Volume 27 Number 3
Negative pressure wound therapy in the management of complex lower limb wounds: a case series highlighting outpatient care with small single-use devices
Leonardo Zandavalli Cordova, Jennifer Martins and Patricia Terrill
Keywords wound healing, Negative-pressure wound therapy, wound closure techniques
For referencing Cordova et al. Negative pressure wound therapy in the management of complex lower limb wounds: a case series highlighting outpatient care with small single-use devices. Wound Practice and Research 2019; 27(3):116-121.
DOI https://doi.org/10.33235/wpr.27.3.116-121
Abstract
Background Traditionally, the treatment of exposed tendon has required vascularised tissue coverage, usually with complex surgical intervention. The introduction of negative pressure wound therapy (NPWT) has challenged this traditional reconstructive concept. Unfortunately, standard NPWT units are usually bulky, noisy and curtail the mobility and lifestyle of patients. The introduction of small, modern, lightweight, portable, single-use NPWT units have therefore revitalised the use of NPWT and provided a solution to the many problems encountered with the larger devices.
Case series This study highlights three cases of patients with complex lower limb wounds with tendon exposure who were successfully treated with single-use NPWT in an outpatient setting. The median time for complete wound granulation was 5–10 weeks. Minor sharp debridement of wounds was required to encourage granulation tissue formation and this was well tolerated in the outpatient setting.
Conclusion Portable NPWT units provide an alternative to surgical reconstruction in patients with lower limb wounds with exposed tendon. These devices can be utilised in a purely outpatient setting, thus avoid long-term hospitalisation. Older patients with high anaesthesia risk or who lack alternative reconstructive options due to vascular co-morbidities are the most appropriate candidates for this treatment option.
Introduction
Traditionally the treatment of wounds with exposed tendon or bone has required the application of a well vascularised flap to achieve healing, especially for lower limb wounds1-3. The introduction of negative pressure wound therapy (NPWT) has challenged this conventional rule of reconstruction and enabled restoration of complex lower limb wounds without the necessity to import tissue to cover such defect4,5. Although NPWT has not replaced the need for reconstructive surgery, it has certainly provided the surgeon with novel approaches, specifically in the treatment of patients with co-morbidities that compromise the vascular tree.
The concept of using NPWT to encourage granulation tissue to form over bare exposed tendon and bone was initially reported in 1999 by Greer and colleagues6 and subsequently in several other case series5,7-12. More recently, studies have reported the use of dermal substitutes combined with NPWT for the cover of complex defects with exposed deep structures with successful outcomes13-22. The use of standard NPWT devices, however, have the disadvantages of being bulky and noisy, therefore curtailing patient mobility and lifestyle23. The narrated pain during dressing changes, repeated visits to the operating theatre, issues with device malfunctioning and long inpatient stays have all been shown to decrease patient satisfaction and lead to therapy discontinuation24. These shortcomings have led to the development of portable devices, especially for treatment of the smaller wound.
The PICO® (Smith and Nephew) consists of a four-layered adhesive dressing pad that has an outer high moisture vapour transmission rate film that allows one way transpiration of exudate, along with two intermediate layers that absorb wound fluid and allow airflow through the dressing for even distribution of negative pressure of 80 mmHg. The inner layer is a silicone adhesive that minimises pain upon removal. The dressing has a maximum fluid handling capacity of approximately 300 ml and it is connected via tubing to a very small and lightweight battery unit that weighs 95 g. The advantages of quietness, very small size and low complexity of use makes it very user-friendly. Wound size and fluid handling capacity can, however, serve as a limiting factors25. To our knowledge, only one report has described its use in complex wounds with tendon or bone exposed26.
In this series, we report three cases where PICO® was successfully used in an outpatient setting for the treatment of complex wounds. Complex wounds were defined as full thickness skin defects involving exposure of tendons without paratenon. Full granulation and subsequent epithelialisation of wounds was successfully achieved in all cases, thus avoiding the need for complex surgery.
