Volume 25 Number 4
Overcoming the challenges of topical antibody administration for improving healing outcomes: a review of recent laboratory and clinical approaches
Natalie E Stevens and Allison J Cowin
Keywords wounds, Antibodies, penetration enhancer, topical delivery.
Abstract
Therapeutic antibodies present numerous opportunities for the treatment of wounds and cutaneous conditions; however, they have not been widely adopted due to the difficulty of administering antibodies through the skin. Local antibody administration to the skin may result in fewer side effects, reduce cost of therapy and be less invasive than systemic methods and recent advances in antibody engineering have addressed many stability and formulation challenges. Penetration of the epidermal barrier is crucial to effective delivery of antibodies and other protein drugs and can be achieved through chemical or physical methods. Chemical penetration enhancement is poorly suited for delivery of large hydrophilic molecules such as antibodies; however, enhancers based on surfactants or terpenes may improve antibody delivery to the dermis and novel cell-penetrating peptides provide opportunities for well-tolerated local antibody delivery. Physical penetration enhancement methods (including electroporation, iontophoresis, microneedles and ultrasound) address many formulation challenges common to chemical penetration enhancers; however, more studies are required to demonstrate effective antibody delivery for clinical translation. While topical antibody administration to the skin remains challenging, advances in antibody engineering and skin penetration enhancement may render antibodies more viable treatment options for improving wound outcomes.
Introduction
This narrative review aims to provide an overview of recent advances in the field of antibody delivery to the skin. Methods that have successfully delivered antibodies or large proteins through the epidermal barrier, particularly in a clinical setting, are described and discussed in the context of wound applications including chronic wounds, chronic skin blistering and scar management.
Poor outcomes from wounds represent an increasing health burden in Australia as populations of diabetic, obese and elderly persons who exhibit deficient wound healing increase. Therapeutic antibodies have the potential to treat many kinds of wounds1; however, antibody therapies for wounds have not been widely adopted due to the difficulty of formulating antibodies for local administration and penetration through the skin. Antibodies are one of the fastest growing categories of biopharmaceuticals today, with over 60 antibodies approved for use as therapeutics and over 50 candidates currently in phase III clinical studies2. Presently, antibodies are employed in the treatment of cancer, inflammatory disease and autoimmune disorders3 and their success is attributed to their high specificity and binding affinity to their target proteins with reduced side effects compared to small molecules4. In recent years, preclinical and clinical research has demonstrated roles for antibodies for a number of cutaneous conditions including psoriasis5,6, atopic dermatitis7,8, skin cancers9 and wound healing10-14. However, the development of antibody therapies for skin conditions is challenging as antibodies exhibit poor tissue permeability15 and are thus not efficiently absorbed through the intact skin barrier. This complicates the use of antibodies for wound applications, as while therapies promoting active healing may be directly administered to open wounds, administration to intact skin would be required for blister or scar management.
Human antibodies, also termed immunoglobulins, are antigen-binding proteins consisting of four polypeptide chains arranged in a Y-shape (Figure 1). Two heavy chains linked by disulfide bridges form the constant (Fc) stem region of the antibody molecule, while a light chain linked with each heavy chain forms two identical antigen binding (Fab) regions. The Fab regions are responsible for the specificity of the antibody, while the Fc region determines the antibody isotype and influences further immune signalling, termed secondary effector functions. Antibodies can function as therapeutics via direct neutralisation of their targets by blocking active sites on the protein, or by binding to the target and triggering its destruction by secondary effector functions (Figure 2) as in the case of many anti-cancer antibody therapies9. The function of antibodies is dependent on their three-dimensional structure, which enables binding to a target antigen; consequently care must be taken when developing antibody therapeutics to ensure antibodies are properly folded and retain binding capacity.
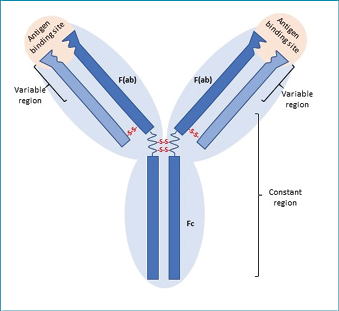
Figure 1: Antibody structure
Human antibodies are composed of four polypeptide chains: two heavy chains (dark blue) and two light chains (light blue). Antigen binding sites are each formed by the variable regions of a light chain and a heavy chain (Fab regions). The constant regions of the heavy chains form the tail (Fc region). The two Fab regions and Fc region are linked by a flexible hinge region that improves the ability of the Ab to bind antigen.
