Volume 25 Number 2
Causes and consideration with chronic wounds: a narrative review of the evidence
Gabrielle Munro
Keywords chronic wound management, debridement and methods, wound pain and comfort, topical analgesics, wound care economics.
Abstract
Background: Chronic wounds are those that fail to move past the inflammatory phase of wound healing in a timely manner. Chronic wounds vary in type and cause and all may present challenges for the clinician, client and the health system. The evidence for current clinical practice needs review without fear or favour to consider how we can improve wound healing.
Aim: To assess the current literature on the management and evaluations of non-healing wounds.
Methods: The electronic databases CINAHL, Medline on Ovid and Cochrane Library were searched for publications from 2005 to 2015, in English on the keywords and MeSH headings including: chronic wound management, debridement and methods, wound pain and comfort, topical analgesics and wound care economics.
Results: Much of the evidence in wound care reports small study sizes, varied methodologies and outcome measures. There is a lack of good-quality evidence to support the most cost-effective method which would minimise psychological effects on the clients. There is insufficient evidence to support which dressing or debridement method should be used. Many of the studies compared different wound types and different treatment options.
Conclusion: There is a critical need for evidence to support efficacy in basic wound care with emphasis in wound bed preparation to promote wound closure with minimal psychological impact on clients.
Introduction
Wound healing closure involves a sequence of phases spanning haemostasis, inflammation, proliferation and maturation. Wounds can fail to progress through these stages in a timely manner. This results in “the chronic wound”, causing delayed wound healing1-6.
When managing chronic wounds, the role for clinicians is to consider the physiological, psychological and economic impact and implement a plan of care to address these key areas. The physiological aspect of the wound considers the preparation of the wound bed to assist to promote the best chance of healing7-9. The psychological and social effects of delayed healing impinge on the overall wellbeing of the patient. Pain, when present, is recognised as a major impact on quality of life7-11. The economic burden affects both the individual and the health system12-14.
A review of the current published literature from CINAHL, Medline on Ovid and Cochrane Library databases searching evidence on assessment and management of the chronic wound with emphasis on debridement, non-healing wounds, wound pain and comfort, topical analgesics and wound care economics highlighted several causes and considerations.
Clinicians are required to utilise their clinical judgement, patient preference and evidence to make critical, cost-effective decisions to provide quality care to patients15-18. The literature reports multiple barriers why this may not occur, including a lack of: knowledge of the available evidence, confidence to perform, knowledge of their scope of practice, mentoring and guidance by more experienced senior clinicians, accountability and outcome measures, organisational culture and change management. In addition, limitations include wrongful interpretation of the evidence and the dearth of a culture of inquiry within the discipline of nursing3,6,9,13,15,16,19,20.
There are numerous international and national guidelines, consensus, and evidence-based practice recommendations in the speciality of wounds21. These guidelines are often related to individual wound types and modalities such as compression, moist wound healing, pressure offloading or interventions such as debridement.
Chronic wounds are frequently classified into the following categories of wounds: pressure injuries; lower limb ulcers; venous, arterial, mixed or lymphoedema, diabetic foot or neuropathic wounds; palliative or malignant wounds; chronic or dehisced surgical wounds. Figure 1 is an example of a chronic wound classified as lower limb ulcers: venous and lymphoedema.

Figure 1: Lower leg ulcers: venous/lymphoedema
Several terms are synonymous with wound causes, including types, mechanism of injury or aetiology and clinicians struggle with which of these to focus on. Regardless of the term used, the primary focus should be the physiological aspect of the wound and imbedding the T.I.M.E principles into practice, making it the gold standard for all wounds and the modalities to improve impairment in that individual client. In Figure 2, the cause of the wound was traumatic, the aetiology was venous leg ulcer. Healing of this wound required the focus to be wound bed preparation and the aetiology which needed compression to heal. It was the lack of wound bed preparation and the delay in identifying local impairment of circulation that delayed healing. In Figure 3 the wound is a pressure injury. Healing of this wound required the focus to be wound bed preparation and the cause requiring the redistribution of pressure to heal.