Cases Series
Case 1
A 67-year-old woman presented to clinic with a delayed wound breakdown over the peroneal compartment of her left leg following the harvest of a free vascularised fibula flap several weeks earlier. This resulted in a 15 x 3 cm area of exposed peroneal tendons that were superficially necrotic on the surface (Figure 1A). The patient declined hospital re-admission for surgical treatment and dressing changes. As a consequence, the superficial desiccated surface of the tendon was sharply debrided in the outpatient setting down to healthy tendon with small punctate bleeding spots (Figure 1B).
NPWT was commenced in the form of PICO® dressing. The patient underwent twice weekly dressings changes with aid from an outreach community nursing program. Over the following month, granulation tissue appeared on the bare tendon (Figure 1C). Sharp debridement was used on further clinic reviews to resect small areas of non-viable tendon and was well tolerated by the patient. The wound had reduced in size to 10 x 1 cm and was completely granulated by 9 weeks, at which point the PICO® was discontinued. The wound continued to heal by epithelialisation from the edges and achieved complete healing by 13 weeks (Figure 1D).
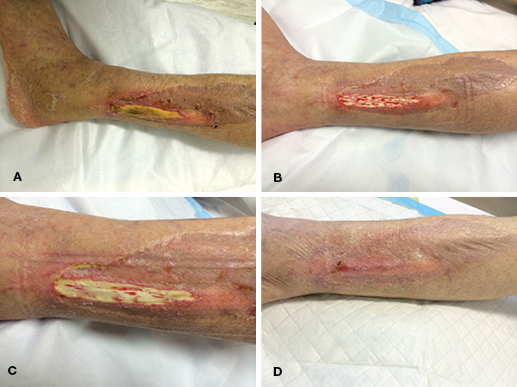
Figure 1. Case 1 treatment progress. (A) Initial necrotic wound over left peroneal compartment; (B) Small punctate bleeding post initial debridement; (C) Small areas of granulation tissue forming over tendon; (D) Wound healed by 13 weeks.
Case 2
A 73-year-old woman with a background of scleroderma underwent a wide-local excision of a squamous cell carcinoma on the dorsum of her right foot, followed by a split-skin graft reconstruction (Figure 2A). The grafted skin had initially taken; however, she developed a Staphylococcus aureus infection, resulting in partial graft loss and exposure of the extensor hallucis longus (EHL) tendon (Figure 2B). She was treated with comparable protocol to Case 1, with initial outpatient superficial wound debridement followed by the application of PICO® dressing. Similarly, serial small outpatient removal of non-viable tendon was required prior to re-application of dressings, promoting granulation tissue formation (Figure 2C). This was also well tolerated.
Once complete granulation was achieved over the bare tendon, dressings were changed to standard moist wound healing products until epithelialisation. Post treatment, the integrity of EHL was maintained to allow functional toe extension. Would healing was complete by 10 weeks (Figure 2D).
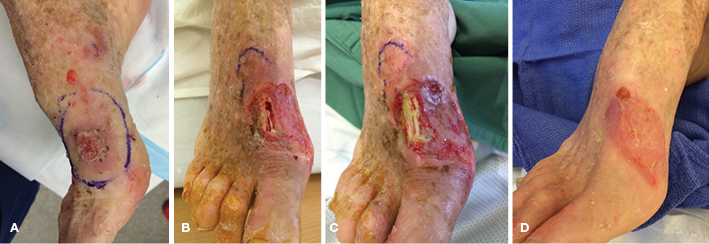
Figure 2. Case 2 treatment progress with exposed extensor hallucis longus. (A) Squamous cell carcinoma prior to operation; (B) Graft failure due to bacterial cellulitis; (C) Small areas of bleeding and granulation forming around and within the tendon; (D) Wound healed after 10 weeks.
Case 3
A non-diabetic 87-year-old woman suffering from peripheral vascular disease presented with a 1-week history of painful, erythematous abscess over her left achilles region. The day prior to admission, the abscess self-expressed, discharging purulent material. On examination, there was a red fluctuant swelling measuring 5 x 3 cm in diameter with surrounding cellulitis. Purulent material was expressed from a sinus over the mass. A doppler ultrasound of her lower limbs identified a small concomitant deep venous thrombosis within the soleus muscle. The wound culture grew methicillin-susceptible Staphylococcus aureus. The wound was debrided and partially closed, with the cavity packed using Aquacel Ag® (ConvaTec, USA). The infection resolved with the administration of intravenous cephalosporin and regular dressing changes.
