Volume 30 Number 1
Cutaneous infection and atopic dermatitis: the importance of early intervention in Indigenous children in Australia
Rhiannon Russell, Dana Slape, Ian McCrossin and John W Frew
Keywords atopic dermatitis, barrier dysfunction, Indigenous, early intervention
For referencing Russell R et al. Cutaneous infection and atopic dermatitis: the importance of early intervention in Indigenous children in Australia. Wound Practice and Research 2022; 30(1):34-39.
DOI
https://doi.org/10.33235/wpr.30.1.34-39
Submitted 10 October 2021
Accepted 7 December 2021
Abstract
There are dramatic disparities in the life expectancy, health and healthcare access between Indigenous and non-Indigenous Australians. The burden of chronic disease is significantly elevated and contributed to by multiple socioeconomic determinants of health. Cutaneous disease, both infectious and inflammatory, have major contributions to the risk of developing chronic disease in later life and contributing to barriers to education, employment and healthcare.
Atopic dermatitis (AD) is a common, burdensome inflammatory skin disease worldwide; however, a paucity of literature exists reviewing the prevalence in Indigenous Australians. Typical therapies of AD are time-consuming and burdensome and can be an additional barrier to treatment compliance. However, multiple novel targeted therapeutics now exist for the effective and safe management of AD which are demonstrated to reduce the risk of cutaneous infections. Effective early and prioritised access to such therapies in the setting of moderate to severe AD may represent a potential intervention, with downstream benefits in the development of chronic disease. Nevertheless, there are multiple outstanding questions regarding the safety of such therapies in the setting of endemic infections such as scabies and strongyloidiasis which require attention, as well as the practicalities for such therapies to be administered in rural and remote communities.
Overall, the appreciation of the role of cutaneous inflammatory and infectious disease as a contributor to health disparities in Indigenous populations is sorely needed. Discussion regarding the risk-benefit and cost-efficacy of targeted therapeutics in moderate to severe AD and consideration of prioritised access to Indigenous patients in need should be undertaken as part of a wider health prevention strategy with community input.
Introduction
Dramatic health disparities exist in Aboriginal and Torres Strait Islander Australians, hereafter respectfully referred to as Indigenous people. Indigenous people in Australia represent 3.3% of the total Australian population1, with most living in regional areas, then major cities, followed by rural and remote areas (44%, 38% and 18% respectively). Health outcome disparities of Indigenous people may be influenced by their geographical location, employment and educational attainment levels2. It is also widely recognised that the impact of racism and intergenerational traumas can also play a role in the discrepancies in Indigenous health when compared to the non-Indigenous counterparts3. These health disparities highlight a life expectancy gap that exists between Indigenous people and non-Indigenous. Although this gap has reduced due to programs such as Close The Gap, there still remains an 8.6-year difference for males and a 7.8-year difference for female Indigenous people compared with non-Indigenous Australians4.
Chronic disease is challenging and complex for Indigenous Australians who have a higher burden of chronic disease compared to their non-Indigenous counterparts1. This can include cardiovascular disease, chronic kidney disease (CKD), ear disease and cutaneous disease. Chronic cutaneous disease can be both infectious and inflammatory. Atopic dermatitis (AD) is a common, burdensome inflammatory skin disease with a predilection to cutaneous infection. AD effects children globally; however, limited data exists regarding the prevalence of AD in particular ethnic origins5. The existing data suggests that individuals in ethnic minorities experience more prevalent and more severe AD; however, it is unclear what is attributable to poor healthcare access and what is due to genetic population variations5.
Multiple novel targeted therapeutics now exist for the effective and safe management of AD which are demonstrated to reduce the risk of cutaneous infections across many different population groups. However, these typical therapies can be time-consuming and burdensome and act as an additional barrier to treatment compliance. Thus, early intervention and prioritised access to appropriate treatment in patients with moderate to severe AD may have downstream benefits in the development of chronic disease.
There are multiple outstanding questions regarding the safety of such therapies in the setting of endemic infections such as scabies and strongyloidiasis which require attention, as well as the practicalities for such therapies to be administered in rural and remote communities. This review aims to discuss the extant evidence surrounding the significant long-term sequelae of cutaneous infectious disease in Indigenous populations, AD and Indigenous Australians, the treatment of AD in order to decrease the risk of infection, whether complex therapeutic regimens for AD undermine patient compliance, and the safety of targeted AD therapies, including the potential for prioritised access to targeted therapeutics to ameliorate the risk of chronic disease associated with such exposures.