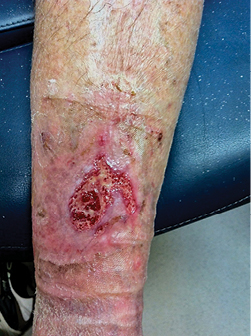
Figure 2: Traumatic wound/venous insufficiency
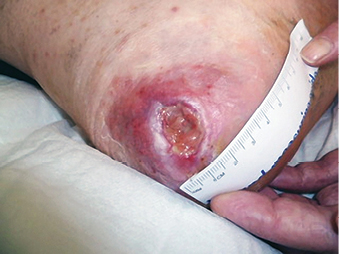
Figure 3: Pressure injury
Physiological Concerns
There are many other causative factors affecting wound care specific to an aetiology or associated disease, but the basic physiology of wounds is the same. Additional to why the wound occurred or why it is not healing optimising timely treatment at the wound bed promotes efficient wound healing7-9,22,23.
Addressing the physiological factors of the chronic wound is crucial in promoting wound healing24. The acronym of T.I.M.E is a ubiquitous framework developed to assist clinicians in assessing, managing and preparing the wound bed to promote healing in the chronic wound. Figure 4 represents the T.I.M.E framework assessment and management of chronic wounds.

Figure 4: TIME framework assessment and management of chronic wounds
The components of T.I.M.E include:
Tissue assessment and management identifying the presence of non-viable tissue and treating using debridement.
Inflammation/infection assessment and management along the continuum that ranges from contamination to systemic infection. When bioburden is present, verified by signs of local infection either covert or overt, debridement methods and antimicrobial dressings should be utilised.
Moisture imbalance for the level of exudate from the wound, managed using dressings.
Edge advancement through assessing the wound size and the surrounding tissue allowing the treatment plan to be reviewed and changed if necessary.
Tissue Assessment and Management
Chronic wounds tend to be delayed in the inflammatory phase and don’t progress through the normal sequence of wound healing, presenting to clinicians as difficult to heal5,24. Often chronic wounds are characterised by the presence of non-viable tissue, which requires debridement for healing to occur24. In Figure 5, non-viable tissue is evidenced by the yellow slough and blackened areas.
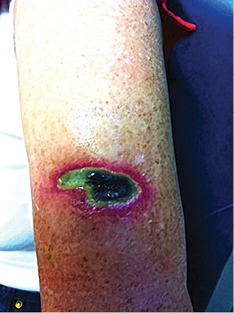
Figure 5: Non-viable tissue
Debridement disturbs the cell cycle at a molecular level. It forces the chronic wound back into an acute wound where the sequence of healing can be resumed, generating normal extracellular formation of granulation tissue, angiogenesis, contraction and epithelialisation3,4,6,25.
In clinical practice, there are several common methods clinicians may choose to debride a wound. These include: autolytic, mechanical, enzymatic, biological, conservative sharp, surgical sharp. Figure 6 shows debrided tissue on the wound bed. Figure 7 demonstrates debrided tissue using the conservative method and the tool used in this case was a curette. More recently debridement techniques such as low-frequency, low-dose ultrasonic and hydrosurgical (lavage), monofilament polyester fibre pad and plasma-mediated bipolar radiofrequency ablation have been developed to potentially benefit wound healing24,25. These newer techniques may challenge traditional methods as the evidence about the best method to debride is insufficient25.
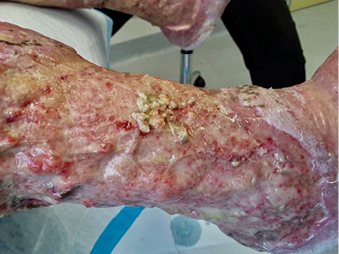
Figure 6: Debrided tissue on wound
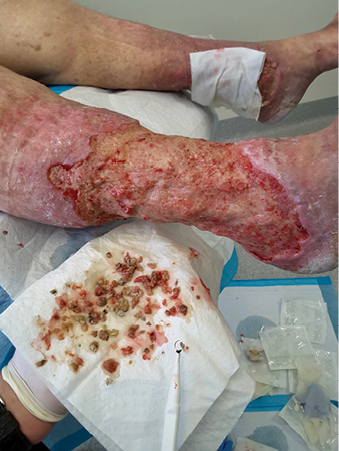
Figure 7: Debrided tissue — method of conservative sharp and tool used curette
Chronic wounds often have elevated levels of matrix metalloproteinases (MMPs) because of the delay in the wound progressing through the phases of healing to closure. The ambition is to have the right substrate and type of MMPs to assist with debridement. More is being understood about the role of MMPs in wound healing. MMPs are crucial, as they are required for normal tissue turnover, breakdown and removal of damaged extracellular matrix (ECM) (autolytic debridement). Excessive MMPs for extended periods of time starts to degrade growth factors and receptors, preventing angiogenesis and the contraction and remodelling of the ECM8. At this stage, the level of proteases needed for actual wound healing is unknown. In a systematic review by Norman et al.8, no evidence or studies were identified to test and treat the level of proteases with modulating therapies.
