Volume 29 Number 4
Risk factors associated with medical device-related pressure injuries in the adult intensive care patient: a scoping review
Paige Weber, Laurel Weaver, Charne Miller
Keywords scoping review, medical device-related pressure injuries, intensive care unit, risk factors
For referencing Weber P et al. Risk factors associated with medical device-related pressure injuries in the adult intensive care patient: a scoping review. Wound Practice and Research 2021; 29(4):219-225.
DOI
https://doi.org/10.33235/wpr.29.4.219-225
Submitted 2 August 2021
Accepted 6 October 2021
Abstract
Aim To explore the risk factors associated with the development of medical device-related pressure injuries (MDRPI).
Method A single reviewer searched electronic databases PubMed, MEDLINE, CINAHL, The Cochrane Library and Google Scholar databases (last searched August 2020) to identify all published studies. Six studies met inclusion criteria and were evaluated for identification of risk factors associated with the development of MDRPI in the adult intensive care (ICU) patient.
Results Nine risk factors emerged as independent predictors associated with the development of MDRPIs from this review – length of stay (LOS), vasopressor administration, low Braden Scale, use of mechanical ventilation, increasing age, admission type, increasing severity of illness, development of a non-device-related hospital-acquired pressure injury (HAPI) and administration of enteral feeds. These results indicate that further research is required to identify risk factors for MDRPI development to guide research and practice.
Conclusion This scoping review has identified a modest evidence base with respect to risk factors for the development of MDRPI in the adult ICU patient. Further research identifying risk factors is required that measures a broad number of potential risk factors, including those identified in this scoping review, to enable further clarification of the relative contribution of these risk factors to MDRPI development.
Introduction
Pressure injury prevention is a long-standing priority for healthcare systems. In 2016, the National Pressure Injury Advisory Panel (NPIAP) redefined the definition of pressure injuries during the NPIAP 2016 Staging Consensus Conference to incorporate medical devices as a source of pressure1–3. A new and updated definition of a pressure injury emerged – “...localised damage to the skin and underlying soft tissue usually over a bony prominence or related to a medical or other device… the injury occurs as a result of intense and/or prolong pressure or pressure in combination with shear”3.
The knowledge of the clinical risk that medical devices present for the development of pressure injuries has been enhanced considerably; however, the literature examining the risks and mitigation remains scant. Medical devices are an integral part of the care for a person within the healthcare setting4. Specifically, patients in intensive care (ICU) have a higher exposure to a variety of medical devices such as oxygen delivery and monitoring devices. These devices include face masks, nasal cannulas, pulse oximetry, bilevel positive airway pressure masks, feeding tubes (e.g. nasogastric, oral gastric, gastric, jejunal tubes), endotracheal devices (oral and/or nasal endotracheal tubes (ETT), tracheostomy and ties), urinary (indwelling urinary catheter) and bowel elimination (faecal containment catheter), and musculoskeletal devices (cervical collar, splints and braces)5. These devices can exert pressure and/or friction on the skin and lead to pressure injuries6. As such, MDRPI are a clinical phenomenon that warrant understanding and a practice response amongst ICU healthcare professionals.
A study conducted in Australia with 179 participants reported that the incidence of developing MDRPI was 27.9%, with 68% of these injuries occurring in the ICU setting7. Of these MDRPI, 42% developed when oxygen tubing behind the ears was in situ and 26% were associated with ETT7. It is reported that the proportion of MDRPI to general pressure injury increases by 2.4 times in the acute care environment8.
However, despite the increased attentiveness of the NPIAP to the development of MDRPI, there remains minimal investigative attention to their development. Consequently, pressure injuries remain stubbornly present in the acute setting, placing an increased burden on the healthcare system estimated at A$983 million per annum9. An appreciation of the risk factors associated with the development of MDRPIs could inform clinical practice in how assessments are undertaken as well as clinical interventions to reduce risk and the subsequent prevalence of these injuries. To inform strategies to effectively prevent MDRPIs and reduce the burden on the healthcare system, a scoping review was undertaken to identify risk factors associated with the development of MDRPI in the adult ICU patient.
