Volume 26 Number 4
Surfaces to enhance matrix deposition for wound healing
T Fernandez, XL Strudwick, S Al-Bataineh, RD Short, AJ Cowin, JD Whittle and LE Smith
Keywords chronic wound, Dermal replacement, electrospinning, plasma polymer, extracellular matrix.
Abstract
Introduction: The next generation of wound dressings will actively participate in the wound healing process, helping the body to heal itself. For deep and/or chronic wounds a dermal scaffold is often required prior to the application of a skin graft or other therapy that encourages re-epithelialisation and the restoration of the barrier function. The aim of this study was to determine if electrospinning and plasma polymerisation could be used to generate a scaffold that would support fibroblast growth and extracellular deposition.
Methods: A totally synthetic electrospun dermal replacement was produced coated with an allylamine plasma polymer and its impact upon cellular processes assessed in vitro.
Results: The scaffold encouraged increased adhesion and rapid migration of human dermal fibroblasts into the scaffold. Fibroblasts rapidly proliferated to populate the scaffold and deposited significantly more collagen I on scaffolds coated with the allylamine plasma polymer than the uncoated poly(lactic acid) scaffold or scaffolds coated with other plasma polymers (acrylic acid or 1,7’ octadiene).
Conclusions: Electrospinning and plasma polymerisation are both versatile and widely used techniques that are also suitable for large-scale manufacture of scaffolds. These properties increase the potential of the scaffold to include its use as a cell delivery device for the delivery of fibroblasts, including their associated extracellular matrix and mitogens to chronic wounds.
Introduction
Wounds create a breach in the cutaneous barrier, thus increasing the susceptibility for infection by invading pathogens. Accordingly, skin possesses a robust regenerative capacity to repair this protective barrier upon injury, and quickly triggers a network of cellular and biochemical processes to heal the wound. However, the dysregulation of these normal healing responses can result in either too little, or in the case of excessive scar formation, too much, healing. Scars are not only unsightly, but often require painful and costly revisionary surgeries due to a functional loss of elasticity, decreased tensile strength and are prone to contracture1. Conversely, the formation of a chronic wound may occur, in which there is an abnormally prolonged healing phase, and may result in a recurrent or non-healing wound. Chronic wounds represent a major burden to healthcare systems and significantly impact sufferers through a loss of mobility, long-term pain and decreased productivity2. Hence, creating innovative and more adequate solutions to manage wound care is a major priority.
Whilst rapid re-epithelialisation and the restoration of barrier function is fundamental, it is apparent that effective wound healing is reliant upon the repair of both the dermal and epidermal layers of the skin. Re-epithelialisation can only occur where there is a substrate or provisional matrix across which the keratinocytes can migrate3. Indeed, the long-term success of skin grafts can be enhanced where an intermediate dermal scaffold is provided4,5. Likewise, the healing of chronic wounds is increased with the application of a dermal substitute to support re-epithelialisation6. Furthermore, the deposition and alignment of collagen fibres by fibroblasts within the newly formed dermis greatly influences the functionality, stability and physical appearance of the scar7.
Apart from addressing the underlying aetiology of chronic wounds and scar formation, several different approaches to enhancing wound healing have been researched and trialled. Cell therapy approaches involve the in vitro expansion of autologous or allogeneic skin cells which are subsequently reintroduced into the wound bed, thus releasing various cytokines and growth factors required for the appropriate repair response8,9. Such cell therapies require specialised cell delivery vehicles, which are able to facilitate both the attachment and growth of cells as well as the transfer of cellular material onto the wound bed10-13. The manufacturing of cell and growth factor therapies for the clinical treatment of wounds requires clearly defined materials and techniques to minimise risks to the patient. The use of animal-derived components in the preparation of such treatments creates the potential for the transmission of pathogens14.
To address this, synthetic biomaterials and surfaces with the capacity to maintain the xeno-free cultivation of human cells have been extensively studied15,16. Synthetic polymers have been used to provide uniform, readily available material for the formation of dermal replacements, effectively removing concerns regarding safety that are seen with human and animal-derived scaffolds4. The deposition of synthetic, ultrathin polymer films with defined surface chemistries has been shown to support cellular growth in the absence of animal-derived culture components17,18. Plasma polymerisation (the fragmentation and deposition of a gaseous monomer to initiate polymerisation and cross-linking onto a surface) has been applied to the in vitro cultivation of various primary and stem cell types. Significantly, surfaces plasma polymerised with acrylic acid and allyl alcohol monomers are favourable for primary keratinocyte attachment at comparable levels to collagen I19.
We investigate here the possibility of using plasma polymerisation to generate a functionally active, temporary scaffold which not only provides an immediate structure for fibroblast attachment, growth and migration within a wound, but also stimulates rapid deposition of native extracellular matrix (ECM), leading to a full and rapid repair of both acute and chronic wounds.