Inflammation/Infection Assessment and Management
In some chronic wounds bacteria may colonise the wound bed without impairing the healing process. These bacteria are planktonic, single, unattached microbes often part of the normal skin flora present in the wound bed. Predominately in acute wound types such as: traumatic, skin tears and surgical wounds, the inflammation phase consists of the body releasing its own response or defences which increases blood supply and protective cells like neutrophils, pro-inflammatory cytokines and proteases to clean up any residual debris or planktonic microbes (process called phagocytosis) allowing the wound to heal in a timely manner27. As the bacterial load increases, the process becomes impaired and the infection can spread to the surrounding tissue24. It is suspected that 60–100% of chronic wounds have biofilm present, yet it is difficult to detect7,23,26,27.
In a chronic wound the biofilm cycle begins. This is reflected in Figure 8. The planktonic microbes start becoming attached, grow and multiply. The extracellular polymeric substance (ESP) released from cells strengthens the cluster of microbes and protects them from the normal host defences of phagocytosis making them difficult to be destroyed. The host identifies the biofilm and continually releases the inflammatory cells in abundance, causing tissue destruction and capillary weakness, thus allowing the biofilm to grow in numbers and strength, so colonies develop, eventually sustaining themselves with water and nutrition26,27.
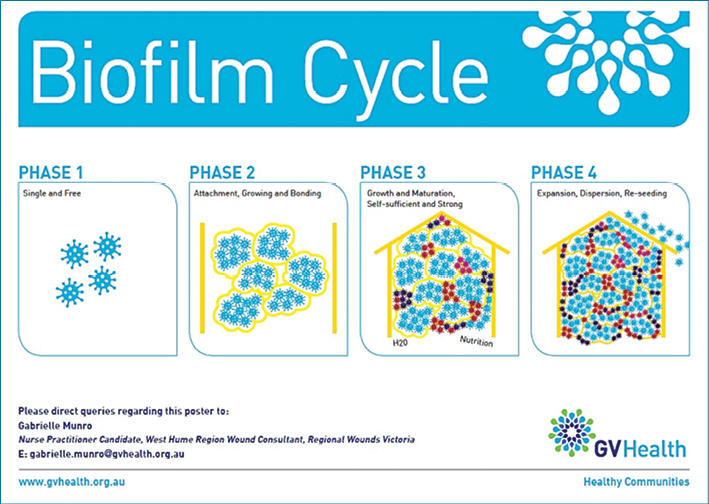
Figure 8: Biofilm cycle
Without destroying the biofilm with some form of debridement and antimicrobial use the biofilms continue to grow, mature and spread, hindering the wound healing process.
When chronic wounds fail to heal due to increasing bioburden the stage in the wound infection continuum needs to be assessed. There are five stage in the infection continuum: contamination, colonisation, local infection, spreading infection and systematic infection. Figure 9 demonstrates the infection continuum. When the microbes are in the planktonic phase (single and unattached) the wound is in the early stage of the wound continuum — contamination and colonisation. These two stages of the infection continuum involve limited proliferation of the microbes and this phase does not create a huge response from the host. Wounds in this stage of the continuum continue to heal. The point of contention for clinicians is at what stage does the wound progress from colonisation to local infection when biofilm is not seen and symptoms may be subtle26,27 (Figure 10).
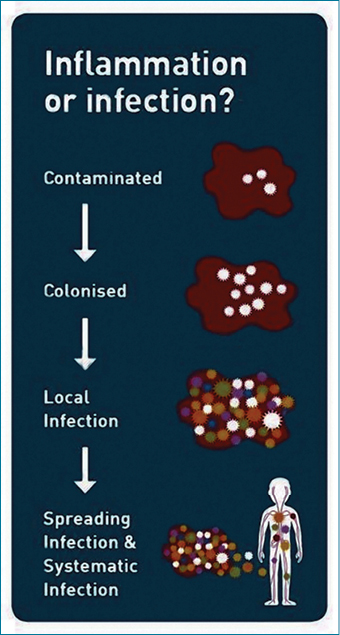
Figure 9: Infection continuum
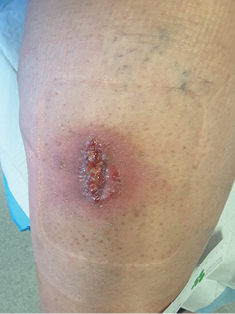
Figure 10: Local infection —hypergranulation, friable tissue, erythema, slough
Local infection causes an inflammatory response from the host and healing is subsequently delayed. With local infection microbes and biofilm grow, mature and become strong, embedding themselves deeper into the wound. If the biofilm is not managed, infective microbes spread further away from the wound to the surrounding tissue and potentially affect the whole body as the infection spreads through to the circulatory and lymphatic systems27.