On the fourth day of her admission, the wound measured 2.5 x 4 cm with exposed achilles tendon and no paratenon present (Figure 3A). The patient underwent an anaesthetic consult and it was concluded that she was not fit for major surgical reconstruction. Furthermore, no local skin flap coverage options were possible due to her peripheral vascular insufficiency. NPWT was therefore commenced with a standard Vacuum-Assisted Closure (V.A.C.®) device with black foam without the use of a contact layer and 125 mmhg of pressure. Over the following 2 weeks, granulation tissue started to appear within the bare tendon. On several occasions, small amounts of superficial non-viable tendon were sharply debrided which exposed small punctate areas of bleeding tissue. These areas, in turn, developed into islands of granulation tissue overlying the body of the tendon. Approximately 50% granulation was achieved by week 4 of admission; however, exposed tendon was still present (Figure 3B). At this stage, it was decided to change the standard NPWT to a small portable PICO® unit which allowed the patient to mobilise and to be discharged. The dressing was changed twice weekly.
The area had completely granulated within 6 weeks following surgery (Figure 3C). The wound area continued to reduce in size and the PICO® was ceased at 8 weeks post-operatively as the wound had reduced to 10 x 15 mm (Figure 3D). Moist wound healing dressings such as Vacutex™ (MPM Medical Inc.), Mesalt® (Mölnlycke Health Care) and honey impregnated alginate were used until full epithelialisation, which was achieved by 12 weeks.
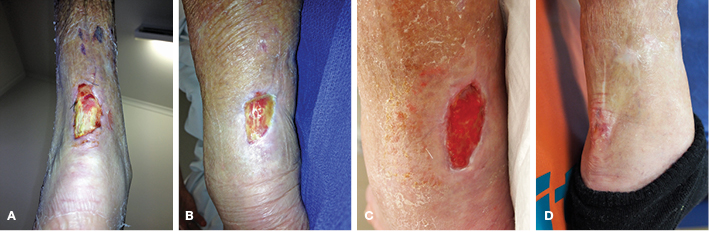
Figure 3. Case 3 treatment progress. (A) Initial necrotic wound over left posterior leg with achilles tendon exposed; (B) 50% granulation achieved post initial debridement and 2 weeks of V.A.C. dressings; (C) Completely granulated wound within 5 weeks following first operation; (D) Wound healed by 12 weeks
Discussion
The rate of complete wound granulation in our case series was between 6–9 weeks. Our results were slower than the described granulation rates with standard foam-based NPWT units which have reported granulating times between 12–46 days9-12,27. The formation of granulation tissue using smaller NPWT units seems to be thinner than those with foam, indicating that the silicone-based contact layer may have properties that resemble the gauze effect28. The integrated silicone contact layer also maintains a slightly moister wound environment compared to standard NPWT, which likely modulates the different granulation rate29. It also obviates the need for a contact layer which is required with standard NPWT with foam. In our practice, wounds with exposed tendon received a non-adherent mesh type dressing under foam such as silicone, knitted cellulose or paraffin impregnated gauze. Based on previous studies findings, infected wounds often require an antibacterial interface, usually a silver impregnated dressing30,31. Theoretically, to speed the rate of granulation tissue formation beneath a PICO® dressing, foam could be used in contact with the wound bed.
In smaller defects, NPWT appears to improve the rate of granulation tissue formation by allowing increased ingrowth from the edges of the defect and reducing wound depth32. In large areas of bare bone, the bone needs to be decorticated or trephine holes need to be made into the cortex to augment the blood supply33. Ohata and colleagues recognised the need to bring vascular supply to the surface of large bare areas of tendon27. The authors reported one case involving a large area of exposed bare achilles tendon through which they made two longitudinal splits to the ventral surface of the tendon and passed the foam of the NPWT through these splits to encourage granulations to rise up from the deep surface. We utilised sharp debridement to expose small punctate areas of bleeding on the tendon surface to the negative pressure dressing. With prevention of desiccation, islands of granulation tissue were able to form and become confluent over the whole tendon surface.
