Volume 27 Number 3
In vitro healing efficacy of stem cell secretome and cord blood platelet lysate on a chronic wound model
Syed Sultan Beevi, Aishwarya Bhale, Nagesh Panchal, Sifa Abdul Wasay Siddiqui, Kavitha Anbarasu and Vinod Kumar Verma
Keywords Chronic wound model, secretome, platelet lysate, relative wound density, bioactive factors
For referencing Beevi et al. In vitro healing efficacy of stem cell secretome and cord blood platelet lysate on a chronic wound model. Wound Practice and Research 2019; 27(3):122-130.
DOI https://doi.org/10.33235/wpr.27.3.122-130
Abstract
Purpose A chronic wound model provides an opportunity to understand fundamental mechanisms that could possibly provide leads for novel diagnostic molecules. An in vitro chronic wound model was created to evaluate the healing potential of stem cell (SC) secretome obtained from human umbilical cord-derived mesenchymal stem cells (hUCMSCs) and cord blood platelet lysate (CBPL).
Methods hUCMSCs were obtained from explant culture of umbilical cord. SC secretome was collected from cells at passage 2 after appropriate SC characterisation. Platelets were isolated from cord blood and exposed to a repeated freeze–thaw cycle to obtain CBPL. An in vitro chronic wound model was created using dimethyl sulfoxide (DMSO) and hypoxia in HEK293 cells through scratch in a cell monolayer. Wounds were exposed to SC secretome and CBPL and their wound closure efficacy was calculated as relative wound density (RWD%) at defined time points.
Results MSCs with typical spindle-shaped morphology were isolated from explant culture that sustained their stemness and morphology until up to passage 10. DMSO efficiently impeded the movement of cells into the wound area to generate a chronic wound model which was efficiently removed upon addition of SC secretome and CBPL.
Conclusion A transient in vitro chronic wound model was created successfully to determine the healing efficiency of SC secretome and CBPL.
Introduction
Chronic wounds are a substantial clinical problem affecting millions of people worldwide. They exemplify a significant burden to patients, healthcare professionals and the entire healthcare system. Chronic wounds notably decrease the quality of life of patients by requiring continuous topical treatment, causing immobility and pain in a high percentage of patients1. They fail to progress through the normal phases of wound healing and often halt in the inflammation phase of healing2. Chronic wounds possess certain attributes such as excessive levels of proinflammatory cytokines, proteases, reactive oxygen species (ROS) and senescent cells, along with the presence of persistent infection and deficient or dysfunctional stem calls (SCs)3. Henceforth, chronic wounds necessitate a multidisciplinary approach to treat them more efficiently and more painlessly for the patient as well as more economically for healthcare funds.
It is not practically viable to study the development of chronic wounds in humans since the primary stage of development is well passed by the time these wounds appear in the clinic. Hence animal models were generated to conduct studies on the genesis of chronic wounds. However, none of the animal models was able to recapitulate the human chronic wound conditions4. Furthermore, there is no such model that could mimic human conditions and therefore evaluate the effect of novel therapeutic agents on chronic wound healing nor, likewise, the understanding of the molecular basis of healing process. In vitro models created until now were of colony biofilm model and used to study the role of flora and fauna in chronic wound healing5-7.
On therapeutic front, SC-based therapy has emerged as an alluring option for chronic wounds owing to its remedial potential8. Human umbilical cord-derived mesenchymal stem cell (hUCMSC) implantation and culture-expanded CD34+ cells of umbilical cord blood have been shown to promote the healing of cutaneous wounds in diabetic rats9,10. However, despite multiple promising results, a low survival rate after transplantation remains one of the major challenges of SC-based replacement therapy11. Secretory molecules of SCs, including growth factors, cytokines and chemokines collectively defined as SC secretome, could be an alternative approach to address the limitations of SC transplantation12,13. Several studies have demonstrated the important role of secretome/conditioned media of the SCs in promoting regeneration and accelerating the repair process in cutaneous wounds13,14. Park et al.15 have demonstrated that secretome isolated from human adipose tissue-derived MSC accelerated wound healing by stimulating the proliferative and migratory abilities of existing skin cells through PI3K/Akt or FAK-ERK1/2 signalling. However, until now, there are no studies that demonstrate the therapeutic effect of secretome from hUCMSCs in wound healing.