Methods
Design
This review was based on the methodological framework for scoping review described by Arksey and O’Malley10. Utilising this framework, a scoping review aims to map the key concepts underpinning a research area and the main sources and types of evidence available regardless of quality10. The five stages are (a) identify the search question (outlined above), (b) identify relevant studies, (c) study selection, (d) charting the data and (e) collating, summarising and report the results. This scoping review followed the recommendations for Preferred Reporting Items for Systematic Review and Meta-analysis Scoping Review (PRISMA-ScR) guidelines11.
Search strategy
Databases searches were conducted with MEDLINE (via Ovid), CINAHL (via EBSCO), PubMed, Google Scholar, COCHRANE and OVID Nursing database. Search terms included “medical device”, “medical device related pressure injury”, “medical device related pressure area”, “medical device related pressure ulcer”, “intensive care”, and “risk factors”. The Medical Subject Heading (MeSH) or Emtree terms of each keyword and combinations by using Boolean operators such as ‘AND’ and ‘OR’ were explored in each database. Reference lists of publication was hand searched for additional studies. All database searches were performed within no specific timeframe.
Selection of studies
Manuscripts were included if they reported any stage of MDRPI in the ICU adult population (≥18 years). Studies were included if they were observational, cohort or longitudinal, either prospective or retrospective, peer-reviewed, and full text published in English. No restriction was placed on the patient’s diagnosis, severity of disease or geographical location. Studies specifically exploring risk factors were included. Studies were excluded if they focused on pressure injuries not occurring as a result of a device and studies that were not specific to ICU or those only exploring the incidence or prevalence of MDRPIs. A PRISMA flowchart of search results and screening process for included studies was created to present the results.
Data collection process
Data extraction was undertaken by a single author (PW). Data extraction compromised (i) the methodological information of the studies: author, year of publication, study design, sample size, number of participants who developed a MDRPI, specific devices implicated as causes of MDRPI, and number of risk factors explored; (ii) reported study outcomes; risk factors of MDRPI.
Critical appraisal (e.g. risk of bias, methodological quality)
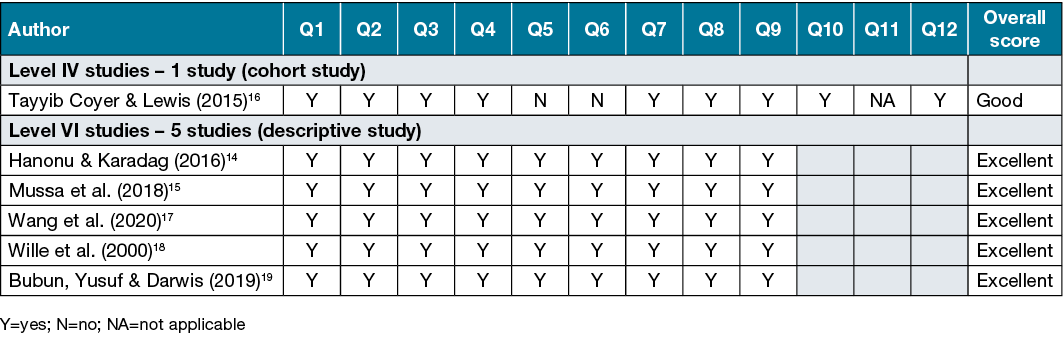
Risk of bias was assessed by a single author using the Joanna Briggs institutional (JBI) critical appraisal tools12,13. The JBI Critical Appraisal Checklists were collaboratively developed and subsequently reviewed and approved by the JBI International Scientific Committee12,13. For each study, the appropriate tool was determined based on study design. There were 12 domains (Chart 2) included in the checklist for cohort studies12 and nine domains (Chart 3) included in the checklist for prevalence13. Criteria were ranked ‘Yes’ or ‘No’ for each study.