Methods
Plasma polymerisation of surfaces
Sterile substrates to be coated were placed into a custom-built plasma reactor, described previously20, comprising a cylindrical stainless steel vacuum vessel with a diameter of 30 cm and a volume of approximately 20 litres. The reactor was pumped down to a base pressure of 1x10-4 mbar, using a two-stage rotary pump with a liquid N2 cold trap. A needle valve (Chell, UK) was used to control the flow of monomer vapour into the reaction chamber.
The monomers underwent three freeze/thaw cycles prior to use to remove dissolved gases. The plasma parameters were optimised for maximum functional group retention. A total monomer flow rate of ca. 4 sccm was used for depositing acrylic acid (AcA) (~1.4x10-2 mbar) and 1,7 octadiene (OD) (~1.9x10-2 mbar) for 20 minutes. A total monomer flow rate of ca. 5 sccm (~1.9x10-2 mbar) was used for deposition of the allylamine (AAm) for 35 minutes21. The flow rates were measured by the method of Yasuda22. The plasma was ignited using a radio frequency generator at 13.56 MHz and a power of 3W (ACA, OD) or 5W (AAM) read from a power meter23 was delivered via an automatic impedance matching network (Coaxial Power Systems Ltd, UK). The flow rate was allowed to stabilise for a few minutes prior to the plasma being ignited and allowed to flow for a further 5 minutes after the power was switched off, before the monomer flow was switched off and the chamber pumped back to base pressure.
X-ray photoelectron spectroscopy (XPS) was used to confirm the modification of the surface chemistry prior to ToF-SIMS analysis. A SPECS SAGE XPS system with a Phoibos 150 hemispherical analyser and MCD-9 detector was used. All results were obtained using a non-monochromated MgKα radiation source (hν: 1253.6 eV) operated at 10 kV and 20 mA (200 W). The analysed area was circular and 5 mm in diameter. Survey spectra (0–1000 eV binding energy) were collected at pass energy of 100 eV with a resolution of 0.5 eV. To determine the chemical functionalities, high-resolution spectra of the C1s core level peak were collected at pass energy of 20 eV with a resolution of 0.1 eV. Resulting spectra were analysed using CasaXPS software (Neal Fairley, UK). A linear background was used, while the full width at half-maximum was set to be between 1.3 and 1.8 eV (and fixed for all peaks in any one fit). The area under the photoelectron peaks was used to calculate the atomic percentage using manufacturer-supplied relative sensitivity factors. The synthetic peak fits used 70% Gaussian/30% Lorentzian peak shapes. All binding energies were referenced to the aliphatic C-C/C-H peak at 285.0 eV.
Time-of-Flight Secondary Ion Mass Spectrometry (ToF-SIMS)
Analysis was performed using a Physical Electronics Inc. PHI TRIFT V nano-ToF instrument equipped with a pulsed liquid metal 79Au+ primary ion gun (LMIG), operated at 30 kV. The extractor current of the ion source was maintained at 3 µA. Surface analyses were performed using ‘unbunched’ Au1 beam settings to optimise spatial resolution and ‘bunched’ Au1 beam settings to optimise mass resolution. Positive ion ToF-SIMS images (250 x 250 µm2) were collected on the cross-sections of untreated PLLA scaffolds (200 µm thickness) and allylamine plasma-modified PLLA scaffolds of different thicknesses, 50 µm, 100 µm and 200 µm. An acquisition time of 5 min was used for all images. Positive ion mass spectra (50 µm x 50 µm) were collected on the same surfaces with an acquisition time of 2 min each. Mass calibration of the spectra was done with CH3+, C2H5+, and C3H7+ ions. Experiments were performed under a vacuum of 10-8 Torr or better and in the static mode (that is, below 1012 ions per cm2) to minimise possible effects arising from sample damage.
Isolation and culture of human fibroblasts
Human dermal fibroblasts were harvested and grown from split-thickness skin grafts obtained from specimens following routine breast reductions and abdominoplasties. All patients gave informed consent for skin to be used for research through a protocol approved by the Ethical Committee at the Queen Elizabeth Hospital and the University of South Australia Human Ethics Committee. Cells were isolated from skin tissue using a previously described protocol24 and cultured in fibroblast culture media (FCM) consisting of DMEM (Glutamax, high glucose) supplemented with 10% (v/v) foetal calf serum, 100 IU/mL penicillin, 100 µg/mL streptomycin and 0.625 µg/mL amphotericin B. Cultures were maintained in humidified incubators at 37°C with 5% CO2 and used between passages 3 and 9.
Culture of fibroblasts for analysis of collagen I deposition
5x104 human dermal fibroblasts were seeded into coated 24 well plate (Corning, Australia) in FCM supplemented with macromolecular crowders (ascorbic acid (1.76 µg/mL) and 37.5 mg/mL of Ficoll 70 and 25 mg/ml of Ficoll 400) to enhance the rate of collagen I deposition similar to protocols described in25 and grown for 8 days at 37°C, 5% CO2, 95% humidity with the media changed after 4 days.