Platelets are widely recognised as having a crucial role in wound healing since they express and release substances that promote matrix remodelling, tissue repair/regeneration, recruitment of progenitor cells and angiogenesis16. Platelets, when lysed, release an assortment of growth factors17 which are known to participate in the activation of the healing processes that are stalled in chronic wounds. Furthermore, it has been found that platelet lysate modulates the proliferation, migration, angiogenesis, tissue repair via ERK activation, and inflammatory response by NFκB18.
With this above background, we utilised sources such as umbilical cord tissue and cord blood platelets, often considered as 'medical waste', to isolate SC secretome and cord blood platelet lysate (CBPL) and employed them as therapeutic intervention for an in vitro-modelled chronic wound. Consequently, we created a cell-based chronic wound model using external factors such as dimethyl sulfoxide (DMSO) and hypoxic condition to ascertain the efficacy of SC secretome and CBPL in chronic wound healing. To the best of our knowledge, this is the first study which evaluated the therapeutic potential of umbilical cord SC secretome and CBPL in chronic wound healing.
Materials and Methods
Dulbecco modified eagle’s medium (L-DMEM & H-DMEM) and foetal bovine serum (FBS) were purchased from Gibco. PenStrep, bFGF, sodium bicarbonate, trypsin and DMSO were procured from Sigma-Aldrich. Antibodies used in this study were procured from Stem Cell Technologies. Human embryonic kidney cells (HEK293) was obtained from NCCS Pune.
Collection of human umbilical cord tissues
Fresh human umbilical cord tissue (2–5 cm) were collected from normal deliveries (gestational period of 36–38 weeks) after informed consent from the mothers by the treating clinician. The umbilical cords (n=10) were collected in serum-free L-DMEM media supplemented with 1% PenStrep and processed within 24 hours. The collections were performed in accordance with the ethical standards of the Institutional Ethics Committee and were approved by our Institution Scientific Advisory Committee and Ethical Board (KFRC/EC/APR/0156/2014) as well as by our Institutional Committee for Stem Cell Research (KFRC/ICSCR/APR/003/2014).
Isolation and culturing of mesenchymal stem cells from the umbilical cord
The umbilical cord was sliced into 0.5–1 mm2 pieces after removal of blood vessels and thorough washing in PBS. Sliced tissues pieces were then carefully placed onto Petri plates and allowed to adhere for 30 minutes before the addition of complete growth media (L-DMEM containing 10% FBS 1% PenStrep and 10ng/mL bFGF). Explant culture was then incubated at 370C in 5% CO2 incubator and left undisturbed for 7 days to allow for migration of cells from explant. On day 7 the media was changed and on day 15 explants were removed and the media was changed thereafter every 48–72 hours. On reaching 70–80% confluency, the cells were passaged in the split ratio of 1:3.
Morphological evaluation of hUCMSCs
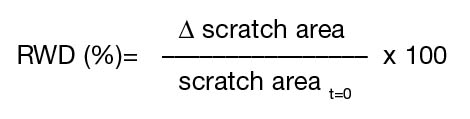
Morphology of the cultured hUCMSCs were evaluated through phase contrast microscopy and Giemsa staining. Briefly, 5 x 105 cells were grown on a coverslip for 18 hours at 370C in 5% CO2. The cells were washed with PBS, fixed with ice cold 100% methanol, and stained with 5% Giemsa solution for 30 minutes at room temperature. Coverslips were then washed with tap water, air-dried and mounted on to microscopic slides.
Growth kinetics and population doubling of hUCMSCs
Growth kinetics of hUCMSCs were evaluated by plating the cells in triplicates at a concentration of 1000 cells per cm2 in 6 cm2 petri-dishes, harvesting them daily up to day 6. Results were plotted on a linear scale as cell number versus time. The population doublings of hUCMSCs was determined by counting the number of adherent cells at the start and end of each passage. The number of population doubling was calculated by the classical formula:
![]()
where N is the number of cells at the end of the culture, N0 is the number of cells seeded, and T is the culture duration in hours.