Literature review
Significant long-term sequelae of cutaneous infectious disease in Indigenous populations
Indigenous Australians have the highest burden of skin disease in the rural and remote primary healthcare setting6. Rates of skin infections are much higher in the Indigenous community, and it had been previously reported to be 15–18% of medical presentations in the primary healthcare setting7. This has since significantly increased with new data reporting in 2018 that skin infections represent 83% of presentations by Indigenous Australians to primary care settings6. These presentations are commonly linked to skin infections including impetigo, pyoderma, scabies and fungal infections, all of which are linked to socioeconomic risk factors including poor living environments due to either overcrowding, limited access to cleaning facilities as well as poverty8. It has been suggested that these risk factors will commonly lead to either scabies or dermatophyte infections which directly cause further skin infections which can have significant long-term complications (Figure 1). These skin infections, whether bacterial, viral or parasitic, have been well documented in the literature and have mostly been described in the central and northern Australian regions9.
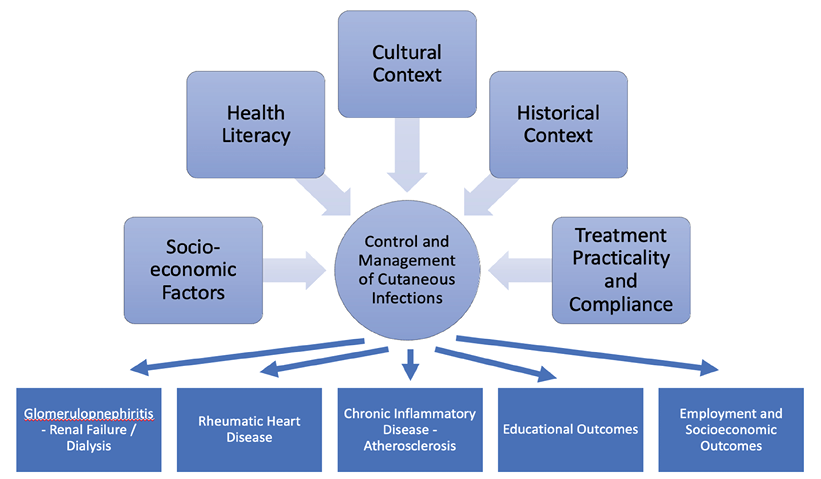
Figure 1. A summary of factors influencing control and management of cutaneous infections and the long-term sequelae of these infections
Impetigo caused by Group A Streptococcus7 pyogenes and Staphylococcus aureus are common. Impetigo can be caused by a direct disruption of the skin barrier from either insect bites or minor trauma, or secondarily from pruritic dermatoses including tinea, lice and scabies10. A recent review of the literature has highlighted that impetigo is found in 45% of Indigenous children in remote Australia, with 33% of these children having concurrent scabies infections10. Previous epidemiological data has suggested that these infections represent 70% of skin infections in rural and remote Australia9. Although research is limited in urban Indigenous people, when reviewing the nationwide hospital statistics, it has been indicated that Indigenous children aged 4 years and under were twice as likely to be admitted to hospital due to skin disease than non-Indigenous children10. This indicates that the disparities may still exist in urban Indigenous children; however, more research is essential in skin infections of urban Indigenous children.
S. aureus has been identified as the primary pathogen causing skin infections, with community-associated methicillin-resistant S. aureus (CA-MRSA) on the rise11. Similar to the previously discussed infections, these too are impacted by the social determinants of health. It has been described that the 5Cs (Crowding, frequent skin-to-skin Contact, Compromised skin integrity, Contaminated items and surfaces, and lack of Cleanliness) have a significant role in the risk profile of acquirement of CA-MRSA12. Complications of CA-MRSA skin infections include “septic joints, necrotising fasciitis, necrotising pneumonia, endocarditis and bacteraemia”13. These serious complications identify strategies appropriate to Indigenous communities are required to improve health outcomes.