Culture of fibroblasts on electrospun scaffolds
Mimetex® poly-L-lactic acid (PLLA) electrospun scaffolds were purchased from the Electrospinning Company, UK. The scaffolds were of three different thicknesses 50 µm, 100 µm and 200 µm with a nominal fibre diameter of 4 µm. Scaffolds were plasma polymerised with different monomers, as detailed above. All scaffolds were incubated in FCM for 24 hours prior to the seeding of fibroblasts.
Immunocytochemistry staining for collagen I
The media was removed from each well and the scaffolds were washed twice with PBS. 200µL of ice-cold methanol was added into each well and the plates were placed in a –20˚C freezer for 10 minutes. After the methanol was removed the plates were left to air dry.
For staining, each well was washed with PBS three times. Surfaces were blocked with 1% BSA (w/v) in PBS-Tween [0.1% (v/v) Tween] for one hour. The cells were incubated for 90 minutes in the primary antibody (C2456 Monoclonal anti-collagen type I Clone Col-1 from mouse ascites fluid (Sigma-Aldrich Australia)) in blocking agent (1:500). The surfaces were then washed thrice with PBS before the secondary antibody (A21237 F(ab’)2-Goat anti-Mouse IgG (H+L) Secondary Antibody, Alexa Fluor® 647 conjugate Invitrogen Life Technologies, Australia) (1:400) and DAPI (4’,6-Diamidino-2-Phenylindole, Dilactate Invitrogen Life Technologies, Australia) (1:1000) in blocking agent was added and incubated for one hour. Following removal of the secondary antibody solution, each well was washed thrice with PBS before being observed with a Nikon A1-R confocal microscope running NIS-Elements. To allow for comparisons to be made between the images, the laser power and gain were kept constant.
Analysis of cell viability
Cell viability was analysed using a resazurin assay, as detailed previously26. Statistical analysis was performed by 2-way ANOVA with a Tukey’s multiple comparisons post-test in GraphPad Prism.
Confocal microscopy
Scaffolds were fixed in 4% paraformaldehyde and stained using phalloidin-Oregon Green (lex = 496nm lem = 520nm) to label intracellular f-actin (1:300) and DAPI (4’,6-Diamidino-2-Phenylindole, Dilactate) (lex = 358nm lem = 461nm) to label the cell nuclei (1:1000) in PBS, using previously published techniques27. The transmission detector was used to visualise the unstained scaffold.
Scanning electron microscopy
Fibroblast-seeded scaffolds were imaged using scanning electron microscopy (SEM) on a FEI Quanta 450 FEG Environmental SEM at Adelaide Microscopy, using previously detailed methods28.
Results
Characterisation of plasma-modified scaffolds
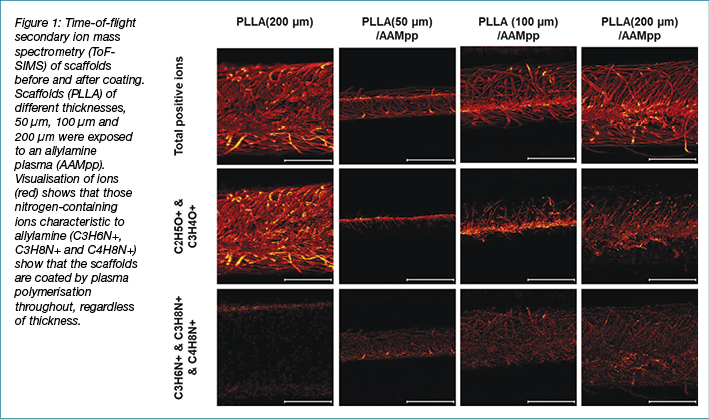
Surface modification of the electrospun poly(L-Lactic Acid) (PLLA) scaffolds was performed using plasma polymerisation of 1,7-Octadiene (OD), Acrylic Acid (AAC) or Allylamine (AAM). Whilst PLLA contains oxygen and carbon, as does AAC and OD, it does not contain nitrogen. Therefore, treatment with an AAM plasma was used to confirm the homogeneity of the plasma polymer coating using ToF-SIMS. Scaffolds of different thicknesses, 50 µm – 200 µm were coated with a plasma polymer of AAM, the scaffolds were then cross-sectioned and imaged via ToF-SIMS (Figure 1). It can be seen that after coating with the AAM plasma polymer, nitrogen-containing fragments (C3H6N+, C3H8N+ and C4H8N+) can be observed throughout the scaffolds, even up to a thickness of 200 µm. This confirms that plasma polymerisation can evenly coat these scaffolds, regardless of the scaffold thickness.