Several studies have also described techniques using NPWT with dermal substitutes, including autologous, cryopreserved homologous and acellular dermal matrices in the treatment of wounds with exposed tendon or bone. Integra®, the most commonly used artificial dermal matrix, has a recommended maturation time of between 2–4 weeks following application. This timeframe has been validated by many authors, with cited periods from the application of Integra® to skin grafting between 10–26 days11,13,16-18,20-22,34-37. The use of V.A.C. dressings overlying dermal substitutes have also been shown to accelerate maturation time, the shorter reported average being of 7.3 days in a series of eight wounds13.
However, despite the possibility to achieve healing without free tissue transfer, many issues have been reported with Integra® use. Failure rates approach 25%, dressing changes need to be made in an aseptic environment, and there is the intrinsic need for a further surgical procedure to replace the superficial silicone layer with split-skin autografts16. Furthermore, it is often necessary to minimise mobility when treating lower limb wounds to prevent shearing of the dermal layer, thus hospital stays are usually lengthy. In view of such drawbacks, the treatment of appropriate complex wounds with single-use V.A.C. dressings may show greater benefit than dermal substitutes in the cohort of patients in which significant morbidity is generated by prolonged hospital stays and immobility.
There are significant cost benefits with outpatient treatment of complex wounds with portable vacuum-assisted devices. The average spent per patient in this series was A$3192, with the weekly costs of PICO® dressings being A$195 and weekly plastic surgery clinic visits amounting to A$204. In contrast, the average cost for an admission involving microvascular tissue transfer sits at A$28,000 with an average 7-day hospital stay in Australia38. Regarding treatment with dermal substitutes, a 10 x 13 cm box of the material is valued at around A$1100, patients usually require prolonged hospital stays at an expense of A$1800 per day, and the operative price of the second stage skin graft is around A$2300. Costs can therefore easily surpass A$15,000 when all fees are accounted for.
Conclusion
Complex reconstruction with distal or local tissue transfer are fraught with difficulty in the leg and are not always suitable. Many patients do not wish for major surgery or prolonged inpatient care and, in older people, prolonged periods of bed rest and immobility may lead to further deconditioning. Portable single-use NPWT can successfully achieve tissue coverage over exposed tendon and has additional cost benefits. In our series, the use of the PICO® device allowed NPWT to continue in the outpatient setting and permitted either earlier discharge or complete avoidance of tertiary care admissions. Future studies with larger cohorts are needed to further explore these findings.
Funding and conflicts of interest
There are no conflicts of interest in the development of this article and no pharmaceutical or industry support was provided.
All authors are in agreement with the content of the manuscript presented. The manuscript has not been published previously and is not under consideration elsewhere.
Consent
Informed consent was obtained from the patients for publication of this case series and all the accompanying images.
Institutional approval was not required to publish the case series as identifiable patient information was completely excluded.
Author(s)
Leonardo Zandavalli Cordova
MBBS
Department of Plastic and Reconstructive Surgery, Frankston Hospital, Peninsula Health, VIC, Australia
Jennifer Martins
MBBS, BDsc
Department of Plastic and Reconstructive Surgery, Frankston Hospital, Peninsula Health, VIC, Australia
Patricia Terrill*
MBBS, FRACS
Department of Plastic and Reconstructive Surgery, Frankston Hospital, Peninsula Health, VIC, Australia
Email: pjterril@bigpond.net.au
* Corresponding author
References
- Melamed E, Melone CP, Jr. Coverage of hand defects with exposed tendons: the use of dermal regeneration template. Am J Orthoped 2018;47(5).
- Chen HC, Buchman MT, Wei FC. Free flaps for soft tissue coverage in the hand and fingers. Hand Clinic 1999;15(4):541–54.
- Armstrong MB, Villalobos RE, Leppink DM. Free-tissue transfer for lower-extremity reconstruction in the immunosuppressed diabetic transplant recipient. J Reconstruct Microsurg 1997;13(1):1–5.