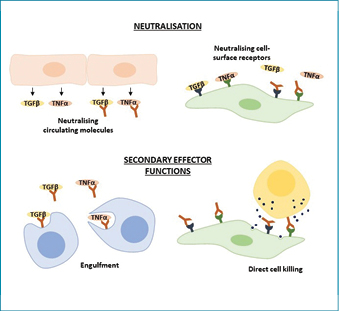
Figure 2: Therapeutic antibody functions
Therapeutic antibodies function through two main routes: direct neutralisation or secondary effector functions. Neutralisation occurs by binding circulating mediators or their receptors and preventing further signalling. Secondary effector functions recruit immune cells to engulf the target or directly kill the target cell.
Antibodies for topical administration
Antibodies have previously been used in the management of multiple cutaneous conditions, including in inflammatory skin diseases, healing wounds or managing scars. Clinical studies have shown improvement in psoriatic lesions after administration of antibodies targeting inflammatory mediators tumour necrosis factor alpha5, interleukin 2316 and interleukin 1717, and atopic dermatitis has been successfully treated with antibodies targeting interleukin 4 receptor alpha18. Antibodies against wound mediators including Flightless I10,19 and tumour necrosis factor alpha20 have been shown to improve healing in a number of wound types, and antibodies against transforming growth factor beta have been used to reduce scarring12,21. Therapeutic antibodies are generally administered by intravenous, subcutaneous or intramuscular injection, as direct oral administration commonly employed for small molecule drugs would result in gastric digestion of the proteins15. While injection of antibodies has been used successfully for cutaneous conditions22-25, direct application to the skin may confer several advantages to systemic methods. Topical administration provides local therapeutic effect while reducing systemic adverse effects26-28, avoids drug metabolism and dilution thereby reducing the quantity of antibody required29 and noninvasively delivers therapeutics to patients with simplified dosage requirements30,31. Antibodies can be prohibitively expensive to use, thus approaches that use lower dosages via topical administration would reduce costs and allow therapies to be available to more patients32. Local administration of antibodies avoids drug metabolism and improves retention within the tissue33, thus antibodies for treatment of chronic wounds or scarring may only require dosing every 1–4 weeks to maintain effective concentrations within the wound. Topical administration of gel-formulated anti-tumour necrosis factor antibody (Infliximab) has previously been effective in the treatment of open chronic wounds20; however, lesions with intact epithelial barriers including blisters, burns, partially re-epithelialised wounds and scars are not readily accessible by antibodies within a gel formulation.
Despite the potential benefits of therapeutic antibodies for cutaneous conditions, protein-based drugs exhibit challenging properties that render their formulation and delivery complex. As correct folding of antibodies is essential to their function, protein stability must be considered when developing antibody-based biotherapeutics. Protein aggregation is the most common stability issue encountered in antibody development34,35 which influences the potency36 and safety of antibody therapies37,38. Consequently, approaches to alter the aggregation of antibodies through glycosylation39, altering protein charge40,41 or the rational design of antibody sequences to remove aggregation-prone regions41-43 are crucial to the formulation of an effective cutaneous antibody therapy. Fragments of antibodies which comprise the Fab region without the Fc region can still exert neutralising function, and may exhibit greater tissue penetration44 and stability45,46 than their whole antibody counterparts. Systemically administered antibodies are generally lyophilised for reconstitution at the bedside; however, topical formulations are often in gel or cream forms, adding additional complexity to maintaining stability of antibodies. Consequently, antibody therapies for skin administration are likely to require multiple re-engineering processes to optimise formulation stability and tissue penetration into the skin.
Challenges of delivering drugs to wounds
Skin acts as a boundary between the body and the environment and effectively protects internal structures from infection, physical and chemical injury and the loss of water or other valuable compounds. Skin also constitutes a barrier to the topical delivery of antibodies, largely due to the hydrophobic nature of the outer keratinised layer, which prevents delivery of hydrophilic and polar compounds such as proteins47.
The epidermis represents the outermost layer of the skin and is highly cellular, consisting primarily of keratinocytes. The top layer of the epidermis is termed the stratum corneum and comprises a 10–15 µm thick layer of dead cornified keratinocytes embedded in a lipid-rich extracellular matrix (ECM). Below the epidermis lies the dermis, comprised of fibrous collagen punctuated with blood vessels, hair follicles, nerves and secretory glands. As the dermis is a source of fibroblasts and immune cells which secrete proliferative and inflammatory wound mediators, this is often a desirable site for drug delivery in cutaneous conditions. In open wounds where the wound bed is exposed, direct delivery of antibodies and other biologicals to dermal tissue or wound edges is feasible; however, the amount of therapy administered largely relies on the size of the exposed area. The epidermis may be intact in blister and burn wounds, and treatment of scars also requires penetration through an intact epidermis. Consequently, penetrating the barriers of the stratum corneum and intact epidermis48 is essential to delivering therapies to certain types of wounds or for managing scarring.