Characterisation of hUCMSCs
Characterisation of hUCMSCs were performed using immunocytochemistry against markers specific for MSCs (CD90 and CD105) as well as markers specific for haematopoietic cells (CD34). Briefly, 5 x 104 cells were grown on a coverslip for 18 hour at 370C in 5% CO2. The cells were washed with PBS, fixed with 4% paraformaldehyde, and permeabilised with 0.25 % TritonX100. After blocking with 1% BSA, cells were incubated with fluorochrome conjugated antibody (anti-CD90-FITC, anti-CD105-FITC and anti-CD34-PE; concentration 1 mg/mL; dilution 1:50) for 1 hour in the dark at room temperature. After a PBS wash, the slides were mounted using VectashieldTM containing DAPI and imaged using an Olympus Fluorescent microscope (BX53) at 408 λ for DAPI, 450 λ for FITC and 540 λ for PE. Images were collected and processed using Olympus Digital Imaging (CellSens Entry).
Collection of SC secretome from hUCMSCs
The entire population of hUCMSCs at passage 2 without positive selection was used for the collection of secretome. Cells were seeded at the rate of 1 x 106 per 75 cm2 flasks containing complete growth medium (L-DMEM containing 10% FBS 1% PenStrep and 10ng/mL bFGF) and allowed to reach 70% confluence. After 18 hours, the cells were washed twice with PBS and incubated with 10 ml L-DMEM without FBS and PenStrep for 72 hours. After incubation, a SC-conditioned medium was collected and centrifuged twice at 1,500 rpm for 3 minutes to eliminate debris and dead cells. Then the medium was filtered through a 0.45 µm syringe filter. The serum-free conditioned media from at least five flasks were pooled and stored at –800C until further use. The total protein content for each pooled sample was determined by the Lowry method.
Preparation of cord blood platelet lysate
CBPL was prepared following the methods of Schallmoser and Strunk19. Briefly, cord blood was collected in acid citrate dextrose (ACD) and centrifuged at 400 g for 16 minutes. Plasma was collected without disturbing its buffy coat and centrifuged at 1900 g for 25 minutes to obtain platelet rich plasma (PRP). Plasma volume was then adjusted to have 1x109 platelets/mL. CBPL was made by subjecting PRP to repeat a freeze–thaw cycle for 30 minutes each which enabled the release of growth factors into the plasma. CBPL was then centrifuged at 900 g to remove platelet fragments and other cellular debris. Supernatant was pooled and stored at –200C until further use. The total protein content for each pooled sample was determined by the Lowry method.
Creation of an in vitro chronic and acute wound model
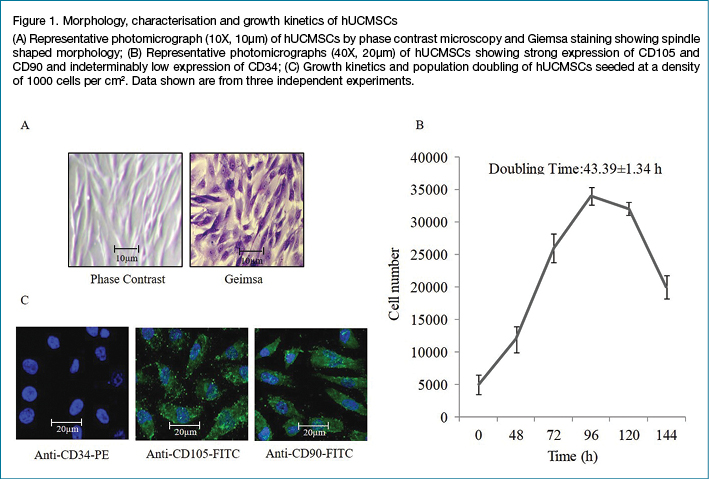
HEK293 cell line was used to create an acute and chronic wound model. Cell line was cultured in H-DMEM containing 10% FBS and 1% PenStrep and maintained at 37°C in humid air with 5% CO2. For the creation of chronic/acute wound model, cells were seeded in a 24-well plate at the rate of 7.0 × 104 cells/well and maintained at 37°C as mentioned above for 18–24 hours until the cell monolayer reached 95% confluency. The confluent monolayer was then scratched with a sterile pipette tip to generate a 12 mm wide area without cells. The cell surface was then washed three times with PBS to remove dislodged cells.