Group A Streptococcus skin infections can contribute to serious sequelae in Indigenous children. This can include acute post-streptococcal glomerular nephritis (APSGN) and acute rheumatic fever (ARF)14, sepsis, and skeletal infections such as osteomyelitis15. A recent study found APSGN was found in 10% of Indigenous patients and osteomyelitis in 32% of Indigenous patients15 presenting with skin infections. APSGN in Indigenous communities is most commonly linked to streptococcal impetigo, with impetigo causing of 87% of APSGN in 200016 which can lead to CKD17. Indigenous people have higher rates of CKD-related deaths18, with these deaths representing 2% of deaths in Indigenous people19. The most common reason of hospital admissions in Indigenous people is to undertake dialysis required for the management of CKD19. Therefore, it is evident that CKD due to skin infections is a serious concern for Indigenous people, particularly those living in rural and remote regions, due to the higher rates of skin infections in these regions20. It is important that, moving forward, holistic and accessible care that is socially and cultural appropriate is promoted to reduce and prevent chronic disease21.
AD and Indigenous Australians
AD is multi-factorial, with a complex inter-relationship between skin barrier development, genetic predisposition, immunological development, skin microbiome composition, environmental and nutritional factors and well as psychological factors22. AD impacts children worldwide and the prevalence is geographically variable. However, there is a lack of research reviewing the long-term effects of eczematous conditions in Indigenous people globally. The inconsistency in estimation of prevalence of AD makes it difficult to identify the impact of ethnicity on the risk of development of AD5. Severe AD has been associated with lower socioeconomic status, family stressors and poor community living23 which have a disproportionate impact on Indigenous people compared with non-Indigenous people. However, there is a paucity of literature as few epidemiological studies have been conducted specifically regarding AD in Indigenous children. A recent audit of 196 Indigenous patients highlighted that eczema was found in with 22 patients presenting to a dermatology Aboriginal health service in a 5-year period24. An additional study conducted in Perth identified that, of 178 Indigenous patients, 21 presented for inflammatory/allergic skin infections which included eczema; however, this data was limited to urban tertiary centres and relied on the accuracy of hospital data25. These two studies show a similar percentage prevalence of AD. Furthermore, Glasgow et al. found that Indigenous children had less eczema than non-Indigenous children; however, this result relied solely on parents to complete a questionnaire, a noted limitation to this study26.
When comparing these studies, it is evident that the rate of Indigenous patients presenting with AD is less than non-Indigenous counterparts. We speculate that this may be the increased primary care services that are available to Indigenous patients or may be due to the under representation of Indigenous patients presenting to dermatological specific services25. This complements the well-documented literature of infectious skin conditions impacting Indigenous communities, the complications associated with this, and their multi-system, and potentially fatal, impact.
The symptoms of AD such as itchiness and sleep impairment may make learning more difficult for children; this has also been seen in children with scabies, thus it is important to recognise the burden this may have on children long-term27. A recent study of the broader community, not Indigenous people alone, the first of its kind, has discussed whether differing severity of AD causes learning disabilities. It was found that with increasing severity, the more likely factor was having a learning disability independent of socioeconomic status, onset of age and other neuropsychiatric disorders28. However, the results of previous studies, which reviewed related outcomes such as educational attainment and cognitive impact, differed. These previous studies identified that people with AD did not have a risk of lower cognitive function or lower level of educational attainment by adulthood when compared to children without AD29.
Not only does AD show potential impacts of learning, it also can have a significant impact on one’s psychological health and quality of life. Many studies have reviewed this poignant issue and identified that patients with AD have a significantly lower quality of life which is directly related to the severity of disease30. It has also been shown to have an unequivocal association with depression, anxiety and ADHD31. Therefore, it is evident that further research is required to identify whether learning disabilities have ongoing consequences for overall cognition and education attainment in transition to adulthood and whether this has any relevance to Indigenous communities specifically.
Differentials of AD are important to recognise such as cutaneous manifestations of human T‑lymphotropic virus type 1 (HTLV‑1). HTVL‑1 is a retrºvirus that preferentially infects CD4+ T‑cells which is highly prevalent in Indigenous people in the northern territory of Australia32. It can present as infective dermatitis, which is diagnosable by skin biopsy, or can present as a chronic inflammatory dermatosis33. Although a rare manifestation, due to the high prevalence of HTLV‑1 in Indigenous people, it is important to screen Indigenous children for HTVL‑1 in rural and remote communities particularly, yet also in urban Indigenous children as this can be easily missed and assumed as AD.