Passive diffusion through the epidermis to the dermis occurs through one or a combination of three main routes: intercellular, transcellular or transappendageal (through dermal appendages, for example, hair follicles, pores; Figure 3). Intercellular diffusion involves diffusion around and between cells, and is the most common transport route for hydrophobic substances as this pathway involves dissolution in lipid-rich ECM. However, transport via this route is slow as substances must diffuse through a convoluted meshwork of ECM which surrounds densely-packed corneocytes — it has been estimated that the path of intercellular diffusion is up to 20 times longer than the thickness of the stratum corneum49. Transcellular diffusion that occurs through cells is the most direct route to the dermis which is more commonly exploited by hydrophilic substances, as they diffuse through the keratin-rich cytosol of keratinocytes. However, these substances must still diffuse through the lipophilic cell membrane and some ECM, and thus substances effectively transported via the transcellular route are ideally of low molecular weight (<600 Da) and have hydrophilic and hydrophobic regions50. While the transappendageal route allows rapid diffusion of hydrophilic molecules, this method is dependent on the number of pores which comprise under 0.1% of total skin surface, thus transappendageal diffusion is not likely to facilitate delivery of proteins in therapeutic doses49.
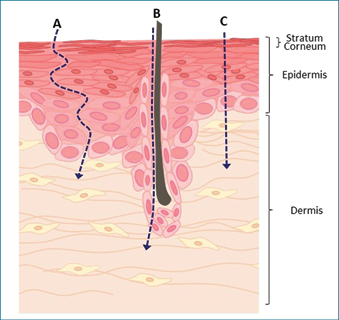
Figure 3: Routes of diffusion through the epidermis
Substances applied on intact skin may reach the dermis through three main routes. (A) Non-polar substances commonly diffuse through the intercellular route via dissolution in lipid-rich ECM. (B) The most efficient transport into the dermis occurs through transappendageal absorption through pores associated with sweat glands, hair follicles and oil glands. However, appendages represent a very small percentage of skin surface area and this reduces the rate of drug penetration. (C) The transcellular pathway through corneocytes represents the shortest distance from the skin surface to the dermis; however, only substances able to diffuse through polar and non-polar environments to efficiently diffuse this way.
Therapeutic antibodies are poorly suited to passive diffusion as they are too hydrophilic for intercellular diffusion, too large for transcellular diffusion and require higher doses than can be delivered through transappendageal diffusion alone. Antibodies are also prone to aggregation and denaturation in hydrophobic solvents which reduces their function. However, recent advances in pharmacological methods to penetrate the stratum corneum may provide the solution for topical antibody administration to skin.
Chemical penetration enhancers
Effective delivery of therapeutic antibodies to intact skin is reliant on the use of penetration enhancer (PE) strategies, which aim to reversibly disrupt the structure of the stratum corneum51. However, lipophilic PEs may impact the stability of antibodies and cause protein aggregation and denaturation51,52. Thus, an ideal chemical PE strategy would exhibit enough hydrophobicity to penetrate the stratum corneum, while protecting protein-based antibodies from denaturation14,53.
Terpene-based PEs function by associating with lipid ECM and increasing the fluidity of this phase. While it has been demonstrated terpene-based PEs with low lipophilicity may stabilise protein drugs, this effect may be dependent on the physiochemical characteristics of the protein51 and further studies are required to determine if terpene PEs are suitable for antibody delivery.
Surfactants have the ability to solubilise hydrophilic molecules in a hydrophobic phase and have successfully been used for delivery of drugs such as insulin54 with no appreciable loss of activity. However, effective doses of surfactants have been demonstrated to cause skin irritation55, thus it is likely surfactants must be combined with another PEs to provide effective delivery and protein stability with reduced irritation56.