For chronic wounds, the cells were then incubated with serum-free H-DMEM media supplemented with 1% DMSO and maintained at 37°C as above for 72 hours. For the hypoxic condition, cells were incubated with serum-free H-DMEM and placed inside a hypoxia bag20. Briefly, the 24-well plate containing cells was placed inside a partially sealed plastic vacuum bag with two openings, one at each edge, to allow for gas to flow in and out of the bag. A gas mixture containing 0% O2 with 5% CO2 and 95% N2 was used to gas the vacuum bag for 5 minutes and the edges were sealed immediately to prevent equilibration with the surroundings. The sealed vacuum bag was incubated for the desired time (72 hours) in an incubator at 37°C. This gassing and sealing process was repeated every 24 hours when the culture plate was taken out for image acquisition.
In the control plate, a scratch was created as above and incubated with only the serum-free H-DMEM at 37°C for 72 hours. For the acute wound model, the wounds were created as before and treated with SC secretome and CBPL immediately.
The scratch area was monitored by acquiring images at 10X magnification at baseline (t=0 hours) and at various time points post-scratch (t=24, 48 and 72 hours) using an Olympus inverted microscope with Olympus digital imaging. Image analysis was performed using ImageJ software. The area of the scratch (μm2) was used to calculate the relative wound density (RWD%) at each time point as follows:
where Δscratch area=scratch area t=0– scratch area t=n
n=test time point
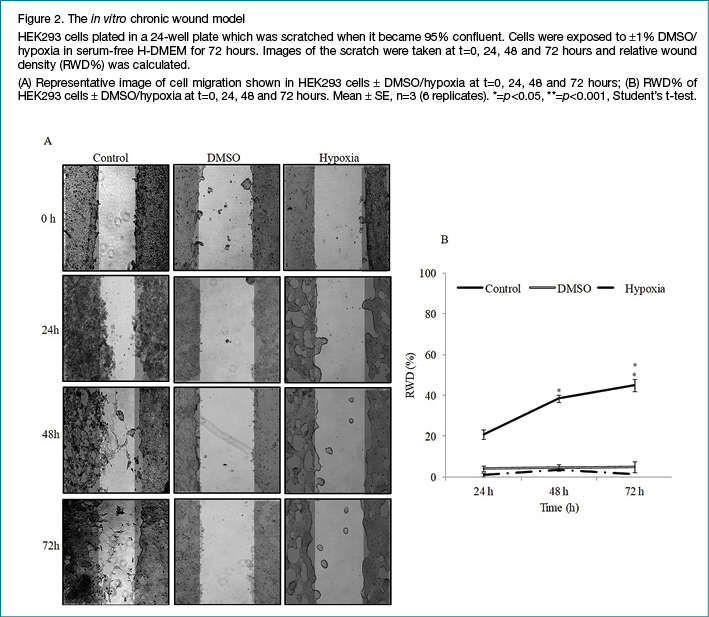
Treatment of acute and chronic wounds
Acute and chronic wounds were created as described above and treated with serum-free H-DMEM containing 0.5 mg protein/ml of either SC secretome or CBPL and maintained at 37°C for 72 hours. For the control plate, wounds were exposed to serum-free H-DMEM without any additives. Wound closure was monitored and analysed as described above.
Rate of healing efficiency was calculated for both chronic and acute wounds as follows:
![]()
Where t0=0 hours; t1=24 hours; t2=48 hours; t3=72 hours
Δt=t1 – t0; t2 – t1; t3 – t2
Statistical analysis
Every experiment was repeated at least three times and all the values were averaged. Data were expressed as mean ± standard error. Student's t-test was used to determine the difference in the efficacy of studied biological materials. A p value of <0.05 was used to establish statistical significance.
Results
Isolation and characterisation of hUCMSCs
Many cells started to migrate from explants and the first adherent cells appeared after 7 days of culturing. Explants were removed after 15 days and cells continued to proliferate and expand with a typical morphology of MSCs (fibroblastic and spindle-shaped) with multiple nucleoli. The cells were passaged in 75 cm2 flasks after 18 days of culturing. Figure 1A illustrates the morphology of hUCMSCs at passage 2 under a Phase Contrast microscope and by Giemsa stain. Cells maintained the same morphological features and exhibited a spindle shape until the 10th passage. Population doubling (PD) was calculated for all the samples collected and plotted on a linear scale as cell number versus time. Our results demonstrated that hUCMSCs showed a higher proliferation rate with the population doubling of 43.39±1.34h (Figure 1B). Furthermore, hUCMSCs showed strong positive staining for SC markers CD90 and CD105, with indeterminably low feeble staining for CD34 (Figure 1C).