Synthesis of results
Data from all eligible studies was synthesised by single author (PW) with interpretation of results facilitated by team review. It was not deemed feasible nor appropriate to perform a meta-analysis due to the high degree of clinical heterogeneity related to the population and predictor variables. The purpose of this review was to identify risk factors rather than to quantify the effect size of the relationship between a given factor and MDRPI; therefore, a narrative synthesis was performed.
The author recorded all factors emerging as independent risk factors for developing a MDRPI. The risk factors which emerged as statistically significant (p<0.05) were identified and reported in the final model as being independently associated with the development of MDRPI. Furthermore, risk factors identified by authors included in the scoping review as being of clinical significance were also documented and reported in this review.
Results
The initial literature search identified 1,396 articles. After screening titles and abstracts, 1,376 articles were removed as they focused on paediatric patients or were non-specific to the development of pressure injury as a result of a medical device. Full text screening was conducted with the remaining 20 articles. Following full text examination and removal of duplicates, and non-English language papers five studies meet the inclusion criteria. A total of six studies (n=1,482 participants, n=262 MDRPI) were included in the review after one additional study was identified after reviewing referencing lists. The screening process of studies is depicted in Figure 1.
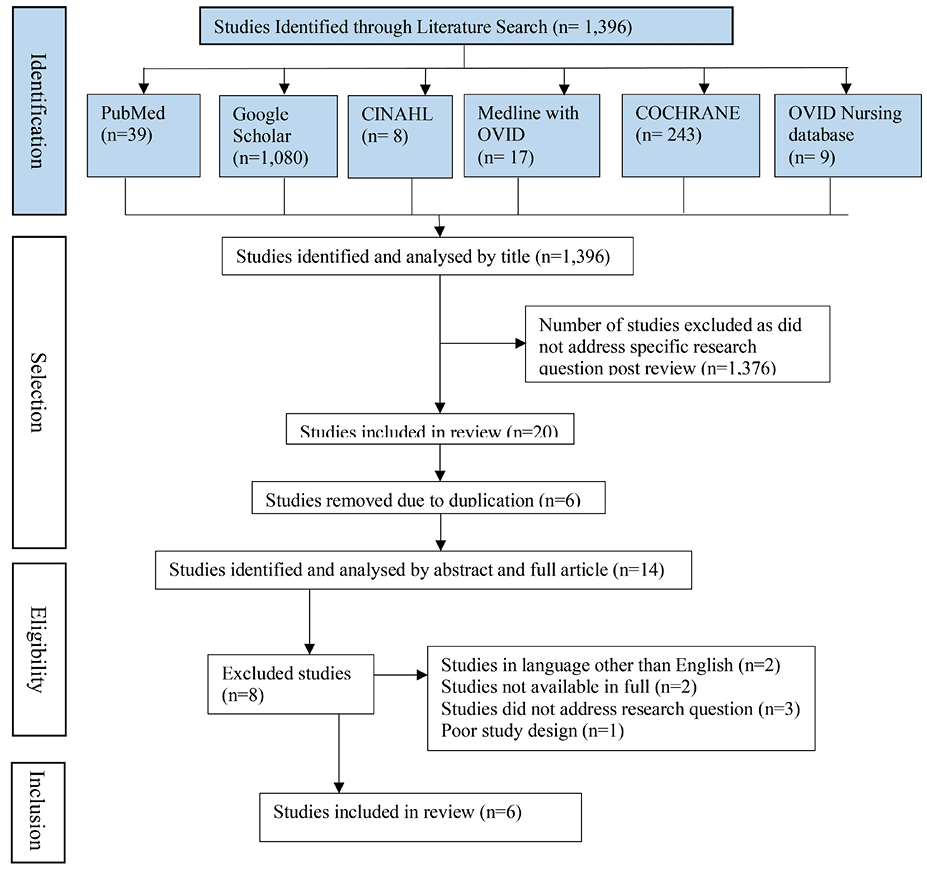
Figure 1. Flowchart of study selection
Study characteristics
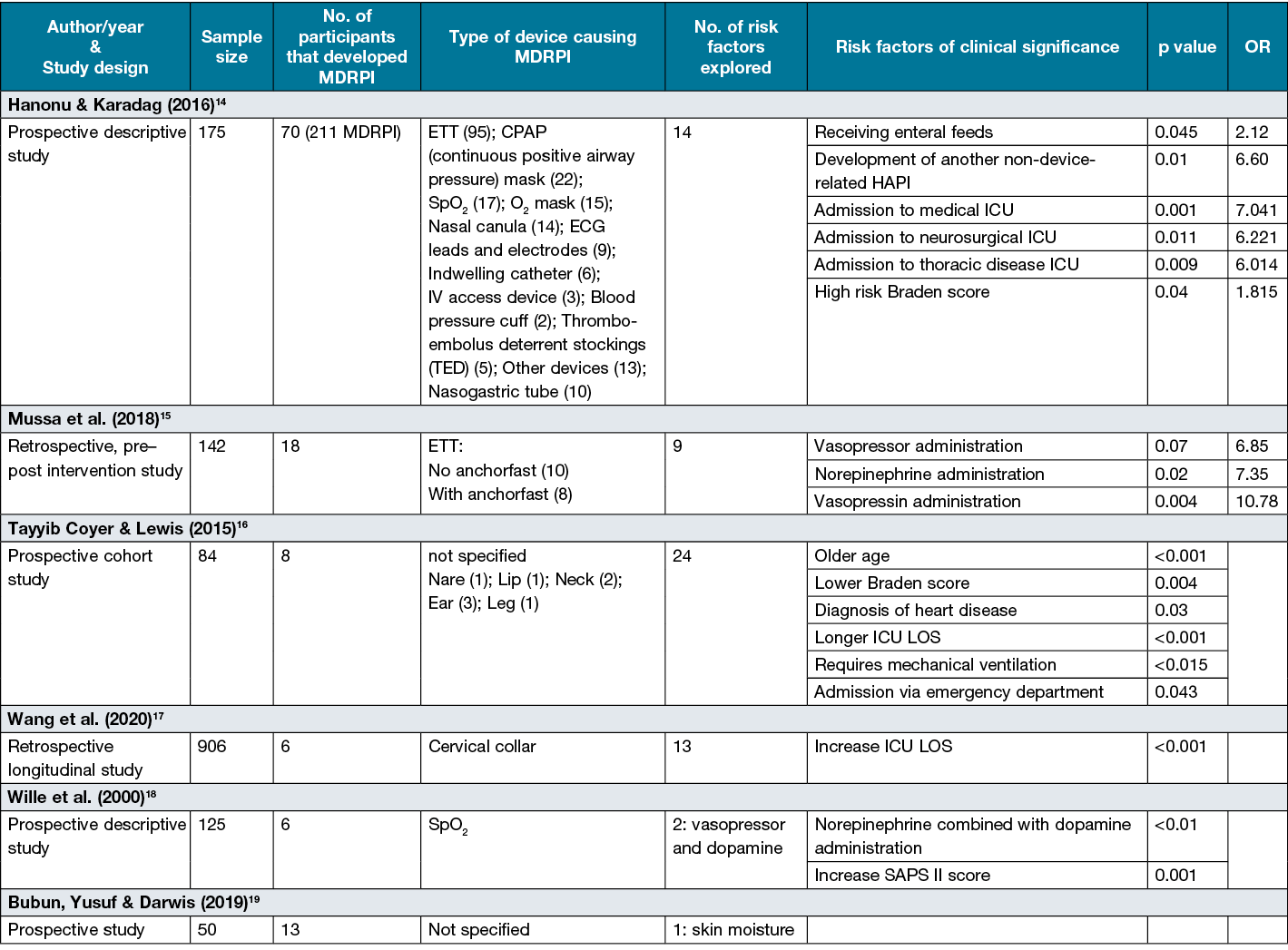
The characteristics of the six included studies are described in Table 114–19. Five of the six studies explored more than one risk factor14–18. The incidence rate of MDRPI varied from 0.7%17 to 40%19 with a variety of different devices responsible for pressure injury development. Two studies explored pressure injuries from a multitude of medical devices14,16, three explored a device specific pressure injuries15,17,18, with the remaining paper not specifying the medical device responsible for the pressure injury19.