Treatment of AD decreases infection risk
Current therapeutics for AD include general measures such as avoiding irritants and allergens, the control and treatment of contributing bacteria, and the use of moisturisers and emollients, topical corticosteroids and topical calcineurin inhibitors. It is well established that anti-inflammatory therapies in AD reduce the risk of cutaneous infection34. Even in the setting of immunosuppressive and immunomodulatory therapies, rates of cutaneous bacterial infections decrease compared with treatment with placebo34. This is largely attributable to the role of T Helper 1 type 2 (Th2) inflammation contributing to a decrease in epidermal barrier function in AD (Figure 2). This alteration in barrier function is an essential component of the cutaneous inflammatory cascade which manifests in AD. Defects in the barrier function of AD lead to decreases in antimicrobial peptides such as Cathelicidin and human Beta Defensins which aid against bacterial skin infections. Therapy with targeted agents such as Dupilumab, Tralokinumab and Janus Kinase inhibitors such as Baricitinib, Upadacitinib and Abrocitinib reduce this Th2 immune axis activation leading to repair of the epidermal barrier function and protection against cutaneous infection35. Ongoing therapy is demonstrated to reverse the barrier dysfunction in the long-term and this translates into decreased rates of cutaneous infections compared with the placebo in the setting of clinical trials35.
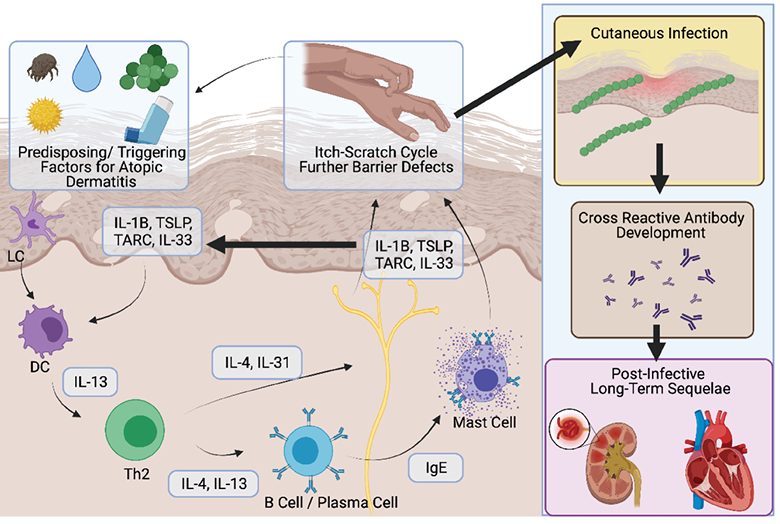
Figure 2. Schematic representation of the pathogenesis of AD and the link between cutaneous infection and chronic long-term disease. Multiple triggering factors lead to the development of T helper 2 (Th2) polarised immune response, characterised by the production of IL‑4 and IL‑13. This is directly associated with the sensitisation of cutaneous neurons leading to intense pruritus and degranulation of MAST cells. The subsequent inflammation in keratinocytes (driven by IL‑1, TSLP, TARC and IL‑33) then lead to a defect in barrier function of the epidermis. This then leads to an increased risk of infection. Infections with pathogens such as streptococcus lead to the development of cross-reactive antibodies which manifest as conditions such as glomerulonephritis and valvular cardiac disease
Complex therapeutic regimens for AD undermine patient compliance
It is well established that factors such as age, literacy and socioeconomic status have a significant influence upon compliance to medical therapy. In the setting of AD, compliance with topical therapies is much lower than other oral or physical therapies. Topical therapy for AD is time-consuming, messy and requires long-term patient compliance to complex regimens36. There is little evidence suggesting that Indigenous people specifically have increased risk of poor adherence to medications, with a few studies indicating that two thirds of Indigenous people report compliance with medications37. However, it has been well documented that poorer health outcomes are associated with risk factors such as smoking, poor physical health, low socioeconomic status, lower levels of education and unemployment38 which can be prevalent in some Indigenous people. Therefore, risk factors should be considered when prescribing complex management regimes, especially in areas of maldistribution of healthcare services.
