Volume 26 Number 4
Evidence summary: Venous leg ulcers: diagnostic tools for venous disease
Emily Haesler
September 2018
Question
What diagnostic tools could be used in an evaluation of venous disease in people with or at risk of venous leg ulcers?
Summary
Venous leg ulcers (VLUs) are ulcers that occur on the lower leg due to venous insufficiency (disease).1, 2 Colour duplex ultrasound, performed by specialists is considered the gold standard for diagnosing venous disease.3, 4 Ankle brachial pressure index (ABPI) or toe brachial pressure index (TBPI) are performed using Doppler ultrasound to identify potential arterial involvement and evaluate eligibility for compression therapy.5 The CEAP Classification System is used internationally and is a valid and reliable method of classifying venous disease. A range of reliable and valid tools that evaluate signs of symptoms of venous disease can be used to determine disease severity, and change over time, particularly when evaluating response to treatment.6-8
Clinical practice recommendations
- Use colour duplex ultrasound to diagnose venous disease and evaluate its progression. (Level A).
- Perform ankle brachial pressure index or toe brachial pressure index using Doppler ultrasound to evaluate eligibility for treatments for venous disease. (Level A) Use the international CEAP system to classify symptoms of venous disease. (Level B)
- Use valid and reliable tools to evaluate venous disease and venous leg ulcer severity and to monitor response to treatment. (Level A)
- Evaluate venous symptoms while the individual is in a standing position. (Level B)
Background
Venous insufficiency describes a condition in which the venous system does not carry blood back to the heart in the most efficient manner, causing blood to pool in the veins of the lower limbs. Venous insufficiency primarily occurs due to:
- previous blood clots,
- impaired valves in the veins in the lower leg do not close sufficiently after each muscle contraction, allowing blood to flow back to a previous section of the vein (venous reflux), and
- calf muscle pump function not adequately assisting in returning blood to the heart. 1, 2
Accurate diagnosis and assessment of vascular disease assists in care planning. Repeated assessments over time can be used to evaluate response to management strategies. Reliable and valid diagnostic tests and assessment tools are available for assessing people who have a VLU or who have venous disease and are at risk of progressing to ulceration.9, 10
Evidence
Evaluation of anatomical abnormalities
Anatomical assessment using colour duplex ultrasound is used for diagnosing and evaluating venous disease.11 Colour duplex ultrasound provides an assessment of disease location and severity through measurement of the amount of retrograde venous blood flow (venous reflux, measured in seconds reflux persists after release of manual calf compression). Colour duplex ultrasound is conducted using specialised machines by specialist health professionals.
- A small case-control study established that reflux persisting beyond 0.5 seconds is indicative of significant anatomical abnormality.3 This finding was confirmed in a second case-control study that found over 96% of superficial and deep veins have retrograde flow persisting less than 0.5 seconds.4 (Level 3)
- The Venous Segmental Disease Score (VSDS) is a used when performing colour duplex ultrasound to calculate a score indicating presence and severity of venous reflux and/or venous obstruction (e.g. thrombosis). Reflux and obstruction are each scored on 10-point scales (maximum VSDS is 20). A significant increase in VSDS (p<0.0001) has been shown to correlate with the CEAP clinical classification (see below)8 (Level 3 - 4).