Cell-penetrating peptides
Peptide-based PEs are of particular interest for the delivery of proteins to tissues, and recent studies have focused on topical application57,58 which may reduce toxicity compared to chemical PEs. Peptide PEs are short amino acid sequences which enter cells without the use of specific receptors or damaging the cell membrane59 and are able to cross the stratum corneum. The mechanism by which these peptides enter cells is not well understood; however, endocytosis, keratin interaction and membrane pore formation have been proposed as potential mechanisms60. Peptide PEs have been used successfully to topically deliver proteins such as elastin61, fluorescein62 and anti-VEGF antibody63 in preclinical studies and topical cell-penetrating peptides are in clinical development for delivery of botulinum toxin64, anti-scarring agents65 and anti-inflammatory cyclosporin A66. While topical skin delivery of antibodies using peptide PEs has not been realised in the clinic, this technology provides clear opportunities for well-tolerated topical antibody administration.
Physical penetration enhancers
Physical methods to disrupt the epithelial barrier use the application of energy to form holes through which therapeutics can enter. In most cases, these methods are used prior to the topical application of aqueous, cream or gel drug formulation; thus formulating antibodies for application using physical PEs may be significantly easier than with chemical-based PEs which involve components that impact protein stability. Some of the most successful physical disruption methods for the delivery of proteins into skin include ultrasound, iontophoresis, electroporation and microneedling67.
Ultrasound induces transient permeability in the stratum corneum through the formation of small bubbles in the targeted tissue, which allow fluids to pass through as they collapse68. This method has successfully been used to deliver molecules up to a size of ~50 kDa, which may make it suitable for the delivery of antibody fragments69,70. Ultrasound delivery is capable of delivering molecules to subcutaneous structures such as joint cartilage71 and transdermally for systemic absorption70. Consequently, antibody fragment delivery by ultrasound may be suited to the treatment of deep or tunnelling pressure ulcers or abscesses, or thickened scar tissue.
Transdermal iontophoresis functions by applying charge to drug molecules and applying an external charge to drive molecules through the stratum corneum72. It has been adopted clinically for dermal delivery of anti-scarring hormones73, local anaesthesia74 and insulin75. While delivery of larger proteins has been trialled in some preclinical studies76 it is unlikely that proteins larger than 10 kDa could be delivered, and delivery of only very small antibody fragments may be achievable using this method. Depth of penetration of proteins using iontophoresis is generally under 150 µm77; thus this method is most suitable for delivery of antibody fragments to scars or for the treatment of inflammatory skin conditions, rather than deeper tissue lesions.
Electroporation methods apply high-voltage pulses across the skin to perturb the barrier and cause transient pores to form. Electroporation is currently used in the clinic for delivery of DNA and small molecules into tissues for cancer therapy78 and vaccination79 and has successfully delivered peptides and small proteins in preclinical studies80,81. While high voltages are required for transdermal protein delivery, dermal delivery may require lower voltages, which may reduce patient discomfort associated with the procedure. Electroporation has also been used preclinically to assist with cosmetic skin regeneration82 and scar resolution83, thus this method may have additional benefits for managing scarring following trauma or burns.
Microneedles are thin micro-scale projections which can pierce the skin to access structures below the stratum corneum. Microneedles can be used either to pretreat the skin to improve drug penetrance or deliver drugs directly into the skin, either via hollow bore needles for drug infusion or needle tips coated with or comprised of dissolvable drug formulation84,85. Microneedling is considered painless as the needles do not stimulate nerves within the dermis, and pores formed by microneedling are within micron range and thus close rapidly (generally within 12 hours)86. Microneedle pretreatment has improved the penetration of peptides87, anti-cancer agents88 and anti-scarring agents89 and seems amenable to the application of large biologics such as antibodies90. Microneedling itself has been shown to initiate healing responses in skin which can reduce scars and thus may serve an additional adjuvant-effect of stimulating wound healing pathways91. However, the use of microneedles as skin permeabilisation agents for topical antibody treatment may be limited by the longevity of pores formed67. As microneedles form larger pores than other physical skin penetration methods, this method is unlikely to be useful in conditions where epidermal layers are poorly adhered, such as blistering conditions. However, microneedle delivery may be particularly useful for delivering therapeutic quantities of antibodies to surgical wound edges or tissue surrounding chronic wounds, and it had been postulated that microneedles may also be a convenient method for antimicrobial delivery to infected wounds92.
Recent research efforts have focused on developing coated or dissolvable microneedles for the direct delivery of antibodies through application of a microneedle patch. The challenge of this delivery method lies in using fabrication methods amenable to maintaining the stability of the antibody — antibodies must be desiccated or solidified to form dissolvable microneedles, which may promote aggregation93. Selection of a compatible microneedle matrix is also crucial to achieving protein stability and ensuring antibodies can disperse into solution. A preclinical study recently demonstrated dissolvable hyalruonic acid-based microneedles can deliver anti-PD1 antibody transdermally to subcutaneous tumours94,95. Clinically, microneedle delivery has been trialled for influenza vaccination and shown to be safe and effective, with few adverse effects at the site96. As microneedle delivery relies on local diffusion from the puncture site, it is most suited to areas where the epidermal barrier is intact, including minor burns, scars or intact wound edges and would not be suitable for blisters. While challenges in stability of formulation still exist, microneedles may represent an effective method for dermal delivery of therapeutic antibodies.