Table 1. Characteristics of included studies (n=6)
Critical appraisal of included studies
Table 2 displays the overall score of each of the criteria of the JBI critical appraisal tool for included studies. The six included studies received yes (Y) for 75% of the checklist tool and a score of good or excellent.
Table 2. Quality assessment of included cohort studies using JBI checklist for cohort studies12 and descriptive studies using JBI checklist for prevalence studies13
Risk factors of statistical significance
All studies explored independent risk factors associated with the development of MDRPI; however, of the six studies, five14–18 reported a level of significance (p<0.05) amongst independent predictions of MDRPI outcomes. A summary of risk factors emerging as independent predictor variables of significance are summarised by studies and displayed in Table 1.
Vasopressor administration
Vasopressor infusions are often administered in periods of shock states and are considered a potent peripheral vasoconstrictor which may result in the development of pressure injuries20. Commonly prescribed vasopressors are norepinephrine, dopamine and vasopressin21. Two studies explored vasopressor administration as a predictor variable, with both studies identifying vasopressor administration as a risk factor15,18. Wille et al.18 evaluated administration of dopamine combined with norepinephrine versus administration of dopamine alone. Patients receiving a combination of norepinephrine and dopamine (5 of 22, 22.7%) were more likely to experience an MDRPI than were patients receiving dopamine alone (1 of 103, 0.009%)18. Similarly, Mussa et al.15 explored the relationship between the development of MDRPI and the use of multiple versus single agent vasopressor therapy. They reported associations between MDRPI occurrences and administration of any vasopressor (p<0.007, OR=6.85, 95% CI=1.37–39.3), administration of vasopressin alone (p<0.004, OR=10.78, 95% CI=2.13–54.44), and administration of norepinephrine alone (p<0.02, OR=7.35, 95% CI=1.37–39.3) as compared to patients not given vasopressors15.
ICU length of stay (LOS)
Two studies16,17 explored and identified ICU LOS as an independent variable that contributed to the development of MDRPI (Table 1). Tayyib, Coyer and Lewis16 reported a mean LOS of 13.3 (SD=8.36) and Wang et al.17 reported a mean of 20.1 (SD=20.3) amongst those developing a MDRPI, suggesting that a prolonged stay in ICU is associated with a higher risk of MDRPI development with a statistical significance of p<0.001 for the development of MDRPI from various medical devices16 and cervical collars17. The variation in results may be due to the different medical devices explored amongst the research papers (Table 1).
Braden score
The Braden Scale22 is an assessment tool designed to predict the risk of pressure injuries. Overall, three studies included the Braden score in their research14–16, with only two14,16 identifying the Braden score22 as an independent risk factor (Table 1). Hanonu and Karadag14 reported that, as Braden score risk increases, the incidence of developing a MDRPI rate doubles (OR=1.815).
Age
Four research papers14–17 explored age as a predictor for MDRPI development. Only one study15 identified age as a statistically significant independent predictor (Table 1). Tayyib, Coyer and Lewis16 found that participants of increasing age were at increased risk of developing a MDRPI.
Mechanical ventilation
Three studies14–16 investigated the influence of mechanical ventilation-related on MDRPI and, amongst those, only one study15 identified increasing duration of mechanical ventilation was an independent predictor for MDRPI (Table 1).
Admission type
Two studies14,16 explored and established a correlation between the patient’s admission source and journey within the acute setting and their risk of developing a MDRPI. Tayyib, Coyer and Lewis16 was the only study to explore the area of pre-admission, emergency and post-operative care, with the researchers establishing admission via the emergency department to be an independent predictor variable (Table 1). In contrast, the researchers found no correlation between the development of MDRPI and admission post-operatively, with no MDRPI developing post-operatively in the sample population.