The recommended treatment for suspected bioburden in a wound is a combination of debridement and antimicrobial dressings. A multitude of antimicrobial dressings exist containing silver, iodine, honey, and polyhexamethylene biguanide (PHMB) to assist in reducing the presence of bioburden without building resistance. Currently there is insufficient evidence to support which antimicrobial dressing is the most effective in wound healing7,23,24,28.
Moisture Imbalance
Despite the lack of current published literature outlining which is the most appropriate dressing, it is recognised that utilising a dressing that maintains optimal moisture balance and promotes autolytic debridement should be chosen. The most common advanced dressings considered beneficial and used to balance the wound bed moisture are traditionally categorised as films, foams, hydrocolloids, alginate and hydrogels29,30.
Moisture balance is managed through the appropriate dressing choice. There are multiple brands varying in costs all claiming to be the best. Some are more absorbent than others with better adherence and individual differences. The more sophisticated absorbent system in the vacuum-assisted closure dressing type can absorb copious amounts of exudate24.
A systematic review by Valle et al.29 compared several advanced wound dressings to compression therapy. Like much of the published literature, the comparison of different treatments (although the overall outcome is to improve healing) serves completely different purposes. In this case, compression therapy is used to re-establish circulation dynamics. Advanced dressings balance the exudate allowing the body to repair itself and thus improve wound healing.
Edge advancement
The edge is the state of the surrounding tissue of the wound, the surface area of the wound as well as any undermining or tunnelling present. These measurements are a great indicator of treatment plan improvement or in need of reassessment7,23.
The edge advancement can be expedited by acellular dermal matrices which act as a skin substitute, epidermal skin harvesting to substitute skin grafting7,23. It is proposed that the edge may be advanced by treatments such as: gas laser therapy to increase cellular proliferation and migration, hyperbaric oxygen therapy and topical oxygen attempting to increase oxygen to the wound to improve healing and stem cells to promote wound healing by migrating across the wound bed2,7. The evidence supporting the benefits of using these new edge advancement treatment modalities is limited23.
Electrical stimulation attempts to stimulate endothelial cells to migrate and promote the release of growth factors facilitating angiogenesis. It was found through a systematic review by Barnes et al.12 that electrical stimulation was generally effective. However, there was no statistical significance due to study size and participants in the review.
Autologous platelet rich plasma (PRP) is a treatment that contains fibrin (makes the blood clot); has high concentration of growth factors, has the potential to promote the regeneration of tissue and promote healing in chronic wounds. Martinez-Zapata et al.1 reported that although PRP may help in healing diabetic foot ulcers, the evidence was ambiguous, and of low quality to justify its effect in healing of other chronic wounds. However, Marti-Carvajal et al.31 acknowledged that growth factors may improve healing in diabetic foot ulcers.
A systematic review of the literature by Aziz and Cullum10 did not find clear evidence to suggest if electromagnetic therapy (EMT) stimulates the number of fibroblasts and macrophages to the wound bed, thus decreasing inflammation and promoting cell proliferation to assist in the healing of chronic wounds.
Edge advancement, has evolved since the implementation of T.I.M.E principles. The indicator to predict healing is the edge advancement equivalent to 20–40% reduction of wound area and improved state of the surrounding tissue following two and four weeks of treatment7,23,32.
Khoo and Jansen32 suggest the most accurate wound size assessment is digital planimetry and considered this more reliable if the wound was small and the assessor was the same for each wound assessment.
Psychological concerns and the wellbeing of clients
Chronic ulcers have a marked impact on patient wellbeing contributing to reduced quality of life, pain, increased stress and anxiety, additional treatment cost, potentially significant changes to the client's existing lifestyle, and in many cases acceptance and maintenance of a client’s health condition5,12-14,23.