Plasma polymerised surfaces enhance fibroblast attachment
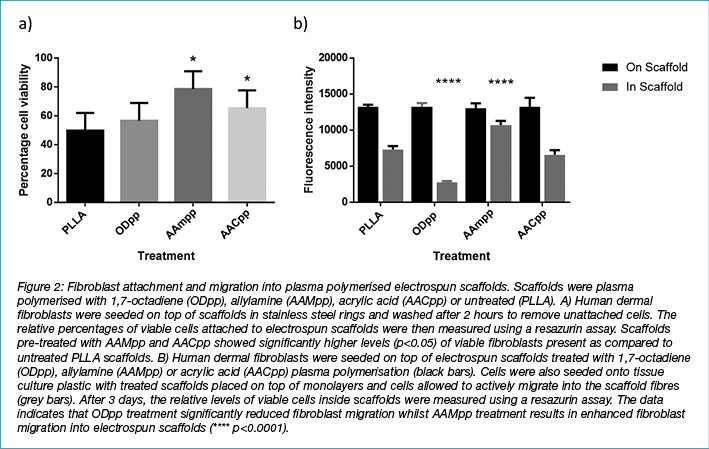
Cell attachment and migration are heavily dependent upon the adhesion of cells to their surrounding ECM. Receptors such as integrin’s are among the cellular components required for migration over a surface, by orchestrating interactions between the extracellular environment and intracellular actin filament networks28. Fibroblast attachment to electrospun scaffolds pre-treated with plasma polymerisation was investigated (Figure 2A). Plasma polymerisation of PLLA scaffolds with allylamine (AAMpp) and acrylic acid (AACpp) resulted in enhanced attachment of fibroblasts to the scaffold fibres. No significant differences were observed between PLLA and 1,7-octadiene-treated scaffolds.
Migration of fibroblasts into plasma polymerised scaffolds
Following injury, fibroblasts actively migrate into the wounded tissue to synthesise ECM and various stimulatory factors necessary for skin regeneration29. We investigated whether plasma polymerisation not only enhanced the attachment of fibroblasts, but also stimulated fibroblasts to migrate into the scaffold fibre network. Fibroblasts were seeded onto tissue culture plastic with treated and untreated scaffolds placed on top of cell monolayers. After 3 days in culture, scaffolds were removed and analysed for the presence of viable fibroblasts (Figure 2B). Scaffolds with fibroblasts seeded on top of the scaffold surface were used to compare passive seeding versus active migration. After 3 days under culture conditions, no significant differences were observed in scaffolds with fibroblasts seeded onto the upper surface. However, in the scaffolds in which fibroblasts were allowed to migrate in, significantly higher levels of viable cells were observed in the AAMpp-treated scaffolds as compared to the untreated controls. Treatment with the ODpp negative control was shown to significantly hamper the migration of cells into the scaffolds. This data indicates that AAMpp creates surfaces that are favourable for fibroblast migration.
Proliferation of fibroblasts cultured in electrospun scaffolds
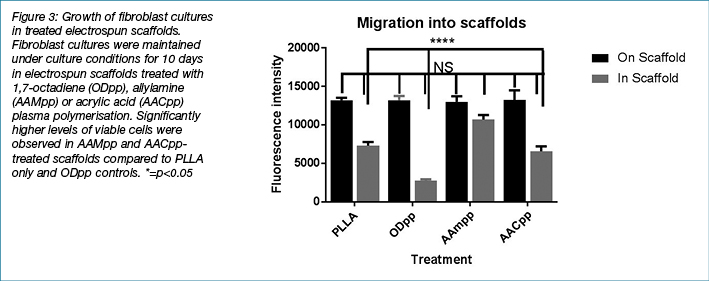
Surface modified electrospun scaffolds showed potential for enhancing fibroblast attachment and migration. Cells cultured in these scaffolds were then assayed over time to determine whether these scaffolds could maintain fibroblast cell growth. Importantly, the capacity for fibroblasts to synthesise ECM material in the electrospun scaffolds is critical for their application as a dressing for deep wounds. Over the course of 10 days, samples of fibroblast-populated electrospun scaffolds with plasma polymerised modified surfaces (as above) were assayed for viability (Figure 3). The data mirrors results shown in the cell attachment study, in which AAMpp and AACpp coated scaffolds were observed to have significantly higher levels of viable fibroblasts at Days 3, 7 and 10 under culture conditions (compared to PLLA only scaffolds).