Conclusion
Poorly healing wounds remain a significant healthcare burden, and antibody therapeutics may fill a clinical niche in treating chronic, non-healing wounds or scars. Local antibody administration has many benefits over systemic methods, including reduced therapeutic cost, higher drug availability at the site and reduced adverse effects associated with local administration. However, dermal delivery of antibodies remains to be realised within the clinic due to the delicate nature of antibody proteins and the formidable barrier of the stratum corneum. Despite these challenges, several new technologies or combinations of these technologies have the potential to render antibody therapeutics a viable treatment option for wound healing.
Acknowledgements
Conflict of interest:The authors declare no conflict of interest.
Funding: AJC is supported by an NHMRC Senior Research Fellowship (GNT#1102617).
Author(s)
Natalie E Stevens
PhD
Future Industries Institute, University of South Australia, Adelaide, SA, Australia
Email natalie.stevens@unisa.edu.au
Allison J Cowin
PhD
Future Industries Institute, University of South Australia, Adelaide, SA, Australia
Email allison.cowin@unisa.edu.au
References
- Turner CT, McInnes SJP, Cowin AJ. Therapeutic antibodies for improved wound healing. Wound Practice & Research 2015;23(1).
- Reichert JM. Antibodies to watch in 2017. MAbs 2017;9(2):167–181.
- Boyman O, Comte D, Spertini F. Adverse reactions to biologic agents and their medical management. Nat Rev Rheumatol 2014;10(10):612–27.
- Mocsai A, Kovacs L, Gergely P. What is the future of targeted therapy in rheumatology: biologics or small molecules? BMC Med 2014;12:43.
- Di Lernia V. Adalimumab for treating childhood plaque psoriasis: a clinical trial evaluation. Expert Opin Biol Ther 2017;1–4.
- Kopp T et al. Clinical improvement in psoriasis with specific targeting of interleukin-23. Nature 2015;521(7551):222–6.
- Kasutani K et al. Anti-IL-31 receptor antibody is shown to be a potential therapeutic option for treating itch and dermatitis in mice. Br J Pharmacol 2014;171(22):5049–58.
- Kovalenko P et al. Exploratory population PK analysis of Dupilumab, a fully human monoclonal antibody against IL-4Ralpha, in atopic dermatitis patients and normal volunteers. CPT Pharmacometrics Syst Pharmacol 2016;5(11):617–624.
- Gilbert SM et al. A phase I clinical trial demonstrates that nfP2X7 targeted antibodies provide a novel, safe and tolerable topical therapy for basal cell carcinoma. Br J Dermatol 2017;177(1):117–124.
- Turner CT et al. Delivery of Flightless I neutralizing antibody from porous silicon nanoparticles improves wound healing in diabetic mice. Adv Healthc Mater 2017;6(2).
- Jackson JE et al. Flii neutralizing antibodies improve wound healing in porcine preclinical studies. Wound Repair Regen 2012;20(4):523–36.
- Shah M, Foreman DM, Ferguson MW. Control of scarring in adult wounds by neutralising antibody to transforming growth factor beta. Lancet 1992;339(8787):213–4.
- Sakimoto T et al. Anti-inflammatory effect of IL-6 receptor blockade in corneal alkali burn. Exp Eye Res 2012;97(1): 98–104.
- Haidari H et al. Development of topical delivery systems for flightless neutralizing antibody. J Pharm Sci 2017;106(7):1795–1804.
- Bruno BJ, Miller GD, Lim CS. Basics and recent advances in peptide and protein drug delivery. Ther Deliv 2013;4(11):1443–67.
- Dong J, Goldenberg G. New biologics in psoriasis: an update on IL-23 and IL-17 inhibitors. Cutis 2017;99(2):123–127.
- Menter A et al. Efficacy of ixekizumab compared to etanercept and placebo in patients with moderate-to-severe plaque psoriasis and non-pustular palmoplantar involvement: results from three phase 3 trials (UNCOVER-1, UNCOVER-2 and UNCOVER-3). J Eur Acad Dermatol Venereol 2017;31(10):1686–1692.
