Volume 25 Number 2
A trial of two prophylactic sacral dressings (2PSD) in the prevention of stage 1 sacral pressure injury in the critically ill patient: a study protocol
Jodie Gordon, Monica Stankiewicz, Hamish Pollock, Martin Christensen, Nicola Barker-Gregory and Joel Dulhunty
Abstract
Pressure injuries (PIs) are highest amongst the critically ill. The impact of PIs is well reported and includes increased length of stay in acute facilities, increased cost of care, decreased quality of life, and pain and disability for the patient. Over the last decade, a considerable amount of research has been undertaken in the area of PI prevention. We now know that the use of prophylactic silicone dressings can assist in reducing the incidence of PIs in critically ill patients. However, there is currently a gap in the literature in comparing the effectiveness of different silicone products available in Australia. This cluster-controlled clinical trial aims to compare the onset of PIs and cost-effectiveness between two silicone products currently available.
Key points:
- The use of prophylactic silicone dressings is considered a preventative strategy to minimise PI occurrence.
- There is little published comparative clinical evidence or cost-effectiveness data on the various silicone dressings with regard to PI prevention. This study aims to fill this gap.
Definitions: Pressure injury (PI): The result of localised injury to the skin or underlying structures usually over a bony prominence due to one or more contributing factors, such as unrelieved pressure and/or shear forces1.
Clinical trial end point definitions: Stage 1 PI: Non-blanchable erythema1.
Introduction
The prevention and management of pressure injuries (PIs) has been emphasised as a critical area of health care in both an Australian and international context2. Hospital-acquired PIs are a concern for every health service and health care professional. Within the Australian health care system, Standard 8 of the National Safety and Quality Health Service Standards specifically establishes minimum standards of care that health service organisations (HSO) must meet and mechanisms that allow HSOs to realise and implement developmental goals3. McInnes et al.4 highlight the need for rigorous attention to prevent PI development in the acute care setting due to the high cost of managing acquired PIs, which is estimated at $24 million per annum in Queensland and Victoria alone.
The prevalence of PIs in Australia ranges from 6% to 48% in acute and subacute health care facilities2,3,5,6. International studies have shown that PI prevalence is higher in patients who have been admitted to intensive care units (ICUs)7-13. This is likely due to a multitude of factors, including decreased mobility, physiological effects of critical illness, requirements for therapeutic and monitoring devices and compromised nutritional status14-16. The most common sites in hospitalised patients for PIs are the sacrococcygeal region, heels, elbows and malleoli5. Graves et al.6 report that people who sustain a PI have an increased median length of four or more days and poorer clinical outcomes, including morbidity and mortality.
Research problem
There have been a number of studies in the previous five years that have demonstrated that silicone wound dressings are effective in reducing the incidence of PI development11,12,14-18. However, all of these studies11,12,14-18 compared the same brand silicone foam dressing (Mepilex® Border, Mölnlycke) against bare skin (that is to say, no other intervention). There have been no studies comparing the relative effectiveness of various silicone foams available on the market.
The aim of this study is to explore whether there is a difference in sacral PI prevention between two available silicone dressings used in the Australian ICU setting: Dressing 1 (Mepilex® Border, Mölnlycke) and Dressing 2 (Allevyn® Life Sacrum, Smith & Nephew). Our null hypothesis is that there is no difference in PI onset with the use of either prophylactic dressing. This article describes the two prophylactic sacral dressings (2PSD) study protocol.
Study design and study site
The 2PSD study is a cluster-controlled clinical trial conducted in a 10-bed ICU located within an outer metropolitan public teaching hospital in Brisbane, Australia. All eligible patients will receive either Dressing 1 or Dressing 2 allocated in alternating three-monthly clusters until the a priori sample size is reached (Figure 1). The allocation strategy was chosen to minimise the impact of seasonal variation9.