The major challenge experienced by clients with a chronic wound is pain. Pain can be categorised into three types: nociceptive pain, neuropathic and emotional pain. Nociceptive pain is a consequence of multiple factors such as tissue damage, the inflammatory phase of normal healing, damaged nerves, infection, skin irritation due to exudate or the application and removal of various dressings, and debriding11,33-36. Neuropathic pain is a consequence of disease or damage to the nerve endings by oedema, external pressure, heavy bedding, allergies, neuropathic ischemia and arthritic pain, nerve stimulation or exposure and thermal insult33-36. Emotional pain is a consequence of the psychological impact of the chronic wound arising from odour, exudate, social isolation and depression. It is purported nurses understand the maximum discomfort experienced by the client is during dressing changes33-36. Assessing pain experienced by clients continues to be a challenge for nurses as they lack knowledge of how to appropriately treat pain. There are several different scales and ways of measuring and managing pain33-36. Using medication to treat the disease may be one of these. The use of diversional factors such as distraction, reassurance and the development of good rapport may minimise psychological factors associated with pain35.
Pain can be reduced by choosing an advanced dressing that is more absorbent, manages infection and requires less frequent changing. Using a silicone border dressing may prevent damage upon removal. Gauze, compared to advanced dressings like foam, is time-inefficient because of dressing frequency and deemed more painful37.
In a systematic review of published and unpublished randomised controlled trials (RCTs), looking at the topical analgesic or anaesthetic effect of pain in the venous leg ulcers, five trials found that a clinically significant reduction in post-procedural pain was demonstrated when using EMLA 5% prior to debriding11. There is sufficient evidence to demonstrate that EMLA is more effective in reducing pain than a nitrous oxide/oxygen mixture. Despite EMLA use, complete debridement was still not possible as it was found that clinicians were asked to stop debriding due to pain11.
Claeys et al.38 found some evidence to suggest that the use of ibuprofen slow-release foam dressings improved pain for clients, but acknowledged more research is recommended due to inconsistent pain score techniques.
There are numerous reasons why the client may choose not to follow the evidence-based best practice. Clinicians often consider the biggest barrier to be client education and engagement followed by concordance to the recommended intervention5,15,39. For instance, debriding is considered best practice but is painful11. The Pan Pacific Venous Leg Ulcer (VLU) guideline identifies that compression therapy is the gold standard for the management of such wounds40. The barriers to compression use are the discomfort and expense. The experiences of the client with compression also affect the concordance and there is limited evidence on strategies to improve this41.
The effect of living with a chronic wound is difficult to quantify. Expert opinion suggests that patient involvement and consideration of their concerns forms a positive experience and partnership in the management plan of healing chronic wounds42.
Oien et al.43 found a significant positive difference using the EuroQual-5Disease (EQ-5D) questionnaire in health-related quality of life assessment in a client with a healed ulcer in favour of an open ulcer. Results also indicated that clients could distinguish between wound pain and other pain, which was reduced significantly in the healed ulcer. Many different scales are available to assess quality of life. They are a self-reported measure of health with questionable validity to ulcer healing as the dimensions often affect many aspects of a client’s experience.
Economic Concerns
Chronic ulcers are a significant burden on health resources. The larger and more chronic the wound, the higher the associated cost8,9,12. It is accepted that the cost of dressings and diagnostic equipment is expensive but the real cost should be considered as an episode of care. This is linked to professional time; hugely affected by wound healing duration, complications such as infection and hospitalisation, and dressing change frequency. It is estimated that nursing time equates to 33%–41% of the total cost of wound care13. Changing the model of care to reduce dressing frequency, provide coordinated evidence-based practice and improve healing times, demonstrates a significant cost savings utilising a valid measurement of wound healing13,37.
It is estimated that the cost to treat chronic wounds such as leg ulcers, foot wounds and pressure injuries is somewhere near $2.5–3 billion per annum, not counting the people who self-treat13,22,44. Graves et al.13 noted that ulcers classified as severe or deteriorating cost two to six times more per week than those classified as progressing well. The chronic wound per se needs to attract the attention of important stakeholders — the consumer (client), health care providers and policy makers — as chronic wounds are not part of the ageing process; they are a chronic disease44.
Discussion on contemporary field in chronic wound management
Despite the reviewed evidence of the causes and concerns of chronic wounds, the reality is: the available evidence is not transitioned into the everyday practice of the average nurse.
Most nurses have had current education on: the assessment and treatment of chronic wounds; T.I.M.E wound bed preparation framework, including debridement (particularly with the methods — mechanical, conservative sharp, and autolytic); the different types of dressings and the implication for use; the importance of measuring and tracking the wound size; and client factors affecting wound healing, such as age, nutrition, co-morbidities and general health. They are aware of the cost of dressings and visits as these costs are transferred to the clients.