Creation of the chronic wound model
The addition of 1% DMSO to the cells significantly delayed the migration of cells to the wounded area until 72 hours as compared to untreated cells. Growing cells inside the hypoxia bag also retarded the cell migration considerably (Figure 2A). RWD reached 45.1% in case of untreated cells, while cells exposed to DMSO and those grown under hypoxia showed relatively lower RWD%, enduring at 4.9% and 1.35% respectively until 72 hours (Figure 2B). Further experiments were continued with DMSO-exposed chronic wound model since hypoxia-exposed cells failed to sustain their viability post 72 hours.
Treatment of chronic and acute wounds
Both chronic and acute wounds were treated with 0.5 mg protein/ml each of SC secretome or CBPL respectively and wound closure was observed for 72 hours with RWD% calculated at 0, 24, 48 and 72 hours. Figures 3 and 4 illustrate the healing efficacy of SC secretome and CBPL respectively. CBPL showed statistically significant wound healing efficacy with RWD% of 67.1 for chronic and 77.5% for acute wounds respectively as compared to untreated cells with RWD of 7.9% for chronic and 45.1% for acute wounds at 72 hours, whereas, SC secretome-treated chronic and acute wounds recorded RWD% of 58.8 and 49.4 respectively. Interestingly, the rate of healing efficiency was comparatively exceptional for chronic wounds than acute wounds at calculated time points for both CBPL and SC secretome.
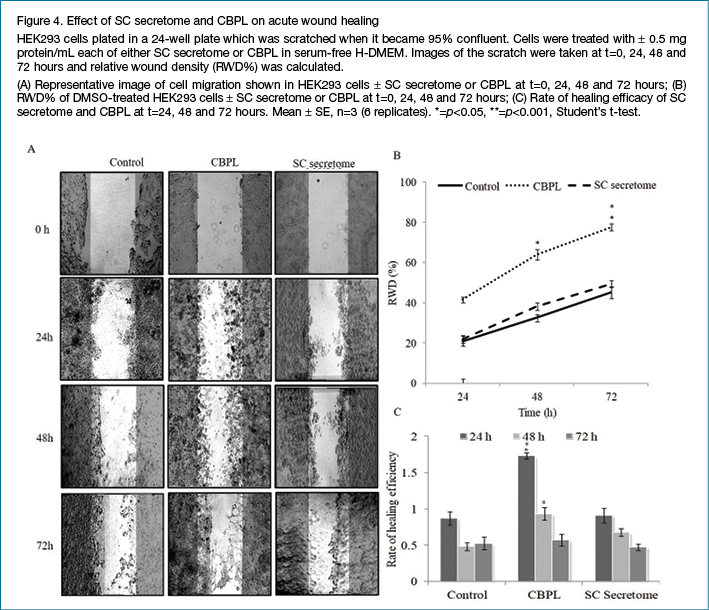
Discussion
Chronic wounds are a challenge to wound care professionals and exhaust inordinate amount of resources around the globe. Even though arduous to treat, explicit attention towards managing these can often lead to efficacious healing3. The development of a chronic wound model is imperative as it provides an opportunity to understand fundamental mechanisms involved in chronic wounds that could possibly lead to ascertaining diagnostic molecules for eventual therapeutic application.
In this study, chronic wounds were created using DMSO and hypoxia as an anti-healing factor in HEK293 cells through scratch in a cell monolayer with the intention of evaluating the healing efficacy of SC secretome and CBPL. In vitro scratch assay is an easy, low-cost and well developed method to measure cell migration in the wound area. This assay is particularly suitable to study the effects of cell–matrix and cell–cell interaction as it mimics cell migration during wound healing in vivo21. It has been reported that DMSO at low concentration prevents cell proliferation, cell migration and cytokine production22. During hypoxia, the healing cascade of inflammation, proliferation and remodelling can become impaired which can stall the wound healing process23. In line with these findings, 1% DMSO significantly inhibited the cell migration into the wound area until 72 hours without any appreciable loss in cell viability. Even though cell migration was arrested significantly under hypoxic condition, there was substantial cell death post 72 hours.