Figure 1: Participant allocation
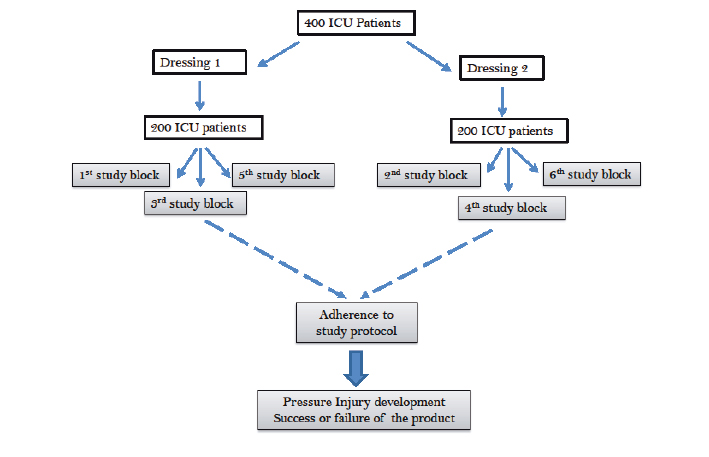
The primary outcome is the incidence of a new sacral PI (Stage 1 or greater) per 100 dressing days in the ICU. The primary outcome will be assessed by skin inspection at least twice a day by trained members of the ICU team and by a member of the research team for all ICU patients at least three times a week or, more frequently, at ICU clinician request. Inspection involves partially removing the dressing from the sacral region to assess the skin for any sign of PI and then reapplying the dressing if no PI is found.
Secondary outcomes include the mean number of dressings per patient, the cost-effectiveness of dressings to prevent a sacral PI and product integrity. Cost-effectiveness is defined as the average cost of dressings per patient in each group divided by the proportion of patients without a sacral PI. Product integrity is defined as reapplication of the product after skin inspection or rolling of edges, no rolling of product edges, the product remaining intact in the appropriate location since the last skin inspection.
Participants
All adult patients (≥18 years of age) admitted to the ICU for >48 hours during the study period will be included in the study. Participants are excluded from the study if the dressing becomes soiled or dislodged more than three times in a 24-hour period or the dressing is unable to be applied for more than 24 hours.
Data will be collected for all study participants within 48 hours of ICU admission until one of the following occurs: the participant develops a Stage 1 (or higher) PI underneath the dressing; the participant or clinician request discontinuation or change of the allocated PI dressing (inclusive of moisture control issues); the participant is discharged from the ICU, or transferred to another clinical area; the participant is withdrawn from the study for any reason.
Study interventions
Dressing 1 is a five-layer product that has a silicone adhesive contact layer (silicone gel adhesive), a hydrocellular foam (polyurethane), an absorbent core (cellulose fibre and polyacylate particles), a protective masking layer (hydrophilic polyester yarn) and a breathable film with padding support (polyurethane film)19.
Dressing 2 is five-layer silicone product that has a perforated silicone wound contact layer (Safetac® technology), an absorbent core made of three layers, a thin sheet of polyurethane foam, a piece of non-woven fabric and a layer of absorbent polyacrylate fibre on a polyurethane film20.
The use of prophylactic dressings (1 or 2) will be in conjunction with standard PI prevention strategies. These strategies include the use of alternating air mattress, repositioning two- to four-hourly, depending on individualised skin assessment and tolerance of the skin including medical stability, use of offloading devices to assist with turning and repositioning, skin hydration and moisturising and regular perianal hygiene to prevent incontinence-related skin damage.
Induction and data collection
Prior to the study commencement, the wound management clinical nurse consultant (principal investigator) will provide in-service education on data collection and the use of both dressing products to all ICU staff involved in the study.
Study variables will be collected by a member of the research team or bedside nurse using a study-specific data collection sheet (Table 1). This includes four questions on dressing usage that will be asked of bedside nursing staff twice a day and recorded as a dichotomous (yes/no) response. The research assistant will work closely with ICU nursing staff to complete skin inspections and assist with completing data collection tools.
Table 1: Study variables
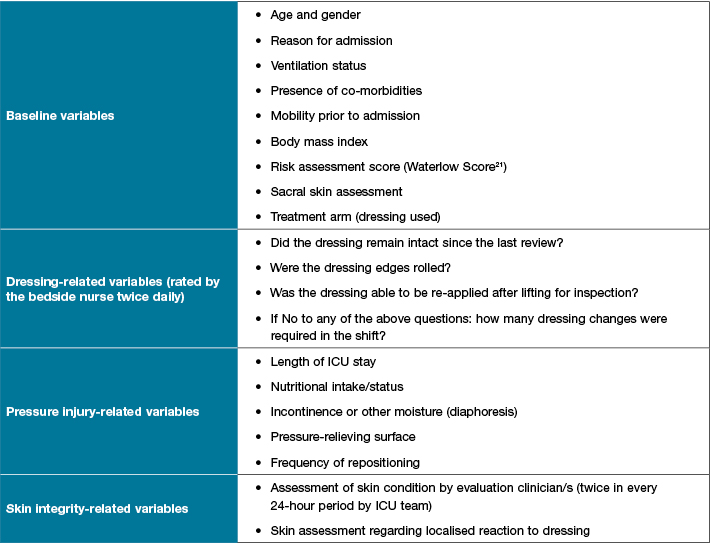
Where required, data variables will be cross-checked with the patient record for completeness and accuracy prior to entry into the study database in a non-identifiable form.