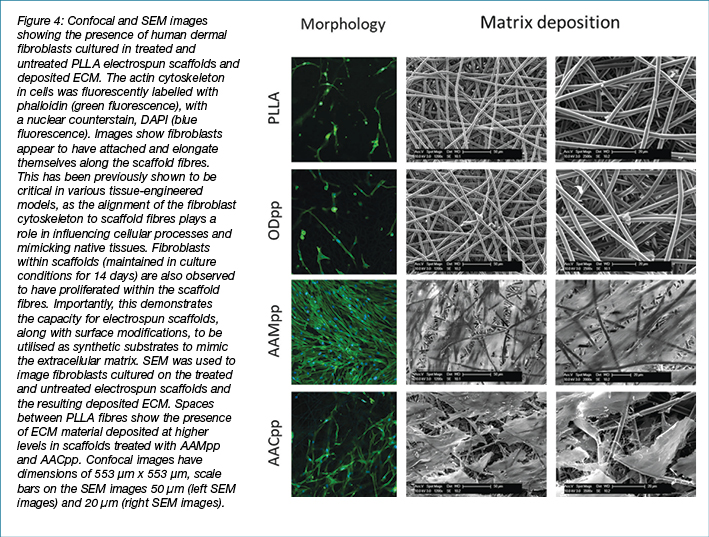
Confocal microscopy techniques were also used to analyse the cell morphology and density of fibroblasts within the various scaffolds (Figure 4). After 3 days in culture, fibroblasts migrated into scaffolds were imaged using immunofluorescent labelling. Significantly elevated cell densities were observed in AAM-treated scaffolds as compared to the other scaffold treatments. SEM was used to analyse the fibres of fibroblast-populated electrospun scaffolds at high magnification and resolution (Figure 4). SEM analysis revealed that AAMpp and AACpp coated scaffolds showed significantly higher levels of cellular matter as compared to ODpp treated and untreated controls after 2 weeks in culture. SEM analysis further highlights the capacity of scaffold fibres modified with incorporation of amine and acid surface chemistry to maintain human dermal fibroblasts under culture conditions.
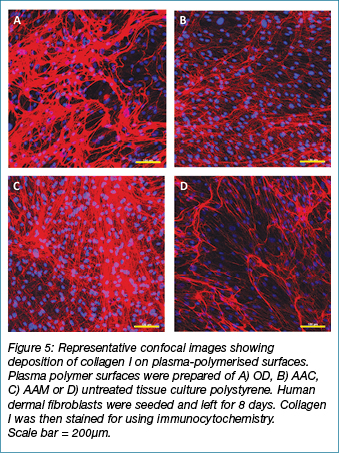
Plasma polymerised surfaces enhance deposition of collagen I
Confocal microscopy was again used to confirm differences in the deposition of extracellular matrix material on the different plasma polymerised surfaces. Due to the challenges inherent with imaging dense 3D structures the plasma polymers of OD, AAM and AAC were prepared on 2D substrates. Immunocytochemistry revealed differences in the amount of collagen I deposited onto the different plasma polymers (Figure 5). The untreated tissue culture polystyrene and the AACpp (D and B, respectively) showed much less collagen deposition than the ODpp (A), which also had fewer cells attached. The AAMpp showed the greatest amount of collagen deposited and the greatest number of cells attached which correlates with the data for the fibroblast attachment to the PLLA scaffolds in Figure 1.
Discussion
Dressings which mirror the role of the ECM in wound healing have previously been developed and trialled. For instance, Promogran® (Acelity), consisting of oxidised regenerated cellulose and collagen, has been applied to diabetic leg ulcers with some success30. Electrospun chitosan and collagen dressings have also been fabricated as substrates for fibroblast migration and the production of ECM components for wound healing31. Other dressings which serve as dermal replacements such as Alloderm (Lifecell), Biobrane (Smith & Nephew), Integra (Integralife) and Dermagraft (Advanced BioHealing) have also been developed32,33. Such dressings provide a structural scaffolding as well as ECM and growth factors required for cell migration. Encouraging stromal cell ingress into scaffolds is still a major challenge in tissue engineering and regenerative medicine. Integra® is often used beneath split-thickness skin grafts to increase the ‘take rate’ and improve surgical outcomes33,34. This bovine collagen/glycosaminoglycan dermal replacement is used to prepare the wound bed prior to the skin graft being placed. However, it still takes a relatively long time for cells to infiltrate35. Moreover, the use of biodegradable, polymeric scaffolds as a provisional dermal replacement usually requires pre-treatment with such biological molecules to enhance and encourage cellular attachment and migration.
In this study, we investigated the potential for plasma polymerisation to modify the surface chemistry of PLLA wound dressings to create a platform upon which cells can migrate and grow. The advantages of plasma polymerisation as a method of surface modification include: 1) the variety of dressing substrates that can be utilised, including those that are already used clinically; 2) properties such as the deposition of specific functional groups, surface chemistry, hydrophilicity and roughness can be controlled to optimise cell adhesion; 3) coatings are thin (nanometre thickness) and generally uniform; and 4) involve an efficient, one-step process without the use of solvents and can polymerise a wide range of polymers. Importantly, plasma polymers can facilitate the adsorption of biomolecules to aid in the migration of cells from the wound bed itself, thus negating the need for prior in vitro autologous cell expansion and transfer21,36. Additionally, we have recently shown that careful selection of surface chemistry can encourage specific cell behaviours37.