SCs promise an emerging opportunity for advancing tissue repair and regeneration and are touted as a solution for chronic non-healing wounds24. Of late, SCs have been utilised topically or as a scaffold/ECM in the management of chronic wounds25. However, although it is known that SCs positively influence wound healing, ethical constraints limit its widespread therapeutic application. Conversely, SC secretome is an important part of the SC niche and contains a complex set of molecules secreted by SCs including a variety of serum proteins, angiogenic factors, growth factors, hormones, cytokines, chemokines and ECM proteins10. Lately, platelet lysate is gradually gaining impetus as serum and growth factor supplement in SC culturing under cGMP compliant cell therapy26. Platelets derived from immune-naïve cord blood contain 10-fold higher growth factor with unaltered cytokine profile than adult platelets27. In addition, this is usually discarded during cord blood processing for SC banking. With an innovative range of growth factors, minimal immunogenicity and less ethical stringency, CBPL has immense potential in the context of wound healing and tissue regeneration.
We effectively isolated, cultured and characterised SCs from umbilical cord tissue as MSC-like cells with typical spindle-shaped morphology along with positive staining for surface markers specific for MSCs like CD90 and CD105 and feeble staining for CD34. Furthermore, we processed hUCMSCs at passage 2 for the collection of SC secretome. Platelets from cord blood was processed to obtain 5x of their initial concentration for their subsequent lysis and release of growth factors.
SC secretome and CBPL significantly stimulated cell migration into the wound area in chronic as well as in acute wounds as compared to untreated cells, implying their potential in wound healing. Interestingly, CBPL displayed more efficient healing ability as compared to SC secretome – that might be due to differences in the composition of cytokines, chemokines and growth factors. It is well known that CBPL and hUCMSCs exude a large number of bioactive molecules and many of them, such as TGF-β, EGF, FGF, VEGF and IGF, favour tissue regeneration28. It is tempting to speculate that the beneficial effect exhibited by SC secretome and CBPL could be due to these bioactive factors.
Argôlo Neto et al.29 have demonstrated that direct administration of MSCs along with PRP at the injury site reduces the reaction time for initiation of downstream effect and also activates the existing SCs in the local environment by activating PI3K/Akt or FAK/ERK1/2 signalling cascades in wound healing. Kim et al.30 have identified several growth factors, including GDF-11 in conditioned media obtained from umbilical cord blood-derived MSCs, that can stimulate skin rejuvenation by increasing extracellular matrix (ECM) production of human derived fibroblasts. Valentini et al.31 have compared cytokine profile and MSCs growth supporting ability of CBPL obtained from adult and cord blood and found that CBPL was more effective in supporting cell proliferation as compared to adult platelet lysate, highlighting a differential content of proteins between adult platelet lysate and CBPL.
Chronic wounds often encompass persistent inflammation, excessive proteolysis, impaired angiogenesis, reduced proliferation and degradation of ECM. Cells residing within the wounds have lost their ability to proliferate and migrate effectively owing to reduced progenitor cells. Impaired angiogenesis leads to wound bed injury and diminished healing, perhaps due to insufficient oxygen and nutrient supply to the cells inhabiting the wound bed32.
We have constructed a working model of chronic wound healing (illustrated as Figure 5) wherein SC secretome and CBPL, by virtue of their growth factor array, both established and non-established, could have alleviated inflammation, decreased the protein degradation, restored the progenitor cells and enhanced the angiogenesis, thereby stalling the ECM degradation and allowing the migration of epithelial cells for eventual healing process.
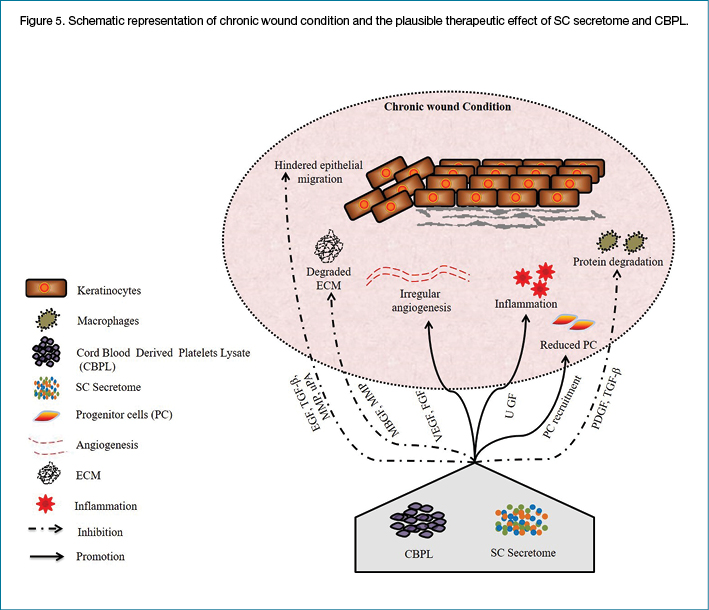
Conclusion
An in vitro chronic wound model was created to evaluate the healing efficiency of CBPL and SC secretome that were sourced from medical waste such as umbilical cord and cord blood platelets. Both CBPL and SC secretome effectively augmented the migration of cells into the wound area. Our study thus uncovers the beneficial role of growth factor-rich CBPL and SC secretome in chronic wound healing that necessitates further studies to profile the growth factors precisely responsible for wound healing as well as to understand the molecular aspect of the healing phenomenon.
