Volume 27 Number 3
Factors contributing to wound chronicity in diabetic foot ulceration
Peta Ellen Tehan, Clare Linton, Kate Norbury, Diane White and Vivienne Chuter
Keywords chronic wound, Diabetic foot ulceration, dietary intake, protocol.
For referencing Tehan et al. Factors contributing to wound chronicity in diabetic foot ulceration. Wound Practice and Research 2019; 27(3):111-115.
DOI https://doi.org/10.33235/wpr.27.3.111-115
Abstract
Wound chronicity in diabetic foot ulceration (DFU) presents a significant cost to the healthcare system and also increases the likelihood of infection and amputation. Factors such as dietary intake, smoking, vascular status and infection have been proposed as contributory factors for chronicity. However, there is limited quality evidence to demonstrate the contribution of these factors in delayed healing in DFU. The aims of this research protocol are therefore to assess factors contributing to healing outcomes and wound chronicity in people with DFU, and to measure dietary intake in patients with DFU in an Australian setting.
Background
Diabetic foot ulceration (DFU) affects 56,000 Australians annually, with 70 Australians undergoing a diabetes-related amputation each week. Diabetes is the leading cause of non-traumatic lower limb amputation, with foot ulcer development preceding amputation in 85% of all lower limb amputation cases1. Recent estimates suggest that DFU and amputations cost the Australian healthcare system over $600 million annually2. A complex pathway leads to the development of a DFU, including the development of peripheral neuropathy (loss of sensation), peripheral arterial disease (PAD) (decreased circulation), and changes to foot shape due to the diabetes disease process3,4. This is further complicated by the increased risk of infection in patients with DFU5.
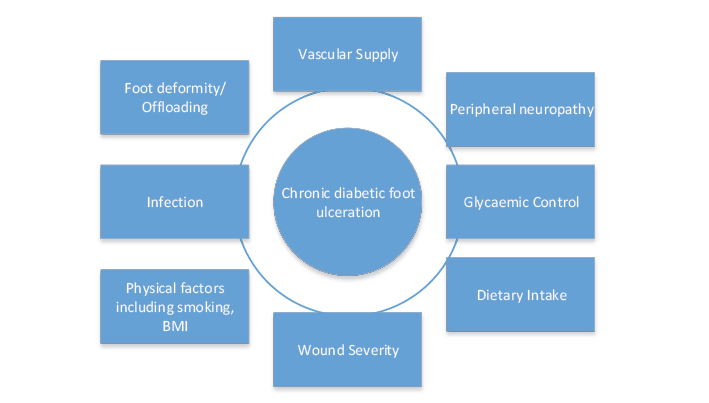
However, whilst the evidence is clear on the risk factors for the development of DFU6, and clinicians can screen and triage patient care appropriately in individuals at high risk of DFU, in relation to risk factors for wound chronicity in DFU the evidence is inconclusive. A number of factors have been proposed to increase the likelihood of chronicity in DFU such as infection, vascular supply and dietary intake (Figure 1); however, there is currently a paucity of good quality, prospective research in this area. For example, a recent systematic review by Lefrancois et al.7 identified that there is currently inadequate or low-quality evidence on a number of factors which have been implicated in wound chronicity in DFU, including dietary intake, smoking, glycaemic control, vascular supply and infection. In particular, a lack of prospective studies investigating detriments to wound healing in DFU was noted. For example, once a patient has an established DFU, it is unclear who will become chronic, defined as >12 weeks' duration8. Chronic DFU patients are more likely to undergo amputation as they are more prone to infection and wound deterioration over time5.
Aims
In order to determine which factors are contributing most to delayed healing in patients with DFU, we need to conduct a prospective cross-sectional study that will measure all of the major factors implicated in wound chronicity in DFU (Figure 1) and determine the effect each of these have on wound healing time and outcomes, including amputation. Therefore the aims of our study are primarily to assess factors contributing to healing outcomes and wound chronicity in people with DFU and secondarily to measure dietary intake in patients with DFU in an Australian setting.
Figure 1. Factors influencing wound chronicity in DFU
Method
Participants with diabetes (type 1 and 2) and current DFU attending a public health podiatry clinic will be included and will attend a single testing session. Exclusion criteria will include a contraindication for placement of the toe cuff around the hallux or second digit, previous bilateral mastectomy preventing brachial blood pressure examination, vasospastic disorders, an inability to adhere to the testing protocol, and/or an inability to give informed consent. During a 12-month period it is expected 144 participants will be recruited from across three sites (Hunter New England High Risk Foot Clinic, Wyong High Risk Foot Clinic and Gosford High Risk Foot Clinic), with 60 participants recruited to date.