- Doppler ultrasound is used to conduct ABPI/TBPI to evaluate presence of peripheral arterial disease. Evaluation of arterial pathophysiology is conducted to screen individuals with venous disease for eligibility for the gold standard treatment for venous disease, compression therapy.9 Brachial systolic pressure and either ankle or toe systolic pressure are measured, and the ratio of the two measurements gives the value of the ABPI or TBPI.9, 10 (The ABPI lower cut-off point of 0.89 has a sensitivity of 97%, a specificity of 99.1% and a positive predictive value of 98.9% as an indicator of peripheral disease. Optimal upper cut-off point is 1.18.5 (Level 4)
- This suggests that individuals with an ABPI between 0.8 to 1.2 can be considered to have good arterial flow when no clinical symptoms are present9 although reproducibility of ABPI is varied.10 (Level 5)
- The ABPI or TBPI are non-invasive tests performed with handheld devices by trained health professionals.5 (Level 5)
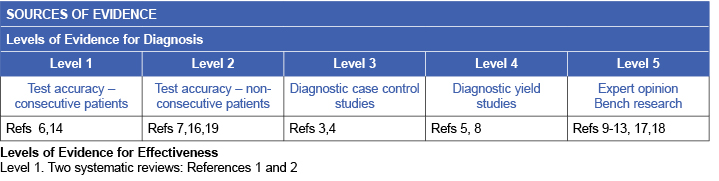
Classification of clinical signs of venous disease
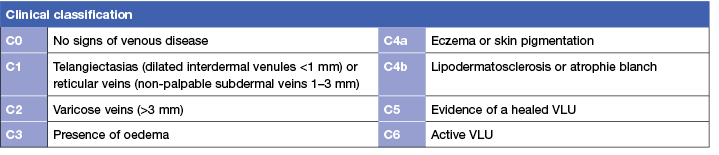
The Clinical-Etiological-Anatomical-Pathophysiological (CEAP) classification scale is an international system for classifying symptoms of venous disease. The system has four sub-scales that are used to classify clinical presentation, primary cause of venous disease, anatomical location of the affected veins and type of disease. The clinical sub-scale of the CEAP classification system consists of seven classifications from C0 to C6 describing severity of venous disease.12, 13 The sub-scale has been validated in studies that show a significant relationship between classification on the scale and both clinical symptoms14, 15 (Level 1) and abnormalities shown on duplex ultrasound.16 (Level 4).
To evaluate venous disease, inspect the lower limb while the individual is in a standing position and document presence of the signs and symptoms listed in Table 117 (Level 5).
Table 1: International CEAP classification system: Clinical sub-scale12, 13, 17, 18
Evaluation of severity of symptoms of venous disease
- The Venous Severity Scoring system (VSS) is commonly used in conjunction with the CEAP scale to evaluate the severity of venous disease. The VSS comprises three reliable and valid tools, the Venous Clinical Severity Score (VCSS), the VSDS (see above) and the Venous Disability Score (VDS). A significant increase in the VCSS score is associated with increases in CEAP categories (p<0.001).8 (Level 4)
- The Venous Clinical Severity Score (VCSS) indicates the severity of disease. Scores on this tool are associated with clinical findings on duplex ultrasound that indicate presence and severity of venous disease (venous reflux, venous flow and outflow resistance).6, 7 (Level 1 & 2) The VCSS is sensitive to changes in clinical condition over time.19 As well as providing a reliable indicator of the severity of clinical symptoms of venous disease, the VCSS is also significantly correlated with the clinical class of CEAP.19 (Level 2)
- The Venous Disability Score (VDS) is a measure of the impact of venous disease on the individual’s functional ability. The tool provides a score from 0 to 3 that describes functional level from asymptomatic to unable to carry out usual work-related activities of daily living. In one study, individuals with C3 to C6 did not have significantly higher VDS scores; however, there was a significant association between VDS and severity of pain (p<0.001).8 (Level 4)
Methodology
The development of this evidence summary is based on the Joanna Briggs Institute methodology.20 A structured database search using variations of the search terms describing VLUs and diagnostic tools was employed. Searches were conducted in EMBASE, Medline, AMED and the Cochrane Library for evidence to May 2018 in English.
Author(s)
Haesler E. for JBI Wound Healing and Management Node
References
- O’Meara S, Cullum N, Nelson E, Dumville J. Compression for venous leg ulcers. Cochrane Database Syst Rev, 2012;Issue 11(Art. No.: CD000265.
- Palfreyman S, Nelson EA, Michaels JA. Dressings for venous leg ulcers: systematic review and meta-analysis. BMJ, 2007;335(7613):244-56.
- Sarin S, Sommerville K, Farrah J, Scurr JH, Coleridge Smith PD. Duplex ultrasonography for assessment of venous valvular function of the lower lim. Br J Surg, 1994;81:1591-5.
- Labropoulos N, Tiongson J, Pryor L, Tassiopoulos AK, Kang SS, Ashraf Mansour M, Baker W. Definition of venous reflux in lower-extremity veins. J Vasc Surg, 2003 38(4):793-8.