Disclosure
The author reports no conflicts of interest in this work.
Funding
The authors received no funding for this study.
Author(s)
Syed Sultan Beevi* PhD
Scientist
KIMS Foundation & Research Center,
KIMS Hospitals, Secunderabad, India
Email: drsyedsultan.b@kfrc.co.in
Aishwarya Bhale
KIMS Foundation & Research Center,
KIMS Hospitals, Secunderabad, India
Nagesh Panchal
KIMS Foundation & Research Center,
KIMS Hospitals, Secunderabad, India
Sifa Abdul Wasay Siddiqui
KIMS Foundation & Research Center,
KIMS Hospitals, Secunderabad, India
Kavitha Anbarasu
KIMS Foundation & Research Center,
KIMS Hospitals, Secunderabad, India
Vinod Kumar Verma* PhD
Scientist
KIMS Foundation & Research Center,
KIMS Hospitals, Secunderabad, India
Email: drvinod.v@kfrc.co.in
* Corresponding authors
References
- Han G, Ceilley R. Chronic wound healing: a review of current management and treatments. Adv Ther 2017;34(3):599–610.
- Guo S, DiPietro LA. Factors affecting wound healing. J Dent Res 2010;89(3):219–229.
- Frykberg RG, Bank J. Challenges in the treatment of chronic wounds. Adv Wound Care (New Rochelle) 2015;4(9):560–582.
- Nunan R, Harding KG, Martin P. Clinical challenges of chronic wounds: searching for an optimal animal model to recapitulate their complexity. Dis Model Mech 2014;7(11):1205–1213.
- Omar A, Wright JB, Schultz G, Burrell R, Nadworny P. Microbial biofilms and chronic wounds. Microorganisms 2017;5(1):9.
- Yadav MK, Chae SW, Go YY, Im GJ, Song JJ. In vitro multi-species biofilms of Methicillin-Resistant Staphylococcus aureus and Pseudomonas aeruginosa and their host interaction during in vivo colonization of an Otitis Media rat model. Front Cell Infect Microbiol 2017;7:125.
- Price BL, Lovering AM, Bowling FL, Dobson CB. Development of a novel collagen wound model to simulate the activity and distribution of antimicrobials in soft tissue during diabetic foot infection. Antimicrob Agents Chemother 2016;60(11):6880–6889.
- Ebrahimian TG, Pouzoulet F, Squiban C, et al. Cell therapy based on adipose tissue-derived stromal cells promotes physiological and pathological wound healing. Arterioscler Thromb Vasc Biol 2009;29(4):503–510.
- Han Y, Sun T, Han Y, et al. Human umbilical cord mesenchymal stem cells implantation accelerates cutaneous wound healing in diabetic rats via the Wnt signaling pathway. Eur J Med Res 2019;24:10.
- 1Whiteley J, Chow T, Adissu H, Keating A, Rogers IM. Topical application of culture-expanded CD34+ umbilical cord blood cells from frozen units accelerates healing of diabetic skin wounds in mice. Stem Cells Transl Med 2018;7(8):591–601.
- Daley GQ. The promise and perils of stem cell therapeutics. Cell Stem Cell 2012;10(6):740–749.
- Vizoso FJ, Eiro N, Cid S, Schneider J, Perez-Fernandez R. Mesenchymal stem cell secretome: toward cell-free therapeutic strategies in regenerative medicine. Int J Mol Sci 2017;18(9):1852.
