.org/
- Plassman P. Measuring wounds. J Wound Care 1995;4(6):269–272.
- Van Korlaar I, Vossen C, Rosendaal F, Cameron L, Bovill E et al. Quality of life in venous disease. Thromb Haemost 2003;90(1):27–35.
- Australian Bureau of Statistics. Australian demographic statistics (No 3101.0). 2012; Retrieved from: http://www.ausstats.abs.gov.au/ausstats/subscriber.nsf/0/A535F84F4393C62FCA257AD7000D113E/$File/31010_jun%202012.pdf.
- Simon DA, Dix FP, McCollum CN. Management of venous leg ulcers. Br Med J 2004;328(7452):1358–62.
- Glass GE, Murphy GF, Esmaeili A, Lai LM, Nanchahal J. Systematic review of molecular mechanism of action of negative-pressure wound therapy. Brit J Surg 2014;101(13):1627–36.
- Chen SZ, Li J, Li XY, Xu LS. Effects of vacuum-assisted closure on wound microcirculation: an experimental study. Asian J Surg 2005;28(3):211–7.
- Khashram M, Huggan P, Ikram R, Chambers S, Roake J et al. Effect of TNP on the microbiology of venous leg ulcers: a pilot study. J Wound Care 2009;18(4):164–7.
- Khashram M, Roake JA, Lewis DR. The effect of vacuum-assisted closure on the tissue oxygenation of venous ulcers: a pilot study. Wounds 2009;21(9):249–53.