Ethical issues
This study has received ethics approval from The Prince Charles Hospital Human Research Ethics Committee (HREC/15/QPCH/6). The need for individual participant consent was waived on the basis that the two products being compared are in standard use within the ICU setting for prevention of PIs in the sacral region.
Sample size and power
A sample of 400 patients (200 in each group) is anticipated to be recruited over a two-year period. This sample was selected to ensure 90% power to detect a difference in the incidence of new onset PIs with a baseline rate of 4/100 dressings days and a hazard rate of 0.5 (that is to say, 2/100 dressing days in the comparator group) using a Poisson regression model with no additional variables of interest and an alpha of 0.05.
Statistical analysis
The primary and secondary outcomes will be evaluated using an intention-to-treat analysis of all eligible patients. The primary outcome, development of a sacral PI, will be compared between the two treatments using Poisson regression. Descriptive analysis will occur for the secondary outcomes, including number of dressing changes within each group, cost difference between groups and product integrity. The mean number of dressings per patient will be compared between groups using a student’s t-test or Mann-Whitney U test, depending on whether or not normality assumptions are achieved. A two-sided p-value <0.05 will be considered evidence of a statistically significant difference in the study outcomes.
Funding
This study was funded by research grants from the Redcliffe Hospital (Private Practice Trust Fund) and Wounds Australia. These funding bodies have no input into the design, conduction or reporting of the trial.
Current status
The study commenced recruitment in February 2016. The expected end date for recruitment is August 2017.
Summary
As a nurse-led initiative, this study puts nursing at the forefront of PI prevention and management in the critical care environment and aims to promote nursing practice and research around PI prevention and maintenance of skin integrity. This study aims to provide a previously unexplored comparison between two silicone dressings available on the Australia market in the prevention of sacral PIs in the critically ill patient.
This study is significant and timely because it will contribute to the growing body of work related to PI prevention in the critically ill. In addition, the increasing cost in avoidance and treatment of PIs is concerning not only in terms of resource management but the wider implications of patient care and quality of life, particularly as health care consumers demand more accountable, transparent and cost-effective health care delivery and services.
Acknowledgements
The authors would like to thank the biostatisticians at Queensland Institute of Medical Research, ICU Medical and Nursing team who have contributed to developing the study protocol.
Conflicts of Interest
The study investigators have no commercial interests in the outcome of the study.
Author(s)
Jodie Gordon
RN, MClinSpec (WM), GradCert STN
Nursing Director, Redcliffe Hospital, Metro North Hospital and Health Service, QLD, Australia
Monica Stankiewicz*
RN, MAppSc (Research), MNSc (NP), GradDip WM, GradCert STN
Clinical Nurse Consultant, Redcliffe Hospital, Metro North Hospital and Health Service;
Nurse Practitioner, Haut Dermatology, Spring Hill
Level 5 Wound Management and Stomal Therapy Department, Redcliffe Hospital, Anzac Avenue, Redcliffe, QLD 4020, Australia
Email monica.stankiewicz@health.qld.gov.au
Tel 07 3883 7633
Hamish Pollock
BM, MRCP, FRCA, DICM (UK), FCICM, FANZCA, PGDipCU
Director, Caboolture — Redcliffe Intensive Care Unit, Redcliffe Hospital, and Staff Specialist Anaesthesia Royal Brisbane and Women’s Hospital, Metro North Hospital and Health Service;
Senior Lecturer, School of Medicine, The University of Queensland, QLD, Australia
Martin Christensen
BSc (Hons), PGCert (ICU), MSc, MA, PhD
Associate Professor, Queensland University of Technology; Redcliffe Hospital, Metro North Hospital and Health Service, QLD, Australia
Nicola Barker-Gregory
RN, GradCert ICU, GradCert Adult & Wkplace Ed, MEdu
Nurse Unit Manager, Intensive Care Consultant, Redcliffe Hospital, Metro North Hospital and Health Service, QLD, Australia
Joel Dulhunty
MBBSm, MTH, PhD
Director of Research and Medical Education, Redcliffe Hospital, Metro North Hospital and Health Service; Senior Lecturer, School of Medicine, The University of Queensland; Adjunct Professor, School of Public Health and Social Work, Queensland University of Technology
QLD, Australia
* Corresponding author
References
- National Pressure Ulcer Advisory Panel (NPUAP), European Pressure Ulcer Advisory Panel (EPUAP) and Pan Pacific Pressure Injury Alliance. Prevention and Treatment of Pressure Ulcers: Clinical Practice Guideline. Osborne Park, Australia: Cambridge Media; 2014.