- Dedmond BT, Kortesis B, Punger K, Simpson J, Argenta J, Kulp B, et al. The use of negative-pressure wound therapy (NPWT) in the temporary treatment of soft-tissue injuries associated with high-energy open tibial shaft fractures. J Orthopaed Trauma 2007;21(1):11–7.
- DeFranzo AJ, Argenta LC, Marks MW, Molnar JA, David LR, Webb LX, et al. The use of vacuum-assisted closure therapy for the treatment of lower-extremity wounds with exposed bone. Plast Reconstr Surg 2001;108(5):1184–91.
- Greer SE, Longaker MT, Margiotta M, Mathews AJ, Kasabian A. The use of subatmospheric pressure dressing for the coverage of radial forearm free flap donor-site exposed tendon complications. Annals Plast Surg 1999;43(5):551–4.
- Repta R, Ford R, Hoberman L, Rechner B. The use of negative-pressure therapy and skin grafting in the treatment of soft-tissue defects over the Achilles tendon. Annals Plast Surg 2005;55(4):367–70.
- Heugel JR, Parks KS, Christie SS, Pulito JF, Zegzula DH, Kemalyan NA. Treatment of the exposed Achilles tendon using negative pressure wound therapy: a case report. Journal Burn Care Rehab 2002;23(3):167–71.
- Bollero D, Carnino R, Risso D, Gangemi EN, Stella M. Acute complex traumas of the lower limbs: a modern reconstructive approach with negative pressure therapy. Wound Repair Regen 2007;15(4):589–94.
- Horch RE, Dragu A, Lang W, Banwell P, Leffler M, Grimm A, et al. Coverage of exposed bones and joints in critically ill patients: lower extremity salvage with topical negative pressure therapy. J Cutan Med Surg 2008;12(5):223–9.
- Lee HJ, Kim JW, Oh CW, Min WK, Shon OJ, Oh JK, et al. Negative pressure wound therapy for soft tissue injuries around the foot and ankle. J Orthopaed Surg Res 2009;4:14.
- Kollrack Y, Mollenhoff G. Vacuum therapy for deep wound infection after Achilles tendon repair. Z Orthop Unfall 2009;147(4):433–8.
- Molnar JA, DeFranzo AJ, Hadaegh A, Morykwas MJ, Shen P, Argenta LC. Acceleration of Integra incorporation in complex tissue defects with subatmospheric pressure. Plast Reconstr Surg 2004;113(5):1339–46.
- Brandi C, Grimaldi L, Nisi G, Silvestri A, Brafa A, Calabro M, et al. Treatment with vacuum-assisted closure and cryo-preserved homologous de-epidermalised dermis of complex traumas to the lower limbs with loss of substance, and bones and tendons exposure. JPRAS 2008;61(12):1507–11.
- Moiemen NS, Yarrow J, Kamel D, Kearns D, Mendonca D. Topical negative pressure therapy: does it accelerate neovascularisation within the dermal regeneration template, Integra? A prospective histological in vivo study. Burns 2010;36(6):764–8.
- Gonzalez Alana I, Torrero Lopez JV, Martin Playa P, Gabilondo Zubizarreta FJ. Combined use of negative pressure wound therapy and Integra(R) to treat complex defects in lower extremities after burns. Annals Burns Fire Disaster 2013;26(2):90–3.
- Hutchison RL, Craw JR. Use of acellular dermal regeneration template combined with NPWT to treat complicated extremity wounds in children. J Wound Care 2013;22(12):708–12.
- Cunningham T, Marks M. Vacuum-assisted closure device and skin substitutes for complex Mohs defects. Dermatolog Surg 2014;40 Suppl 9:S120–6.
- Kang GC, Por YC, Tan BK. In vivo tissue engineering over wounds with exposed bone and tendon: autologous dermal grafting and vacuum-assisted closure. Annals Plast Surg 2010;65(1):70–3.
- Weigert R, Choughri H, Casoli V. Management of severe hand wounds with Integra(R) dermal regeneration template. J Hand Surg 2011;36(3):185–93.