In order to determine which factors are contributing to delayed healing outcomes, a number of variables will be measured. A current general medical history will be obtained from the participants’ general practitioner, including current medication, concurrent chronic disease status, overall general medical history and diabetes duration, type (1 or 2), and current HbA1c levels. HbA1c levels are reflective of glycaemia over 2-3 months and are associated with wound healing rates in patients with diabetes. Higher HbA1c levels are associated with poorer healing outcomes – with each 1.0% increase in HbA1c, a daily reduction in wound healing of 0.028 cm2 can be expected9. Demographic data, including age, gender and smoking status, will also be collected. Nicotine is a vasoconstrictor which reduces skin blood flow, resulting in localised tissue ischaemia and impaired healing capability10. Smoking has therefore been clinically associated with general delayed healing; however, extensive controlled studies are yet to be undertaken in DFU. This study will determine if – and to what level – smoking has an effect on healing outcomes in DFU.
Lower limb vascular assessment will include systolic toe pressure measurement along with a toe-brachial pressure index, both of which have been demonstrated as accurate indicators of PAD in patients with diabetes11,12. The presence of PAD and its negative impact on healing capacity is frequently underestimated, with the presence of ischaemia within a DFU not always obvious. That is, whilst purely ischaemic ulcers are readily identified by their characteristic appearance, symptoms and location, neuro-ischaemic DFUs can be more subtle in presentation13,14. This study will therefore aim to establish how much impact PAD has on healing outcomes in DFU.
An assessment of dietary intake will also be conducted by using the Australian Eating Survey (AES), a valid and reproducible method of quantifying dietary intake15 that assesses usual food and nutrient intake over the preceding 3-6 months. Wound healing requires adequate dietary intake, with poor healing outcomes associated with deficiencies in nutrition16. Nutrition deficiencies can negatively impact wound healing by prolonging the inflammatory phase, decreasing fibroblast proliferation, and altering collagen synthesis17. However, dietary intake in patients with DFU has not yet been determined in an Australian cohort. With such variation in regional dietary intake, it is important that this can be established.
Presence of infection will be determined by the International Working Group for the Diabetic Foot (IWGDF) guidelines for infection and will be collected by the treating podiatrist. Infection remains the most frequent diabetes complication requiring hospitalisation, and the most common precipitating event leading to lower extremity amputation18. This study will determine how much impact infection has on healing outcomes in DFU.
Self-reported physical activity levels will be determined using the International Physical Activity Questionnaire (IPAQ), a validated research tool to determine physical activity levels19. Weight-bearing activity influences the amount of mechanical trauma in the plantar surface of the foot, and is a contributor to DFU20. Presence of foot deformity will also be collected, along with offloading intervention type, and wound education will be given. Current guidelines recommend that patients with DFU reduce their weight-bearing activities, in addition to wearing an offloading device to assist with healing21.
Waist circumference will be determined using a tape measure, and weight and height measurements will also be taken to determine patients' body mass index. Obesity is associated with higher rates of post-operative wound infection, with reductions in tissue perfusion proposed as one contributing factor – adipose tissue is poorly vascularised and a reduction in collagen production is also frequently seen22. However, it is currently unclear how obesity impacts wound healing in patients with DFU.
Neurological assessment will be performed using a combination of two tests, including a four-site monofilament test and measurement of vibration perception threshold by a neurothesiometer at the hallux. Ascertaining neurological status will help inform the aetiology of the DFU.
Wound grade will be determined using University of Texas wound grade classification for DFU which involves assessing the size, depth and duration of the wound. Higher wound grades in chronic DFU are associated with higher rates of non-healing23. Follow-up with participants regarding their wound healing will be completed at regular intervals through a clinical note audit at 3 and 6 months after the initial assessment, where wound size will be compared to the initial wound measurement.
Statistical Analysis
To identify the relative contribution of each of these variables and their association with delayed wound healing, univariate analyses will be undertaken (Pearson’s product moment and Spearman’s Rho correlation coefficients) to identify significant associations with DFU chronicity, for example time to healing or amputation (major or minor). A Cox proportional hazards model will be used to determine the relative risks of increased time to healing, and minor and major amputation and variables identified as significantly associated in the univariate analysis. To quantify the dietary intake of people with DFU in an Australian setting, descriptive analysis will be undertaken. In addition, the role of dietary intake in chronicity of wound healing will be investigated via binomial regression analysis, with classification of the wound as normal (<12 weeks to healing) or chronic (>12 weeks to healing). The model will use factors known to influence wound chronicity such as vascular and neurological status and dietary intake as predictor variables. The level of significance will be set at 0.05 for all analyses.