Although nurses have received education on the infection continuum, the very complex nature of inflammation and biofilm is still emerging and not fully understood. Biofilm cannot be seen, so the easiest way to presume biofilm is in the wound is to measure and track the advancing edge. The issue then lies in being able to identify which biofilm or which cluster of microbes is responsible for the delayed wound healing. Taking a swab, which is common practice in community nursing and medical fields, is inefficient as many microbes such as Pseudomonas aeruginosa imbed deeper into the wound bed. Taking a biopsy requires a skill that many nurses do not have and with the disproportion of microbes across the wound bed, the biopsy may also be inefficient. Potentially a combination of the two will be adequate. There is also the concern if testing available at standard laboratories is capable of testing for a small number of potential microbes. Further, laboratories are reliant on the specimen reaching them within four hours, but the distance between services is a barrier27.
Identifying and understanding the infection continuum and the implication of biofilm on the wound is complex and difficult for the average clinician to understand. Clinicians need to accept: chronic wounds have biofilm, healing is affected by the ability or strength of the host to minimise the impact of the biofilm and the treatment of biofilm consists of debridement and antimicrobials and antibiotics if infection is spreading beyond the wound27. The costs associated with the T.I.M.E wound bed principles and treatment and management of microbes and biofilm is complex and onerous on the clinician, client and services.
If we have all the evidence supporting how to best assess and identify, manage, debride, dress and minimise the overall impact on clients within a cost-efficient service, the contemporary issue of imbedding this evidence into every day practice will remain26,27.
Summary with recommendation for improvement based on the current literature identified
The conundrum of what is best practice for chronic wounds continues. The aim of wound care for the clinician is to achieve complete wound closure. This needs to be provided through evidence-based, timely, client-focused and cost-efficient care7,9. There is a lack of good-quality, comparative evidence in wound care. RCTs are the measure of change because of an intervention15. The challenge in wound care is to correlate the research question to measure the wound in response to multiple interventions over the duration of care, accompanied by vigorous data, all with the provision of care reflecting patients and clinical goals45. In many cases, due to lack of good-quality, small study sizes, poorly designed methodology and unbiased results, it is accepted that consensus-based recommendations are used in wound care45.
The T.I.M.E principles for wound bed preparation remains an appropriate assessment and management tool to guide clinical practice22. Debridement remains the most efficient method to remove devitalised tissue. Selection of which method, new or old, is influenced by many factors. In 2010, Edwards and Sapley46 concluded that evidence was poor and more research needs to be undertaken on debridement per se and the different methods of debridement to achieve wound healing. The ability to detect and identify the presence and amount of bioburden remains unclear. There may be some benefits of multiple methods to manage bioburden, at different stages of the healing continuum using a combination of methods, in conjunction with the appropriate dressing. The choice of dressing needs to balance the moisture in the wound. Frequent wound measurement is indicative of progress. Utilising these T.I.M.E principles provides challenges for the clinician, especially without organisational support, policy and clinical mentoring23.
There is limited evidence to support time frames for healing, but it is acknowledged timely interventions are required to prevent delay in healing. More research is required to assess the effectiveness of care that nurses provide, ensuring that the standard of care is not deviated due to weaknesses of an organisation and/or its clinicians. Therefore, a change in the way wound care services are delivered and managed is required to enhance healing and improve outcomes9.
More research is needed in the evidence-based care delivered through services that safeguards the client’s wellbeing from unnecessary and preventable social and emotional consequences when managing chronic wounds. Constant monitoring and evaluation of the care provided by nurses is needed to suit the client’s individual needs.
Lastly, more research and data is needed on measuring the cost efficacy of the care provided. A holistic approach to the cost of treating chronic wounds is required9,13.
Chronic wound management is impacted by a lack of research, client variables and sector capacity to manage effectively leaving a gap in the current practice. As a result, the physiological, psychological and economic causes of chronic wounds need further consideration.
Acknowledgement
Dr Maria Murphy, School of Nursing and Midwifery, La Trobe University.
Author(s)
Gabrielle Munro
Nurse Practitioner Candidate, GradDipNurs, BN
Affiliations: Goulburn Valley Health, VIC, Australia
West Hume Region Wound Consultant— Regional Wounds Victoria;
Email Gabrielle.Munro@gvhealth.org.au
Tel 0419 761 730
References
- Martinez-Zapata MJ, Arti-Carvajal AJ, Solà I et al. Autologous platelet-rich plasma for treating chronic wounds. Cochrane Database Syst Rev 2016; Issue 5. Art. No: CD006899. DOI: 10.1002/14651858.CD006899.pub.3.
- Atkin L. Understanding method of wound debridement. Br J Nurs (Tissue Viability Suppl) 2014;23(12).