Hanonu and Karadag14 investigated the area of ICU admission in relation to MDRPI development. A direct correlation between MDRPIs and patients admitted in the internal medicine, neurosurgical and thoracic disease ICU was discovered. No association was found for cardiovascular surgery and anaesthesia-resuscitation ICU14.
Severity of illness
Three studies16–18 included different measures of illness severity in their assessment of MDRPI risk factor. The SOFA (Sepsis-related Organ Failure Assessment) score23 was developed to assess the acute morbidity of critical illness at a population level24 compared to the APACHE II score25 which is a tool used to assess the patient’s risk of mortality in first 24 hours of admission26. The SAPS II tool24 (Simplified Acute Physiology Score) calculates a severity score using the worst values measured during the initial 24 hours in the ICU for 17 variables27. One study identified the severity of illness as an independent predictor, with increased SAPS II score associated with the development of MDRPIs related to SpO2 (saturation peripheral oxygen) devices (p<0.001)18. No statistically significant association were found between SOFA scores and MDRPI at various anatomical locations16 or APACHE II and MDRPIs specifically associated with cervical collars17.
Other factors
Hanonu and Karadag’s14 study found that the administration of enteral feeding (p<0.045) and the development of another non-device-related hospital-acquired pressure injury (HAPI) (p<0.01) were a risk factor for the development of MDRPI.
Discussion
To the authors’ knowledge, this is the first scoping review of risk factors related to the development of MDRPI. The aim of this scoping review was to identify risk factors associated with the development of MDRPI in the adult ICU patient. Inconsistency amongst studies and the lack of homogeneity in descriptive values has impacted the interpretation of results and the use of data in further analysis. A need for consistency in future research studies would facilitate synthesis of the body of evidence. Researchers should, therefore, avoid over interpreting the results from any single study. Furthermore, a strength of this study was that each included publication was subject to a quality assessment, allowing researchers and clinicians to consider quality of evidence when interpreting the results. This review would suggest that although there are few studies examining predictors of MDRPIs in the ICU context (n=6 studies, n=1,482 participants, 262 MDRPIs), the quality of the evidence is good to excellent.
Seven risk factors – LOS, vasopressor administration, Braden score, mechanical ventilation, age, admission type, administration of enteral feed and development of a non-device-related HAPI – emerged as independent predictors of the development of MDRPI amongst five studies14–18. LOS16 and vasopressor administration15,17 were the only independent predictor variables that emerged in all studies in which they were evaluated (not included in all studies). Increasing age14–17, mechanical ventilation14–16 and severity of illness18 emerged as significant predictors in some studies; however, they were not consistently identified by studies including these measures as statistically significant. This inconsistency amongst the variables explored and findings highlights the importance of future research in this area to enhance confidence as to the contribution of these variables to MDRPI development.
Nevertheless, this review highlights important limitations with the current evidence, subsequently creating challenges in conducting and interpreting results. One of the key limitations is the diversity of risk factor variables explored and lack of consistency across studies which impacts interpretation. This, therefore, highlights the need for an internally agreed minimal data set. Overall, the study quality was generally good; however, the study with the highest level of evidence also had the lowest quality score by not considering confounding factors, increasing the potential that this study may have yielded false positive results.
The lack of meta-analysis may also be perceived as a limitation; however, performing a meta-analysis was not possible. A significant issue identified is that the patient inclusion criteria and dependent variables were not standardised across the studies. Therefore, a narrative synthesis of risk factors that independently predicted the development of MDRPI amongst the adult ICU patient was undertaken, focusing on studies utilising rigorous statistical methods.
Given that medical devices are increasingly used amongst the ICU population, providing life-saving treatments and managing patient care28, and the growing incidence of MDRPI7, healthcare providers must develop effective prevention strategies to reduce the patient and healthcare system burden arising from MDRPIs. Such screening tools and prevention strategies can only be created once risk factors have been identified.