- Walter MN, Wright KT, Fuller HR, MacNeil S, Johnson WE. Mesenchymal stem cell-conditioned medium accelerates skin wound healing: an in vitro study of fibroblast and keratinocyte scratch assays. Exp Cell Res 2010;316 (7):1271–1281.
- Zhou BR, Xu Y, Guo SL, et al. The effect of conditioned media of adipose-derived stem cells on wound healing after ablative fractional carbon dioxide laser resurfacing. BioMed Res Int 2013;2013:519126.
- Park SR, Kim JW, Jun HS, et al. Stem cell secretome and its effect on cellular mechanisms relevant to wound healing. Mol Ther 2017;26(2):606–617.
- Etulain J. Platelets in wound healing and regenerative medicine. Platelets 2018;29(6):556–568.
- Ranzato E, Mazzucco L, Patrone M, Burlando B. Platelet lysate promotes in vitro wound scratch closure of human dermal fibroblasts: different roles of cell calcium, P38, ERK and PI3K/AKT. J Cell Mol Med 2009;13(8B):2030–2038.
- Barsotti MC, Losi P, Briganti E, et al. Effect of platelet lysate on human cells involved in different phases of wound healing. PLoS One 2013;8(12):e84753.
- Schallmoser K, Strunk D. Preparation of pooled human platelet lysate (pHPL) as an efficient supplement for animal serum-free human stem cell cultures. J Vis Exp 2009;(32):1523.
- Bakmiwewa SM, Heng B, Guillemin GJ, Ball HJ, Hunt NH. An effective low-cost method for achieving and maintaining hypoxia during cell culture studies. BioTech 2015;59(4):223–229.
- Liang CC, Park AY, Guan JL. In vitro scratch assay: a convenient and inexpensive method for analysis of cell migration in vitro. Nat Protoc 2007;2(2):329–333.
- Costa LA, Ottoni MHF, Santos MG, et al. Dimethyl sulfoxide (DMSO) decreases cell proliferation and TNF-α, IFN-γ, and IL-2 cytokines production in cultures of peripheral blood lymphocytes. Molecules 2017;22(11):1789.
- Kröger K, Dissemond J, Storck M, Risse A, Engels P. Chronic wounds: hypoxia prevents healing. Wound Manag 2012;6(5):212–217.
- Leavitt T, Hu M, Marshall C, Barnes L, Longaker M, Lorenz P. Stem cells and chronic wound healing: state of the art. Wound Care Manag Res 2016;3:7–27.
- Dash BC, Xu Z, Lin L, et al. Stem cells and engineered scaffolds for regenerative wound healing. Bioengineering 2018;5:23–42.
- Becherucci V, Piccini L, Casamassima S, et al. Human platelet lysate in mesenchymal stromal cell expansion according to a GMP grade protocol: a cell factory experience. Stem Cell Res Ther 2018;9:124.
- Shirzad N, Bordbar S, Goodarzi A, et al. Umbilical cord blood platelet lysate as serum substitute in expansion of human mesenchymal stem cells. Cell J 2017;19(3):403–414.
- Ferreira JR, Teixeira GQ, Santos SG, Barbosa MA, Almeida-Porada G, Goncalves RM. Mesenchymal stromal cell secretome: influencing therapeutic potential by cellular pre-conditioning. Front Immunol 2018;9:2837.
- Argôlo Neto NM, Del Carlo RJ, Monteiro BS, et al. Role of autologous mesenchymal stem cells associated with platelet-rich plasma on healing of cutaneous wounds in diabetic mice. Clin Exp Dermatol 2012;37(5):544–553.
- Kim YJ, Seo DH, Lee SH, et al. Conditioned media from human umbilical cord blood-derived mesenchymal stem cells stimulate rejuvenation function in human skin. Biochem Biophys Rep 2018;16:96–102.
- Valentini CG, Nuzzolo ER, Bianchi M, et al. Cord blood platelet lysate: in vitro evaluation to support the use in regenerative medicine. Mediterr J Hematol Infect Dis 2019;11(1):e2019021.
- Demidova-Rice TN, Hamblin MR, Herman IM. Acute and impaired wound healing: pathophysiology and current methods for drug delivery. Part 1: normal and chronic wounds: biology, causes, and approaches to care. Adv Skin Wound Care 2012;25(7):304–314.