- Harris RJ. The nursing practice of conservative sharp wound debridement: promotion, education and proficiency. Wound Care Canada 2009;7(1):22–30.
- Lebrun E, Tomic-Canic M, Kirsner R. The role of surgical debridement in healing diabetic foot ulcers. Wound Repair Regen 2010;18(5):433–438. DOI: 10.1111/j.1524-475x.2010.00619.x
- White R. Hard-to heal wounds: results of an international survey. Wounds UK 2011;7(4).
- Wounds UK. Effective debridement in a changing NHS: a UK consensus. London: Wounds UK; 2013. Assessed from: www.wounds-uk.com
- Harries R, Bosanquet D, Harding K. Wound bed preparation: TIME for an update. Int Wound Journal 2016;13(Suppl S3):8–14.
- Norman G, Westby MJ, Stubbs N, Dumville JC, Cullum N. A ‘test and treat’ strategy for elevated protease activity for healing in venous leg ulcers (Review). Cochrane Database Syst Rev 2016; Issue 1. Art. No.: CD011753. DOI: 10.1002/14651858. CD011753.pub.2.
- Vowden P, Vowden K. The economic impact of hard-to-heal wounds: promoting practice change to address passivity in wound management. Wounds Int 2016;7(2).
- Aziz Z, Cullum N. Electromagnetic therapy for treating venous leg ulcers. Cochrane Database Syst Rev 2015; Issue 7. Art. No.: CD002933. DOI: 10.1002/14651858.CD002933.pub.6.
- Briggs M, Nelson EA, Martyn-St James M. Topical agents or dressings for pain in venous leg ulcers. Cochrane Database Syst Rev 2012; Issue 11. Art. No. CD001177. DOI: 10.1002/14651858.CD001177.pub.3.
- Barnes R, Shanin Y, Gohil R & Chetter I. Electrical stimulation vs standard care for chronic ulcer healing: a systematic review and meta-analysis of randomised controlled trials. European Journal of Clinical Investigation. 2014; 44:429–440. DOI: 10.1111/edi.12244.
- Graves N, Finlayson K, Gibb M, O’Reilly M, Edwards H. Modelling the economic benefits of gold standard care for chronic wounds in a community setting. Wound Practice & Research 2014;22(3):163–168.
- Greer N, Forman MacDonald R, Dorrain J, Fitzgerald P, Rutks I, Wilt T. Advanced wound care therapies for non-healing diabetic, venous, and arterial ulcers: a systematic review. Ann Intern Med 2013;159:532–542.
- Courtney M, McCutcheon H. Using evidence to guide nursing practice. 2nd edn. Chatswood, Australia: Churchill Livingstone; 2010.
- Flodgren G, Rojas-Reyes MX, Cole N, Foxcroft DR. Effectiveness of organisational infrastructures to promote evidence-based nursing practice. Cochrane Database Syst Rev 2012; Issue 2. Art. No.: CD002212. DOI: 10.1002/14651858. CD002212. pub.2.
- Heslop L, Lu S. Nursing –sensitive indicators: a cohort analysis. J Adv Nurs 2014;70(11):2469–2482. DOI: 10.1111/jan.12503
- International Council of Nursing (ICN). Nursing Matters Fact Sheet. Genera; 2009. Assessed from: http://www.icn.ch/images/stories/documents/publications/fact_sheets/15c_FS-Nursing_Sensitive_Outcome_Indicators.pdf
- Neilson ER, Harris C. Conservative sharp wound debridement: an overview of Canadian education, practice, risk and policy. J Wound Ostomy Continence Nurs 2003;40(6). DOI: 10.1097/WON.0b013e3182a9ae8c. pp. 594–601.
- DiCenso A, Guyatt G, Ciliska D. Evidence-based nursing, a guide to clinical practice. St Louis: Elsevier; 2005.
- Regional Wounds Victoria. Best Practice links. 2016. Assessed from http://www.grhc.org.au/vic-wound-man-cnc-project/links/36-groups-left-menu/95-wm-best-practice-consensus-documents
- Conduit C, Free B, Sinha S. TIME-H in clinical practice — a pilot study. Wound Practice & Research 2013;21(4).
- Leaper DJ, Schultz G, Carville K, Fletcher J, Swanson T, Drake R. Extending the TIME concept: what have we learned in the past 10 years? Int Wound J 2012;9(Suppl 2)1–19.