PLLA has a relatively hydrophobic surface with advancing water contact angles of 80° reported38. The application of an AAMpp to the surface reduces this to a more biocompatible 60°, whilst the application of AACpp resulted in an extremely hydrophilic surface with a water contact angle of essentially 0°37. This change in surface chemistry is homogeneously distributed throughout the scaffold. Coating the scaffold with an AAMpp has previously been shown to increase the rate of fibroblast migration across planar substrates37, the assumption then being that this would translate into a more rapid population of scaffolds for dermal replacements. The application of the AACpp and AAMpp to the scaffold significantly increased the number of fibroblasts attached to the scaffold, additionally the fibroblasts migrated into the scaffold faster. This resulted in a scaffold that became densely populated faster. The fibroblasts that populated the AACpp and AAMpp coated scaffolds were also still metabolically active, producing extracellular matrix proteins, that is collagen I. However, the use of the AAMpp coating resulted in significantly more ECM deposition. Scaffolds with this surface modification therefore show the greatest potential for mimicking the dermal structure of skin and for use in the treatment of full-thickness wounds.
Dermagraft®6,39 comprises a synthetic polyglactin (poly(glycolic acid) [PGA]) woven scaffold populated and conditioned with human dermal fibroblasts. Whilst PGA already has a water contact angle of approximately 60°40, it is the amine functionality, imparted by the AAMpp onto the scaffold used in this study, that encourages rapid fibroblast ingress rather than changes to the hydrophobicity alone37. Therefore, the application of the AAMpp to other biodegradable scaffolds to produce cell therapies similar to Dermagraft® could potentially decrease production times, resulting in a superior, more readily available product.
Blackwood et al. showed that PLLA scaffolds implanted subcutaneously into the backs of rats were still visible histologically after 12 months41. Whilst for minor injuries a dermal scaffold may not be required to remain in place for such an extended period of time, for more severe injuries and chronic wounds a longer lasting dermal scaffold could prove beneficial. The plasma polymerisation process is also substrate independent and therefore the chemistry of the bulk polymer generally does not affect the final surface chemistry. Therefore, these coatings could also be applied to scaffolds produced from polymers that biodegrade over shorter time frames.
Conclusions
The scaffold production described herein is completely synthetic, devoid of animal products, unlike the bovine collagen used in Integra®. Electrospinning and plasma polymerisation are also both extremely scalable technologies, which provide the opportunity for the cost-effective manufacture of an enhanced wound dressing. This study combines the separate processes of electrospinning with plasma polymerisation to create a scaffold with novel surface chemistry properties, which are tailored to enhance infiltration into the scaffold and matrix deposition by fibroblasts. The AAMpp coated electrospun dressing could potentially be used as an acellular dermal matrix into which the patient’s own stromal cells will migrate and populate secreting ECM to replace it as it degrades. Alternatively, the application of the AAMpp to the scaffold could be used to more rapidly populate scaffolds with stromal cells to provide a tissue engineering solution for chronic wounds.
Conflicts of interest
There are no conflicts to declare.
Funding
The authors received funding from the Wound Management Innovation Cooperative Research Centre to support this study.
Acknowledgements
The authors would like to thank the group of Professor Michael Roberts at the School of Pharmacy, University of South Australia for help with the sourcing and collection of the skin. The authors acknowledge the facilities, and the scientific and technical assistance, of the Australian Microscopy & Microanalysis Research Facility at the South Australian Node, University of South Australia.
Author(s)
T Fernandez#
PhD
Future Industries Institute, University of South Australia, Mawson Lakes, Adelaide
SA 5095, Australia
Wound Management Innovation Cooperative Research Centre, West End, QLD 4101, Australia
XL Strudwick#
PhD
Future Industries Institute, University of South Australia, Mawson Lakes, Adelaide
SA 5095, Australia
Wound Management Innovation Cooperative Research Centre, West End, QLD 4101, Australia
S Al-Bataineh
PhD
Future Industries Institute, University of South Australia, Mawson Lakes, Adelaide
SA 5095, Australia
Wound Management Innovation Cooperative Research Centre, West End, QLD 4101, Australia
RD Short
PhD
Future Industries Institute, University of South Australia, Mawson Lakes, Adelaide
SA 5095, Australia
Wound Management Innovation Cooperative Research Centre, West End, QLD 4101, Australia
AJ Cowin
PhD
Future Industries Institute, University of South Australia, Mawson Lakes, Adelaide
SA 5095, Australia
Wound Management Innovation Cooperative Research Centre, West End, QLD 4101, Australia
JD Whittle
PhD
Future Industries Institute, University of South Australia, Mawson Lakes, Adelaide
SA 5095, Australia
School of Engineering, University of South Australia, Mawson Lakes, Adelaide
SA 5095, Australia
Wound Management Innovation Cooperative Research Centre, West End, QLD 4101, Australia
LE Smith*
PhD
Future Industries Institute, University of South Australia, Mawson Lakes, Adelaide
SA 5095, Australia
Wound Management Innovation Cooperative Research Centre, West End, QLD 4101, Australia
Email louise.smith@unisa.edu.au
# Authors contributed jointly
* Corresponding author
References
- Amadeu TP, Coulomb B, Desmouliere A, Costa AM. Cutaneous wound healing: myofibroblastic differentiation and in vitro models. Int J Low Extrem Wounds 2003;2(2):60–8.