- Australian Commission on Safety and Quality in Health Care. Safety and Quality improvement guide. Sydney: ACSQHC; 2012.
- Australian Commission on Safety and Quality in Health Care. Safety and Quality Improvement Guide Standard 8: Preventing and Managing Pressure Injuries. Sydney: ACSQHC; 2012.
- McInnes E, Chaboyer W, Allen T, Murray E, Webber L. Acute care patient mobility patterns and documented pressure injury prevention – an observational study and survey. Wound Practice & Research 2013;21(3):116–125.
- Australian Wound Management Association. Pan Pacific Clinical Practice Guideline for the Prevention and Management of Pressure Injury. Osborne Park: Cambridge Media; 2012.
- Miles S, Fulbrook P, Nowicki T, Franks C. Decreasing pressure injury prevalence in an Australian general hospital: a 10-year review. Wound Practice & Research 2013;21(4):148–156.
- Graves N, Birrell F, Whitby M. Modelling the economic losses from pressure ulcers among the hospitalized patients in Australia. Wound Repair Regen 2005;13(5):462–467.
- Shahin E, Dassen T, Halfens R. Incidence, prevention and treatment of pressure ulcers in intensive care patients: A longitudinal Study. Int J Nurs Stud 2009;46:413–421.
- Monzano F, Navarro M, Roldan D et al. Pressure ulcer incidence and risk factors in ventilated intensive care patients. J Crit Care 2010;25:469–476.
- Alderden J, Whitney J, Taylor S, Zaratkiewicz S. Risk Profile characteristics associated with outcomes of hospital-acquired pressure ulcers: A retrospective review. Crit Care Nurs 2011;31 (4):30–43.
- Walsh N, Blanck A, Smith L, Cross M, Andersson L, Polito C. Use of a sacral silicone border foam dressing as one component of a pressure ulcer prevention program in an intensive care unit setting. J Wound Ostomy Continence Nurs 2012;39(2):146–149.
- Chaiken N. Reduction of sacral pressure ulcers in the intensive care unit using a silicone border foam dressing. J Wound Ostomy Continence Nurs 2012;39(2):143–145.
- Apostolopoulou E, Tselebis A, Terzis K, Kamarinou E, Lambropoulos I, Kalliakmanis A. Pressure ulcer incidence and risk factors in ventilated intensive care patients. Hlth Sci J 2014;8(3):333–342.
- Brindle T, Malhotra R, O’Rourke S et al. Turning and repositioning the critically ill Patient with hemodynamic instability: A literature review and Consensus recommendations. J Wound Ostomy Continence Nurs 2013;40(3):254–267.
- Elliott R, McKinley S, Fox V. Quality improvement program to reduce the prevalence of pressure ulcers in an intensive care unit. Am J Crit Care 2008;17(4):328–334.
- Tayyib N, Coyer F, Lewis P. A Two-arm cluster randomised control trial to determine the effectiveness of a pressure ulcer prevention bundle for critically ill patients. J Nurs Scholarsh 2015;47(3): 237–247. doi.10111/jnu.12136
- Santamaria N, Gerdtz M, Sage S et al. A randomised controlled trial of the effectiveness of soft silicone multilayered foam dressings in the prevention of sacral and heel pressure ulcers in trauma and critically ill patients: the border trial. Int Wound J 2013; doi: 10.1111/iwj.12101
- Walker R, Aitken L, Huxley L, Juttner M. Prophylactic dressing to minimize sacral pressure injuries in high-risk hospitalized patients: a pilot study. J Adv Nurs 2015;71(3):688–696.
- Smith & Nephew Contract Briefing Document, Allevyn Life Sacrum. Retrieved on 1 June 2014, from http://www.smith-nephew.com/allevynhome/our-products/allevynlifesacrum/
- STML Dressings Datacard, Mepilex Border Sacrum. Retrieved on 1 June 2014, from http://www.dressings.org/Dressings/mepilex-border-sacrum.html
- Mahalingam S, Gao L, Nageshwaran S, Vickers C, Bottomley T, Grewal P. Improving pressure ulcer risk assessment and management using the Waterlow scale at a London teaching hospital. J Wound Care 2014; 1;23(12):613–22.