- Strowd LC, Feldman SR. Dupilumab for atopic dermatitis. Lancet 2017;389(10086):2265–2266.
- Kopecki Z. et al. Topically applied Flightless I neutralizing antibodies improve healing of blistered skin in a murine model of Epidermolysis bullosa acquisita. J Invest Dermatol 2013;133(4):1008–1016.
- Streit M, Beleznay Z, Braathen LR. Topical application of the tumour necrosis factor-alpha antibody infliximab improves healing of chronic wounds. Int Wound J 2006;3(3):171–9.
- Mead AL et al. Evaluation of anti-TGF-beta2 antibody as a new postoperative anti-scarring agent in glaucoma surgery. Invest Ophthalmol Vis Sci 2003;44(8):3394–401.
- Boehncke WH, Prinz J, Gottlieb AB. Biologic therapies for psoriasis. A systematic review. J Rheumatol 2006;33(7):1447–51.
- Goffe B et al. An integrated analysis of thirteen trials summarizing the long-term safety of alefacept in psoriasis patients who have received up to nine courses of therapy. Clin Ther 2005;27(12):1912–21.
- Graves JE, Nunley K, Heffernan MP. Off-label uses of biologics in dermatology: rituximab, omalizumab, infliximab, etanercept, adalimumab, efalizumab, and alefacept (part 2 of 2). J Am Acad Dermatol 2007;56(1):e55–79.
- Cranmer LD, Engelhardt C, Morgan SS. Treatment of unresectable and metastatic cutaneous squamous cell carcinoma. Oncologist 2010;15(12):1320–8.
- Liu L, Li Y. The unexpected side effects and safety of therapeutic monoclonal antibodies. Drugs Today (Barc) 2014;50(1):33–50.
- Poku E et al. The safety of intravitreal bevacizumab monotherapy in adult ophthalmic conditions: systematic review. BMJ Open 2014;4(7):e005244.
- Fransen MF et al. Controlled local delivery of CTLA-4 blocking antibody induces CD8+ T-cell-dependent tumor eradication and decreases risk of toxic side effects. Clin Cancer Res 2013;19(19):5381–9.
- Vugmeyster Y et al. Pharmacokinetics and toxicology of therapeutic proteins: Advances and challenges. World J Biol Chem 2012;3(4):73–92.
- Bolge SC et al. Patient experience with intravenous biologic therapies for ankylosing spondylitis, Crohn’s disease, psoriatic arthritis, psoriasis, rheumatoid arthritis, and ulcerative colitis. Patient Prefer Adherence 2017;11:661–669.
- Walling HW, Swick BL. Update on the management of chronic eczema: new approaches and emerging treatment options. Clin Cosmet Investig Dermatol 2010;3:99–117.
- Jones RG, Martino A. Targeted localized use of therapeutic antibodies: a review of non-systemic, topical and oral applications. Crit Rev Biotechnol 2016;36(3):506–20.
- Christ O et al. Efficacy of local versus systemic application of antibody-cytokine fusion proteins in tumor therapy. Clin Cancer Res 2001;7(4):985–98.
- Chakroun N et al. Mapping the aggregation kinetics of a therapeutic antibody fragment. Mol Pharm 2016;13(2):307–19.
- Casaz P et al. Resolving self-association of a therapeutic antibody by formulation optimization and molecular approaches. MAbs 2014;6(6):1533–9.
- Roberts CJ. Protein aggregation and its impact on product quality. Curr Opin Biotechnol 2014;30:211–217.
- Ratanji KD et al. Immunogenicity of therapeutic proteins: influence of aggregation. J Immunotoxicol 2014;11(2):99–109.
- Rombach-Riegraf V et al. Aggregation of human recombinant monoclonal antibodies influences the capacity of dendritic cells to stimulate adaptive T-cell responses in vitro. PLoS One 2014;9(1):e86322.
- Kayser V et al. Glycosylation influences on the aggregation propensity of therapeutic monoclonal antibodies. Biotechnol J 2011;6(1):38–44.
- van der Kant R et al. Prediction and reduction of the aggregation of monoclonal antibodies. J Mol Biol 2017;429(8):1244–1261.
- Courtois F et al. Rational design of therapeutic mAbs against aggregation through protein engineering and incorporation of glycosylation motifs applied to bevacizumab. MAbs 2016;8(1):99–112.
- Skamris T et al. Monoclonal Antibodies Follow Distinct Aggregation Pathways During Production-Relevant Acidic Incubation and Neutralization. Pharm Res 2016;33(3):716–28.