- Weragoda J, Rohini Seneviratne R, Weerasinghe MC, Wijeyaratne SM. ABPI against colour duplex scan: A screening tool for detection of peripheral arterial disease in low resource setting approach to validation. Int J Vasc Med, 2016:Article ID 1390475.
- Garcia-Gimeno M, Rodriguez-Camarero S, Tagarro-Villalba S, Ramalle-Gomara E, Ajona Garcia JA, Gonzalez Arranz MA, Lopez Garcia D, Gonzalez-Gonzalez E, Vaquero Puerta C. Reflux patterns and risk factors of primary varicose veins’ clinical severity. Phlebology, 2013;28(3):153-61.
- Nicolaides A, Clark H, Labropoulos N, Geroulakos G, Lugli M, Maleti O. Quantitation of reflux and outflow obstruction in patients with CVD and correlation with clinical severity. Int Angiol, 2014. June;33(3):275-81.
- Gillet JL, Perrin MR, Allaert FAA. Clinical presentation and venous severity scoring of patients with extended deep axial venous reflux. J Vasc Sug, 2006;44(3):588-94.
- Scottish Intercollegiate Guidelines Network (SIGN). 2010. Management of chronic venous leg ulcers. A national clinical guideline.
- O’Donnell TF, Passman MA, Marston WA, Ennis W, Dalsing M, Robert L, Kistner RL, al. e. Management of venous leg ulcers: Clinical practice guidelines of the Society for Vascular Surgery® and the American Venous Forum. J Vasc Sug 2014;60:3S-59S.
- Maeseneer M, Pichot O, Cavezzi A, Earnshaw J, van Rij A, Lurie F, Smith PC. Duplex ultrasound investigation of the veins of the lower limbs after treatment for varicose veins - UIP consensus document. Eur J Vasc Endovasc Surg, 2011;42:89e102.
- Anonymous. Classification, severity scoring systems and terminology of chronic venous disorders. Int Angiol, 2014. April;33(2):104-10.
- Eklöf B, Rutherford R, Bergan J, et al. Revision of the CEAP classification for chronic venous disorders: consensus statement. J Vasc Surg, 2004;40(6):1248-52.
- Vuylsteke ME, Thomis S, Guillaume G, Modliszewski ML, Weides N, Staelens I. Epidemiological study on chronic venous disease in belgium and luxembourg: prevalence, risk factors, and symptomatology. Eur J Vasc Endovasc Surg, 2015;49(4):432-9.
- Marston WA, Vasquez MA, Lurie F, Wakefield TW, Rabe E, Shortell CK, Lohr JM, Passman MA, McLafferty RB. Multicenter assessment of the repeatability and reproducibility of the revised Venous Clinical Severity Score (rVCSS). Journal of Vascular Surgery: Venous and Lymphatic Disorders, 2013. July;1(3):219-24.
- Matic PA, Vlajinac HD, Marinkovic JM, Maksimovic MZ, Radak DJ. Chronic venous disease: Correlation between ultrasound findings and the clinical, etiologic, anatomic and pathophysiologic classification. Phlebology, 2014. 01 Sep;29(8):522-7.
- Fitridge R. Varicose veins: Natural history, assessment and management. Aust Fam Phy, 2013;42(6):380-84.
- O’Donnell TF, Passman MA, Marston WA, Ennis WJ, Dalsing M, Robert L, Kistner RL, Fedor Lurie F, Henke PK, Gloviczki ML, Eklöf BG, Stoughton J, Raju S, Shortell CK, Raffetto JD, Partsch H, Pounds LC, Cummings ME, Gillespie DL, McLafferty RB, Murad MH, Wakefield TW, Gloviczki P. Management of venous leg ulcers: Clinical practice guidelines of the Society for Vascular Surgery® and the American Venous Forum. J Vasc Sug, 2014;60:3S-59S.
- Lattimer CR, Kalodiki E, Azzam M, Geroulakos G. Responsiveness of individual questions from the Venous Clinical Severity Score and the Aberdeen Varicose Vein Questionnaire. Phlebology, 2014b;29(1):43-51.
- The Joanna Briggs Collaboration. Handbook for Evidence Transfer Centers – Version 4. The Joanna Briggs Institute, Adelaide. 2013