- Hayn E. Successful treatment of complex traumatic and surgical wounds with a foetal bovine dermal matrix. Int Wound J 2014;11(6):675–80.
- Milcheski DA, Chang AA, Lobato RC, Nakamoto HA, Tuma P, Jr., Ferreira MC. Coverage of deep cutaneous wounds using dermal template in combination with negative-pressure therapy and subsequent skin graft. Plast Reconstr Surg 2014;2(6):e170.
- Vig S, Dowsett C, Berg L, Caravaggi C, Rome P, Birke-Sorensen H, et al. Evidence-based recommendations for the use of negative pressure wound therapy in chronic wounds: steps towards an international consensus. J Tissue Viability 2011;20 Suppl 1:S1–18.
- Fagerdahl AM. The patient’s conceptions of wound treatment with negative pressure wound therapy. Healthcare 2014;2(3):272–81.
- Renasys SNI. PICO negative pressure wound therapy clinical guidelines 2012. Available from: https://www.smith-nephew.com/global/assets/pdf/products/wound/us/npwt-renasys-and-pico-clinical-guidelines.pdf.
- Sharp E. Single-use NPWT for the treatment of complex orthopaedic surgical and trauma wounds. J Wound Care 2013;22(10 Suppl):S5–9.
- Ohata E, Yuzuriha S, Mishima Y, Matsuo K. Longitudinal slit procedure in addition to negative pressure wound therapy for a refractory wound with exposed achilles tendon. Eplasty 2015;15:e9.
- Fraccalvieri M, Zingarelli E, Ruka E, Antoniotti U, Coda R, Sarno A, et al. Negative pressure wound therapy using gauze and foam: histological, immunohistochemical and ultrasonography morphological analysis of the granulation tissue and scar tissue. Preliminary report of a clinical study. Int Wound J 2011;8(4):355–64.
- Rippon MG, Ousey K, Cutting KF. Wound healing and hyper-hydration: a counterintuitive model. J Wound Care 2016;25(2):68, 70–5.
- Bukovcan P, Koller J, Hajska M, Zahorec P. Clinical experience with the use of negative pressure wound therapy combined with a silver-impregnated dressing in mixed wounds: a retrospective study of 50 cases. Wounds 2016;28(8):255–63.
- Baharestani M, Amjad I, Bookout K, Fleck T, Gabriel A, Kaufman D, et al. V.A.C. therapy in the management of paediatric wounds: clinical review and experience. Int Wound J 2009;6 Suppl 1:1–26.
- Schwartz JA, Goss SG, Facchin F, Gendics C, Lantis JC. Single-use negative pressure wound therapy for the treatment of chronic lower leg wounds. J Wound Care 2015;24 Suppl 2:S4–9.
- Caputo WJ, Ganime CD, Fahoury G, Longobardi S. Healing diabetic foot ulcers using cortical bone fenestration and cell therapy. Surgical Technol Int 2008;17:85–8.
- Helgeson MD, Potter BK, Evans KN, Shawen SB. Bioartificial dermal substitute: a preliminary report on its use for the management of complex combat-related soft tissue wounds. J Orthopaed Trauma 2007;21(6):394–9.
- Wollina U. One-stage reconstruction of soft tissue defects with the sandwich technique: collagen-elastin dermal template and skin grafts. J Cutan Aesthet Surg 2011;4(3):176–82.
- Violas P, Abid A, Darodes P, Galinier P, de Gauzy JS, Cahuzac JP. Integra artificial skin in the management of severe tissue defects, including bone exposure, in injured children. J Ped Orthoped 2005;14(5):381–4.
- Leitsch S, Koban KC, Pototschnig A, Titze V, Giunta RE. Multimodal therapy of ulcers on the dorsum of the hand with exposed tendons caused by Pyoderma gangrenosum. Wounds 2016;28(3):E10–3.
- Independent Hospital Pricing Authority. National hospital cost data collection: Australian public hospitals cost report 2013–2014 Round 18 [Internet]. Sydney: Independent Hospital Pricing Authority; 2014. Available from https://www.ihpa.gov.au/sites/default/files/publications/nhcdc-round18.pdf