- Wysocki AB. Wound measurement. Int J Dermatol 1996;35(2):82–91.
- Clark RAF, Lanigan JM, DellaPelle P, Manseau E, Dvorak HF, Colvin RB. Fibronectin and fibrin provide a provisional matrix for epidermal cell migration during wound reepithelialization. J Investig Dermatol 1982;79(5):264–9.
- Zhong SP, Zhang YZ, Lim CT. Tissue scaffolds for skin wound healing and dermal reconstruction. Wiley Interdiscip Rev Nanomed Nanobiotechnol 2010;2(5):510–25.
- Shahrokhi S, Arno A, Jeschke MG. The use of dermal substitutes in burn surgery: acute phase. Wound Repair Regen 2014;22(1):14–22.
- Hart CE, Loewen-Rodriguez A, Lessem J. Dermagraft: use in the treatment of chronic wounds. Adv Wound Care (New Rochelle) 2012;1(3):138–41.
- Ehrlich HP, Krummel TM. Regulation of wound healing from a connective tissue perspective. Wound Repair Regen 1996;4(2):203–10.
- Hanson SE, Bentz ML, Hematti P. Mesenchymal stem cell therapy for nonhealing cutaneous wounds. Plast Reconstr Surg 2010;125(2):510–6.
- Cherubino M, Rubin JP, Miljkovic N, Kelmendi-Doko A, Marra KG. Adipose-derived stem cells for wound healing applications. Ann Plast Surg 2011;66(2):210–5.
- Zhu N, Warner R, Simpson C, Glover M, Hernon C, Kelly J et al. Treatment of burns and chronic wounds using a new cell transfer dressing for delivery of autologous keratinocytes. Eur J Plast Surg 2005;28(5):319–30.
- Moustafa M, Simpson C, Glover M, Dawson RA, Tesfaye S, Creagh FM et al. A new autologous keratinocyte dressing treatment for non-healing diabetic neuropathic foot ulcers. Diabet Med 2004;21(7):786–9.
- Chu B, Hsiao B, Hadjiargyrou M, Fang D, Zong X, Kim K. inventorCell delivery system comprising a fibrous matrix and cells; 2004.
- Wood F. Advances in isolation and expansion of human cells for clinical applications. Skin Tissue Engineering and Regenerative Medicine 2016;299–315.
- FDA. Guidance for FDA Reviewers and Industry Medical Devices Containing Materials Derived from Animal Sources (Except for In Vitro Diagnostic Devices): US Department of Health and Human Services, Food and Drug Administration, Center for Devices and Radiological Health, CDRH BSE Working Group; 2018 [updated 13/03/2018 Available from: www.fda.gov/RegulatoryInformation/Guidances/ucm073810.htm.
- Sittinger M, Bujia J, Rotter N, Reitzel D, Minuth WW, Burmester GR. Tissue engineering and autologous transplant formation: practical approaches with resorbable biomaterials and new cell culture techniques. Biomaterials 1996;17(3):237–42.
- Sakiyama-Elbert SE, Hubbell JA. Functional biomaterials: design of novel biomaterials. Annu Rev Mater Res 2001;31(1):183–201.
- Notara M, Bullett NA, Deshpande P, Haddow DB, MacNeil S, Daniels JT. Plasma polymer coated surfaces for serum-free culture of limbal epithelium for ocular surface disease. J Mater Sci Mater Med 2007;18(2):329–38.
- Higham MC, Dawson R, Szabo M, Short R, Haddow DB, MacNeil S. Development of a stable chemically defined surface for the culture of human keratinocytes under serum-free conditions for clinical use. Tissue Eng 2003;9(5):919–30.
- France RM, Short RD, Dawson RA, Macneil S. Attachment of human keratinocytes to plasma co-polymers of acrylic acid/octa-1,7-diene and allyl amine/octa-1,7-diene. J Mater Chem 1998;8(1):37–42.
- Michelmore A, Charles C, Boswell RW, Short RD, Whittle JD. Defining plasma polymerization: new insight into what we should be measuring. ACS Appl Mater Interfaces 2013;5(12):5387–91.