- Powers J, Higham C, Broussaard K, Phillips T. Wound healing and treating wounds — chronic wound care and management. Am Acad Dermatol 2005; DOI: org/10.1016/j.jaad.2015.08.070
- Madhok B, Vowden K, Vowden P. New techniques for wound debridement. Int Wound J 2013;10:247–251.
- World Union of Wound Healing Societies (WUWHS). Florence Congress. Position Document. Management of Biofilm. Wounds Int 2016.
- International Wound Infection Institute (IWII). Wound Infection in clinical practice. Wounds Int 2016.
- Lo S, Hayter M, Chang C, Hu W, Lee L. A systematic review of silver releasing dressings in the management of infected chronic wounds. J Clin Nurs 2008;17:1973–1985. DOI: 10.1111/j.1365–2705.2007.02264x
- Valle F, Maruther N, Wilson L et al. Comparative effectiveness of advanced wound dressings for patients with chronic venous leg ulcers; A systematic review. Wound Repair Regen 2014;22:193–204. DOI: 10.1111/wrr.12151.
- Heyer K, Augustin M, Protz K, Herberger K, Spehr C, Rustenbach SJ. Effectiveness of advanced versus conventional wound dressing on healing of chronic wounds; systematic review and meta-analysis. Dermatology 2013;226:172–184. DOI: 10.1159/000348331.
- Marti-Carvajal AJ, Gluud C, Nicola S, Simancas-Racines D, Reveiz L Oliva P, Cederio-Taborda J. Growth factors for treating diabetic foot ulcers (Review). Cochrane Database Syst Rev 2015; Issue 10. Art. No.: CD008548 DOI: 10.1002/14651858. CD008548.pub.2.
- Khoo R, Jansen S. The evolving field of wound measurement techniques. Wounds 2016;28(6):175–181.
- Bell C, McCarthy G. The assessment and treatment of wound pain at dressing change. Br J Nurs (Tissue Viability Suppl) 2010;19(11).
- Fletcher J. Managing wound pain during application and removal of dressing. Br J Nurs (Tissue Viability Suppl) 2010;19(20.
- Richardson C, Upton D. Managing pain and stress in wound care. Wounds UK 2011;7(4).
- Vuolo J. Wound-related pain: key sources ad triggers. Br J Nurs (Tissue Viability Suppl) 2009;18(15).
- Vermeulen H, Ubbink DT, Goossens A, De Vos R, Legemate DA, Westerbos S. Dressing and topical agents for surgical wound healing by secondary intention. Cochrane Database Syst Rev 2004; Issue 1. Art. No.: CD003554. DOI: 10.1002/14651858. CD003554.pub.2.
- Claeys A, Gaudy-Marqueste C, Pauly V et al. Management of pain associated with debridement of leg ulcers: a randomised, multicentre, pilot study comparing nitrous oxide-oxygen mixture inhalation and lidocaine-prilocaine cream. J Eur Acad Dermatol Venereol 2011;25:138–144. DOI: 10.111/j.1468-3083.2010.03720x.
- National Health and Medical Research Council (NHMRC). Better informed health care through better clinical guidelines. An NHMRC Discussion Paper. Commonwealth of Australia; 2015.
- Australian Wound Management Association (AWMA). Australian and New Zealand Clinical Practice Guideline for Prevention and Management of Venous Leg Ulcers. Australia: AWMA; 2011. ISBN Online: 978-0-9807842-2-0. Assessed from: http://www.awma.com.au/publications/2011_awma_vlug.pdf.
- Weller CD, Buchbinder R, Johnston RV. Interventions for helping people adhere to compression treatment for venous leg ulceration. Cochrane Database Syst Rev 2016; Issue 3. Art. No.: CD008378. DOI: 10.1002/14651858. CD008378. pub.3.
- Grothier L, Pardoe A. Chronic wounds: management of healing and well-being. Br J Nurs (Tissue Viability Suppl) 2013;22(12).
- Oien R, Akesson N, Frossell H. Assessing quality of life in patients with hard to heal ulcers using the EQ-5D questionnaire. J Wound Care 2013;22(8).
- Kapp S, Santamaria N. Chronic wounds should be one of Australia’s National Health Priority Areas. Australian Health Review 2015;39:600–602.
- White R, Grocott P, Young T, Ali O. Evidence in wound care: the recent NICE guidance on Debrisoft monofilament debridement pad for use in acute and chronic wounds. Wounds UK 2014;10(2).
- Edwards J, Stapley S. Debridement of diabetic foot ulcers. Cochrane Database Syst Rev 2010; Issue 1. Art: CD003556.DOI: 10.1002/14651858. CD003556.pub.2.