Limitations of the current scoping review include that the reviewing process was undertaken by a single reviewing which may increase the risk of bias. In addition, studies reported in languages other than English were excluded. Two articles were excluded based on this criteria and could have further informed the study outcomes. Finally, most of the included studies reported risk factors for medical devices broadly and not in relation to specific categories or types of devices this prevented.
Conclusion
Results from this review of MDRPI risk factors amongst the adult ICU patient has highlighted a number of factors associated with MDRPI. Patient characteristics such as advanced age, lower Braden scores, and increased SAPS II severity of illness scores may make them more prone to the development of MDRPI. Interventions such as vasopressor administration, mechanical ventilation and enteral feedings may also serve as contributing factors. Treatment characteristics such as admission through the emergency department, overall LOS, and admission to specific critical care units (medical, neurosurgical or thoracic) may also influence MDRPI development. An association of MDRPI with other non-device-related HAPIs may be related to shared risk factors. Further research identifying risk factors is required that measures a broad number of potential risk factors, including those identified in this scoping review, to enable further clarification of the relative contribution of these risk factors to MDRPI development.
Conflict of interest
The authors declare no conflicts of interest.
Funding
The authors received no funding for this study.
Author(s)
Paige Weber GradDip ICU*1
Email paige_weber@hotmail.com
Laurel Weaver M.Ed1,2
Charne Miller PhD1
1La Trobe University, Melbourne, VIC, Australia
2Intensive Care Unit, University Hospital, 272–322 Ryrie Street, Geelong, VIC, Australia
* Corresponding author
References
- Edsberg LE, Black JM, Goldberg M, McNichol L, Moore L, Sieggreen M. Revised National Pressure Ulcer Advisory Panel pressure injury staging system: revised pressure injury staging system. J WOCN 2016;43(6):585–97.
- Mitchell A. Adult pressure area care: preventing pressure ulcers. Br J Nurs 2018;27:18. doi:10.12968/bjon.2018.27.18.1050
- Schank J. The NPUAP meeting - this was no consensus conference. J Am Coll Clin Wound Spec 2016;7(1–3):19–24. doi:10.1016/j.jccw.2016.07.001
- Percival S, Suleman L, Vuotto C, Donelli G. Healthcare associated infections, medical devices and biofilms: risk, tolerance and control. J Med Microbio 2015;64:4. doi:10.1099/jmm.0.000032
- Makic M. Medical device-related pressure ulcers and intensive care patients. Perianesth Nurs 2015;30(4):336–337. doi:10.1016/j.jopan.2015.05.004
- Black J, Kalowes P. Medical device-related pressure ulcers. Chron Wound Care Manage Res 2015;3:91–99. doi:10.2147/CWCMR.S82370
- Barakat-Johnson M, Barnett C, Wand T, White K. Medical device-related pressure injuries: an exploratory descriptive study in an acute tertiary hospital in Australia. J Tissue Viab 2017;26(4):246–253. doi:10.1016/j.jtv.2017.09.008.
- Barakat-Johnson M, Lai M, Wand T, Li M, White K, Coyer F. The incidence and prevalence of medical device-related pressure ulcers in intensive care: a systematic review. J Wound Care 2019;28:8. doi:10.12968/jowc.2019.28.8.512
- Nguyen K, Chaboyer W, Whitty J. Pressure injury in Australian public hospitals: a cost-of-illness study. Aust Health Rev 2015;39(3):329–336. doi:10.1071/AH14088
- Arksey H, O’Malley L. Scoping studies: towards a methodological framework. J Society Res Method 2005;8(1):19–32.
- Tricco A, Lille E, Zarin W, O’Brien K, Colquhoun H, Levac D, et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR). Ann Internal Med 2018;169(7):467–473. doi:10.7326/M18-0850
- Joanne Briggs Institute. Checklist for cohort studies. 2020 [cited 2020 Sep 1]. Available from: http://joannabriggs.org/sites/default/files/2020-08/Checklist_for_Cohort_Studies.pdf.