Results
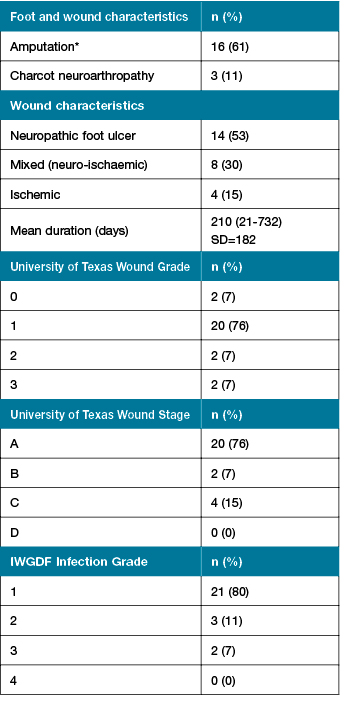
A total of 60 participants have been recruited to the study so far. Some of the descriptive statistics from the initial phase of the project, the first 26 participants recruited from Wyong High Risk Foot Clinic, are presented in Table 1. The majority of participants were male (n=25, 96%) with type 2 diabetes (n=23, 92%) and with a mean diabetes duration of 24.52 years (SD=10). The majority of foot wounds were neuropathic (n=14, 61%) as presented in Table 2. A small number of participants were currently smoking (n=2, 8%). Microvascular complications such as neuropathy (n=18, 73%), nephropathy (n=11, 44%), and retinopathy (n=10, 40%) were frequent. Symptomatic PAD was reported in 36% of participants (n=9). Diet quality was poor overall (Australian recommended food score mean 30.83, SD=9.43), with the intake of non-core (discretionary or ‘junk’) higher than recommended (37% of energy intake) and a lack in the variety in core foods (healthful foods). There were higher than recommended levels of proportion of energy derived from saturated fat (mean 14.2%) which may have cardiovascular implications. Participants demonstrated adequate levels of protein (19.6% proportion of energy intake) and zinc (15.7 mg/day) which is essential to wound healing. Mean levels of vitamin A (1281 µg/day) and vitamin C (150 mg/day) were inadequate, both which may have a negative impact on healing outcomes.
Table 1: Participant characteristics
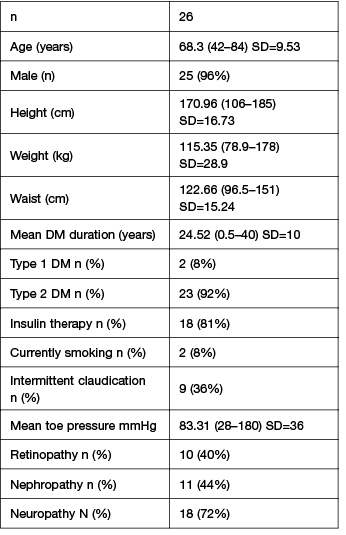
Table 2: Foot and wound characteristics
Discussion and conclusion
On completion, this prospective study will help quantify the relative contribution of factors involved in the presence of wound chronicity in patients with DFU, including providing preliminary data on the role of nutritional intake. Through identifying key risk factors for DFU chronicity more targeted research on risk factor assessment and modification will be possible. Furthermore, results from this research will help guide clinicians' assessment and management of patients with chronic DFU and, consequently, direct resources to address underlying contributors of wound chronicity in DFU patients. This will in turn reduce the number of preventable amputations in patients with DFU which is currently at a higher rate in Australia than other developed countries.
Conflict of Interest
The authors declare no conflicts of interest.
Funding
The authors received no funding for this study.
Ethics
This project has ethical approval from the Hunter New England Ethics committee HNEHREC: 17/09/20/5.06.
Author(s)
Peta Ellen Tehan*
School of Health Sciences, Faculty of Health and Medicine, University of Newcastle, NSW, Australia
Email: peta.tehan@newcastle.edu.au
Clare Linton
Gosford Hospital High Risk Foot Clinic,
Central Coast Local Health District, NSW, Australia
Kate Norbury
Wyong Hospital High Risk Foot Clinic,
Central Coast Local Health District, NSW, Australia
Diane White
John Hunter Hospital High Risk Foot Clinic, Hunter New England Local Health District, NSW, Australia
Vivienne Chuter
School of Health Sciences, Faculty of Health and Medicine, University of Newcastle, NSW, Australia
* Corresponding author
References
1. Moulik PK, Mtonga R, Gill GV. Amputation and mortality in new-onset diabetic foot ulcers stratified by etiology. Diabetes Care 2003;26(2):491–494.