- Jefferis R. Posttranslational modifications and the immunogenicity of biotherapeutics. J Immunol Res 2016;5358272.
- Brereton HM et al. Influence of format on in vitro penetration of antibody fragments through porcine cornea. Br J Ophthalmol 2005;89(9):1205–9.
- Li W et al. Antibody aggregation: insights from sequence and structure. Antibodies 2016;5(3):19.
- Dumoulin M et al. Single-domain antibody fragments with high conformational stability. Protein Sci 2002;11(3):500–15.
- Pham QD et al. Chemical penetration enhancers in stratum corneum — Relation between molecular effects and barrier function. J Control Release 2016;232:175–87.
- Andrews SN, Jeong E, Prausnitz MR. Transdermal delivery of molecules is limited by full epidermis, not just stratum corneum. Pharm Res 2013;30(4):1099–1109.
- Hadgraft J. Modulation of the barrier function of the skin. Skin Pharmacol Appl Skin Physiol 2001;14(Suppl 1):72–81.
- Ruela ALM et al. Evaluation of skin absorption of drugs from topical and transdermal formulations. Brazilian Journal of Pharmaceutical Sciences 2016;52:527–544.
- Varman RM, Singh S. Investigation of effects of terpene skin penetration enhancers on stability and biological activity of lysozyme. AAPS PharmSciTech 2012;13(4):1084–90.
- Karande P et al. Design principles of chemical penetration enhancers for transdermal drug delivery. Proc Natl Acad Sci U S A, 2005;102(13):4688–93.
- Marwah H et al. Permeation enhancer strategies in transdermal drug delivery. Drug Deliv 2016;23(2):564–78.
- Pillai O, Panchagnula R. Transdermal delivery of insulin from poloxamer gel: ex vivo and in vivo skin permeation studies in rat using iontophoresis and chemical enhancers. J Control Release 2003;89(1):127–40.
- Som I, Bhatia K, Yasir M. Status of surfactants as penetration enhancers in transdermal drug delivery. J Pharm Bioallied Sci 2012;4(1):2–9.
- Karande P, Mitragotri S. Enhancement of transdermal drug delivery via synergistic action of chemicals. Biochim Biophys Acta 2009;1788(11):2362–73.
- Gautam A et al. Topical delivery of protein and peptide using novel cell penetrating Peptide IMT-P8. Sci Rep 2016;6:26278.
- Nasrollahi SA et al. Cell-penetrating peptides as a novel transdermal drug delivery system. Chem Biol Drug Des 2012;80(5):639–46.
- Agrawal P et al. CPPsite 2.0: a repository of experimentally validated cell-penetrating peptides. Nucleic Acids Res 2016;44(D1):D1098–103.
- Kumar S et al. Peptides as skin penetration enhancers: mechanisms of action. J Control Release 2015;199:168–78.
- Nasrollahi SA et al. A peptide carrier for the delivery of elastin into fibroblast cells. Int J Dermatol 2012;51(8):923–9.
- Lee H, Park J, Kim YC. Enhanced transdermal delivery with less irritation by magainin pore-forming peptide with a N-lauroylsarcosine and sorbitan monolaurate mixture. Drug Deliv Transl Res 2017.
- de Cogan F et al. Topical delivery of anti-VEGF drugs to the ocular posterior segment using cell-penetrating peptides. Invest Ophthalmol Vis Sci 2017;58(5):2578–2590.
- Glogau R et al. Results of a randomized, double-blind, placebo-controlled study to evaluate the efficacy and safety of a botulinum toxin type A topical gel for the treatment of moderate-to-severe lateral canthal lines. J Drugs Dermatol 2012;11(1):38–45.
- Lopes LB et al. Cell permeant peptide analogues of the small heat shock protein, HSP20, reduce TGF-beta1-induced CTGF expression in keloid fibroblasts. J Invest Dermatol 2009;129(3):590–8.
- Guidotti G, Brambilla L, Rossi D. Cell-penetrating peptides: from basic research to clinics. Trends Pharmacol Sci 2017;38(4):406–424.
- Schoellhammer CM, Blankschtein D, Langer R. Skin permeabilization for transdermal drug delivery: recent advances and future prospects. Expert Opin Drug Deliv 2014;11(3):393–407.
- Tezel A et al. Topical delivery of anti-sense oligonucleotides using low-frequency sonophoresis. Pharm Res 2004;21(12):2219–25.
- Mitragotri S, Blankschtein D, Langer R. Ultrasound-mediated transdermal protein delivery. Science 1995;269(5225):850–3.