- Joanne Briggs Institute. Checklist for prevalence studies. 2020. [cited 2020 Sep 1]. Available from: https://joannabriggs.org/sites/default/files/2020-08/Checklist_for_Prevalence_Studies.pdf.
- Hanonu S, Karadag A. A prospective, descriptive study to determine the rate and characteristics of and risk factors for the development of medical device-related pressure ulcers in intensive care units. Ostomy Wound Manage 2016;62(2):12–22. Available from: https://pubmed.ncbi.nlm.nih.gov/26901386/.
- Mussa C, Meksraityte E, Li J, Gulczynski B, Liu J, Kuruc A. Factors associated with endotracheal tube related pressure injury. SM J Nurs 2018;4(1):1018. Available from: https://smjournals.com/nursing/fulltext/smjn-v4-1018.pdf.
- Tayyib N, Coyer F, Lewis P. Saudi Arabian adult intensive care unit pressure ulcer incidence and risk factors: a prospective cohort study. Int Wound J 2015;13(5):912–919. doi:10.1111/iwj.12406
- Wang H, Campbell J, Doubrovsky A, Singh V, Collins J, Coyer F. Pressure injury development in critically ill patients with a cervical collar in situ: a retrospective longitudinal study. Int Wound J 2020;17(4):944–956. doi:10.1111/iwj.13363
- Wille J, Braams R, Van Haren W, Van der Werken C. Pulse oximeter-induced digital injury: frequency rate and possible causative factors. Crit Care Med 2000;28(10):3555–3557. doi:10.1097/00003246-200010000-00036
- Bubun J, Yusuf S, Darwis M. Relationship between skin moisture and medical device related pressure injury (MDRPI) in intensive care units: prospective study. Enferm Clin 2019;30(S4):420–423. doi:10.1016/j.enfcli.2019.11.009
- Cox J. Pressure ulcer development and vasopressor agents in adult critical care patients: a literature review. Ostomy Wound Manage 2013;50(4):50–60. Available from: https://pubmed.ncbi.nlm.nih.gov/23562874/.
- VanValkinburgh D, Kerndt C, Hashmi M. Inotropes and vasopressors. StatPearl, 2020 [cited 2020 Sep 1]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK482411/.
- Bergstrom N, Braden BJ, Laguzza A, Holman V. The Braden Scale for predicting pressure sore risk. Nurs Res 1987;(4):205–210. PMID:3299278.
- Vincent J-L, Moreno R, Takala J, Willatts S, De Mendonca A, Bruining H, et al. The SOFA (sepsis-related organ failure assessment) score to describe organ dysfunction/failure: on behalf of the working group on sepsis-related problems of the European Society of Intensive Care Medicine. Intensive Care Med 1996;22(7):707–710. doi:10.1007/BF01709751
- Lambden S, Laterrem P, Levy M, Francois B. The SOFA score- development, utility and challenges of accurate assessment in clinical trials. Crit Care 2019;23:374 doi:10.1186/s13054-019-2663-7
- Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med 1985;13(10):818–829. doi:10.1097/00003246-198510000-00009
- Lee H, Lim C, Hong H, Ju J, Jeon Y, Hwang J, Park H. Efficacy of the APACHE II score at ICU discharge in predicting post-ICU mortality and ICU readmission in critically ill surgical patients. Anaesth Intensive Care 2015;43(2):175–86. doi:10.1177/0310057X1504300206.
- Saleh A, Ahmed M, Sultan I, Abdel-Iateif A. Comparison of the mortality prediction of different ICU scoring systems (APACHE II and III, SAPS II, and SOFA) in a single-center ICU subpopulation with acute respiratory distress syndrome. Egyptian Egypt J Chest Dis Tuber 2015;64(4):843–848. doi:10.1016/j.ejcdt.2015.05.012
- Percival S, Suleman L, Vuotto C, Donelli G. Healthcare associated infections, medical devices and biofilms: risk, tolerance and control. J Med Microbiol 2015;64:4. doi:10.1099/jmm.0.000032