2. Lazzarini P, et al. Diabetes foot disease: the Cinderella of Australian diabetes management? J Foot Ankle Res 2012;5(1):24.
3. Boulton AJM, et al. The global burden of diabetic foot disease. Lancet 2005;366(9498):1719–1724.
4. Singh N, Armstrong DG, Lipsky BA. Preventing foot ulcers in patients with diabetes. JAMA 2005;293(2):217–228.
5. Lavery LA, et al. Risk factors for foot infections in individuals with diabetes. Diabetes Care 2006;29(6):1288–1293.
6. Al-Rubeaan K, Al Derwish M, Ouizi S, Youssef AM, Subhani SN, Ibrahim HM, et al. Diabetic foot complications and their risk factors from a large retrospective cohort Study. PLoS ONE 2015;10(5) e0124446.
7. Lefrancois T, et al. Evidence based review of literature on detriments to healing of diabetic foot ulcers. Foot Ankle Surg 2017;23(4):215–224.
8. Sheehan P, et al. Percent change in wound area of diabetic foot ulcers over a 4-week period Is a robust predictor of complete healing in a 12-week prospective trial. Diabetes Care 2003;26(6):1879–1882.
9. Christman AL, et al. Hemoglobin A1c predicts healing rate in diabetic wounds. J Investigat Dermatol 2011;131(10):2121–2127.
10. Sørensen LT. Wound healing and infection in surgery: the clinical impact of smoking and smoking cessation: a systematic review and meta-analysis. Smoking and Surgical Wound Healing. JAMA Surg 2012;147(4):373–383.
11. Tehan P, Barwick AL, Sebastian M, Chuter VH. Diagnostic accuracy of resting systolic toe pressure for diagnosis of peripheral arterial disease in people with and without diabetes: a cross-sectional retrospective case-control study. J Foot Ankle Res 2017;10(58).
12. Tehan PE, Bray A, Chuter VH. Non-invasive vascular assessment in the foot with diabetes: sensitivity and specificity of the ankle brachial index, toe brachial index and continuous wave Doppler for detecting peripheral arterial disease. J Diabetes Complications 2016;30(1):155–160.
13. Edmonds M, Foster A. Diabetic foot ulcers. BMJ 2006;18(332):407–410.
14. Lavery La, Armstrong DG, Vela SA, Quebedeaux TL, Fleischli JG. Practical criteria for screening patients at high risk for diabetic foot ulceration. Arch Int Med 1998;158(2):157–162.
15. Collins CE, et al. The comparative validity and reproducibility of a diet quality index for adults: the Australian Recommended Food Score. Nutrients 2015;7(2):785–98.
16. Himes, D. Protein-calorie malnutrition and involuntary weight loss: the role of aggressive nutritional intervention in wound healing. Ostomy Wound Manag 1999;45(3):46–51, 54–5.
17. Stechmiller JK. Understanding the role of nutrition and wound healing. Nutr Clin Pract 2010;25(1):61–68.
18. Prompers L, et al. High prevalence of ischaemia, infection and serious comorbidity in patients with diabetic foot disease in Europe. Baseline results from the Eurodiale study. Diabetologia 200;. 50(1):18–25.
19. Hallal P, Victoria C. Reliability and validity of the International Physical Activity Questionnaire (IPAQ). Med Sci Sports Exerc 2004;36(3): 556.
20. Maluf KS, Mueller MJ. Comparison of physical activity and cumulative plantar tissue stress among subjects with and without diabetes mellitus and a history of recurrent plantar ulcers. Clin Biomech 2003;18(7):567–575.
21. Bus, SA, et al. IWGDF guidance on footwear and offloading interventions to prevent and heal foot ulcers in patients with diabetes. Diabetes/Metabol Res Rev 2016;32(S1):25–36.
22. Armstrong M. Obesity as an intrinsic factor affecting wound healing. J Wound Care 1998;7(5):220–221.
23. Margolis DJ, Allen-Taylor L, Hoffstad O, Berlin JA. Diabetic neuropathic foot ulcers: the association of wound size, wound duration, and wound grade on healing. Diabetes Care 2002;25(10):1835–9.