- Oberli MA et al. Ultrasound-enhanced transdermal delivery: Recent advances and future challenges. Ther Deliv 2014;5(7):843–57.
- Nieminen HJ et al. Delivering agents locally into articular cartilage by intense MHz ultrasound. Ultrasound Med Biol 2015;41(8):2259–2265.
- Rawat S et al. Transdermal delivery by iontophoresis. Indian J Pharm Sci 2008;70(1):5–10.
- Schmidt JB et al. New treatment of atrophic acne scars by iontophoresis with estriol and tretinoin. Int J Dermatol 1995;34(1):53–7.
- Maloney J. Local anesthesia obtained via iontophoresis as an aid to shave biopsy. Arch Dermatol 1992;128(3):331–332.
- Rossi M et al. Skin vasoreactivity to insulin iontophoresis is reduced in elderly subjects and is absent in treated non-insulin-dependent diabetes patients. Biomed Pharmacother 2004;58(10):560–565.
- Dubey S et al. Noninvasive transdermal iontophoretic delivery of biologically active human basic fibroblast growth factor. Mol Pharm 2011;8(4):1322–31.
- Bai Y et al. Transdermal delivery of proteins using a combination of iontophoresis and microporation. Ther Deliv 2014;5(5):525–36.
- Cadossi R, Ronchetti M, Cadossi M. Locally enhanced chemotherapy by electroporation: clinical experiences and perspective of use of electrochemotherapy. Future Oncol 2014;10(5):877–90.
- Lambricht L et al. Clinical potential of electroporation for gene therapy and DNA vaccine delivery. Expert Opin Drug Deliv 2016;13(2):295–310.
- Chang SL et al. The effect of electroporation on iontophoretic transdermal delivery of calcium regulating hormones. J Control Release 2000;66(2–3):127–33.
- Zhao YL et al. Induction of cytotoxic T-lymphocytes by electroporation-enhanced needle-free skin immunization. Vaccine 2006;24(9):1282–90.
- Golberg A et al. Skin rejuvenation with non-invasive pulsed electric fields. Sci Rep 2015;5:10187.
- Golberg A et al. Preventing Scars after Injury with Partial Irreversible Electroporation. J Invest Dermatol 2016;136(11):2297–2304.
- Nguyen J et al. The influence of solid microneedles on the transdermal delivery of selected antiepileptic drugs. Pharmaceutics 2016;8(4).
- Badran MM, Kuntsche J, Fahr A. Skin penetration enhancement by a microneedle device (Dermaroller) in vitro: dependency on needle size and applied formulation. Eur J Pharm Sci 2009;36(4–5):511–23.
- Kalluri H, Kolli CS, Banga AK. Characterization of microchannels created by metal microneedles: formation and closure. AAPS J 2011;13(3):473–81.
- Zhang S, Qiu Y, Gao Y. Enhanced delivery of hydrophilic peptides in vitro by transdermal microneedle pretreatment. Acta Pharm Sin B 2014;4(1):100–4.
- Naguib YW, Kumar A, Cui Z. The effect of microneedles on the skin permeability and antitumor activity of topical 5-fluorouracil. Acta Pharm Sin B 2014;4(1):94–99.
- Kumar A et al. A method to improve the efficacy of topical eflornithine hydrochloride cream. Drug Deliv 2016;23(5):1495–501.
- Li G et al. In vitro transdermal delivery of therapeutic antibodies using maltose microneedles. Int J Pharm 2009;368(1–2):109–15.
- Singh A, Yadav S. Microneedling: Advances and widening horizons. Indian Dermatol Online J 2016;7(4):244–54.
- Caffarel-Salvador E et al. Methylene blue-loaded dissolving microneedles: Potential use in photodynamic antimicrobial chemotherapy of infected wounds. Pharmaceutics 2015;7(4):397–412.
- Ita K. Transdermal delivery of drugs with microneedles-potential and challenges. Pharmaceutics 2015;7(3):90–105.
- Francis DM, Thomas SN. Progress and opportunities for enhancing the delivery and efficacy of checkpoint inhibitors for cancer immunotherapy. Adv Drug Deliv Rev 2017;114:33–42.
- Wang C et al. Enhanced cancer immunotherapy by microneedle patch-assisted delivery of anti-PD1 antibody. Nano Lett 2016;16(4):2334–40.
- Rouphael NG et al. The safety, immunogenicity, and acceptability of inactivated influenza vaccine delivered by microneedle patch (TIV-MNP 2015): a randomised, partly blinded, placebo-controlled, phase 1 trial. Lancet 2017;390(10095):649–658.