- Robinson DE, Al-Bataineh SA, Farrugia BL, Michelmore A, Cowin AJ, Dargaville TR et al. Plasma polymer and biomolecule modification of 3D scaffolds for tissue engineering. Plasma Process Polym 2016.
- Yasuda HK. Plasma Polymerization. London: Academic Press; 1985.
- Whittle JD, Steele DA, Short RD. Reconciling the physical and chemical environments of plasma: a commentary on “Mechanisms of plasma polymerisation — reviewed from a chemical point of view”. Plasma Process Polym 2012;9(9):840–3.
- MacNeil S, Shepherd J, Smith L. Production of Tissue-Engineered Skin and Oral Mucosa for Clinical and Experimental Use. In: Haycock JW, editor. 3D Cell Culture. Methods in Molecular Biology™. 695: Humana Press; 2011. pp. 129–53.
- Chen CZC, Peng YX, Wang ZB, Fish PV, Kaar JL, Koepsel RR et al. The Scar-in-a-Jar: Studying potential antifibrotic compounds from the epigenetic to extracellular level in a single well. Br J Pharmacol 2009;158(5):1196–209.
- Anoopkumar-Dukie S, Carey JB, Conere T, O’Sullivan E, van Pelt FN, Allshire A. Resazurin assay of radiation response in cultured cells. Br J Radiol 2005;78(934):945–7.
- Ali MJ, Mariappan I, Maddileti S, Ali MH, Naik MN. Mitomycin C in dacryocystorhinostomy: the search for the right concentration and duration — a fundamental study on human nasal mucosa fibroblasts. Ophthal Plast Reconstr Surg 2013;29(6):469–74.
- Mikael PE, Amini AR, Basu J, Josefina Arellano-Jimenez M, Laurencin CT, Sanders MM et al. Functionalized carbon nanotube reinforced scaffolds for bone regenerative engineering: fabrication, in vitro and in vivo evaluation. Biomed Mater 2014;9(3):035001.
- Rodriguez-Menocal L, Salgado M, Ford D, Van Badiavas E. Stimulation of skin and wound fibroblast migration by mesenchymal stem cells derived from normal donors and chronic wound patients. Stem Cells Transl Med 2012;1(3):221–9.
- Veves A, Sheehan P, Pham HT. A randomized, controlled trial of Promogran (a collagen/oxidized regenerated cellulose dressing) vs standard treatment in the management of diabetic foot ulcers. Arch Surg 2002;137(7):822–7.
- Chen J-P, Chang G-Y, Chen J-K. Electrospun collagen/chitosan nanofibrous membrane as wound dressing. Colloids Surf A Physicochem Eng Asp 2008;313–314(0):183–8.
- Pham C, Greenwood J, Cleland H, Woodruff P, Maddern G. Bioengineered skin substitutes for the management of burns: A systematic review. Burns 2007;33(8):946–57.
- Alnababtah K. Management of the deeper wound with Integra® tissue regenerative products. Wounds UK 2010;6(3):72–6.
- Wisser D, Steffes J. Case report: Skin replacement with a collagen based dermal substitute, autologous keratinocytes and fibroblasts in burn trauma. Burns 2003;29:375–80.
- García-Gareta E, Ravindran N, Sharma V, Samizadeh S, Dye JF. A novel multiparameter in vitro model of three-dimensional cell ingress into scaffolds for dermal reconstruction to predict in vivo outcome. BioResearch Open Access 2013;2(6):412–20.
- Robinson DE, Smith LE, Steele DA, Short RD, Whittle JD. Development of a surface to enhance the effectiveness of fibroblast growth factor 2 (FGF-2). Biomater Sci 2014;2(6):875–82.
- Smith LE, Bryant C, Krasowska M, Cowin AJ, Whittle JD, MacNeil S et al. Haptotatic plasma polymerized surfaces for rapid tissue regeneration and wound healing. ACS Appl Mater Interfaces 2016;8(48):32675–87.
- Kiss E, Bertoti I, Vargha-Butler EI. XPS and wettability characterization of modified poly(lactic acid) and poly(lactic/glycolic acid) films. J Colloid Interface Sci 2002;245(1):91–8.
- Ehrenreich M, Ruszczak Z. Update on tissue-engineered biological dressings. Tissue Eng 2006;12(9):2407–24.
- Wang B, Zhang P, Song W, Zhao L, He C. Modification of polyglycolic acid and poly lactic-co-glycolic acid fibers by ultrasonic treatment for enhancing hydrophilicity and cytocompatibility. J Industrial Textiles 2016;45(4):516–30.
- Blackwood KA, McKean R, Canton I, Freeman CO, Franklin KL, Cole D et al. Development of biodegradable electrospun scaffolds for dermal replacement. Biomaterials 2008;29(21):3091–104.