Specific interventions could be used to reduce the impact of socioeconomic factors on health outcomes, whilst improving health literacy in Indigenous people. Improving Indigenous community engagement via the use of Aboriginal healthcare works, co-designing interventions with appropriate use of language, whilst creating and providing culturally appropriate care may have the potential to increase health literacy, thus improve compliance in the long-term39. However, further research is required for these ideas as they are built upon the premise that the behaviour of the patient needs to change, rather than an alternative complimentary approach to change the therapy to one more suitable.
Monoclonal antibody therapies have dramatically altered the treatment landscape for moderate to severe AD34. These treatments are subcutaneously administered at infrequent intervals and have high levels of safety and efficacy in adult, adolescent and, more recently, paediatric populations.
Safety of targeted AD therapies
Certain knowledge gaps exist such as the effect of chronic infections like scabies and strongyloidiasis in patients that are using therapies targeting the Th2 axis. Case reports have been documented on the use of targeted therapies in the setting of scabies infection with no adverse effects; however, there is a lack of sufficient large-scale safety data to ensure that no worsening of systemic infection or infestation would occur in the setting of targeted therapy in AD. In the Australian setting, screening in an urban population has identified an 17% prevalence of asymptomatic strongyloidiais40. Given the varying rates of high prevalence in rural and remote Indigenous communities ranging from 35–60%41 compared with urban Indigenous people, co-treatment with ivermectin would be recommended unless further safety data emerges regarding the risk of reactivation or worsening of such conditions.
It is, however, important to note that medications targeting Th2 axis would be difficult to access in rural and remote areas and would require refrigeration of medication and administration at a community health centre, in line with other therapies for chronic health conditions. Nevertheless, given the ability for many of these biologic agents to be at room temperature for less than 24 hours without loss of efficacy, some flexibility in the time and place of administration may be possible. Therefore, separate considerations would be made for urban versus rural Indigenous healthcare settings with greater practicalities of availability and administration possible in urban settings rather than remote communities. Further community consultation would be needed to establish the most effective method of treatment delivery. The Central Australian Rural Practitioners Association (CARPA) manuals are an excellent source of information for treating skin infections in Indigenous people in rural and remote Australia as social determinant factors are taken into consideration42.
Conclusions and recommendations
The skin is an important portal of entry for chronic infections which have significant downstream impacts upon chronic disease in Indigenous populations. Defects in the barrier function of the skin secondary to AD can be a contributor to the risk of cutaneous infection. Current therapeutic approaches to dermatitis can be potentially impacted by social, economic and health literacy disadvantages. Whilst most therapeutic approaches aim at intervening these social determinants, an argument can be made for selecting the therapeutic option with the highest efficacy that is not impacted by these pre-existing factors. However, consideration of the disparity in the maldistribution of dermatological services can make these therapeutic options difficult to access. Healthcare-administered targeted therapeutics provide a potential opportunity to effectively treat AD, circumvent complex therapeutic regimens, and provide early intervention to reduce the risk of chronic disease caused by cutaneous infections. Further discussions and research are sought regarding appropriate care for Indigenous children in all rural, remote and urban areas to minimise the long-term consequences, both medical and psychosocial, in chronic skin conditions and subsequently reduce the risk of long-term chronic health conditions caused by cutaneous infections.
Conflict of interest
JWF has conducted advisory work for Janssen, Boehringer-Ingelheim, Pfizer, Kyowa Kirin, LEO Pharms, Regeneron and UCB, participated in trials for UCB, Pfizer and Eli Lilly, and received research support from Ortho Dermatologics.
Ethics statement
An ethics statement is not applicable.
Funding
The authors received no funding for this study.
Author(s)
Rhiannon Russell1, Dana Slape2, Ian McCrossin3 and John W Frew*2–4
1The Wollongong Hospital, Wollongong, NSW, Australia
2Department of Dermatology, Liverpool Hospital, Sydney, NSW, Australia
3Laboratory of Translational Cutaneous Medicine, Ingham Institute of Applied Medical Research, Sydney, NSW, Australia
4University of New South Wales, Sydney, NSW, Australia
* Corresponding author Email john.frew@unsw.edu.au
References
- Australian Institute of Health and Welfare. Profile of Indigenous Australians. Canberra: AIHW; 2021.
- Crawford H, Biddle N. Changing associations of selected social determinants with Aboriginal and Torres Strait Islander Health and Wellbeing, 2002 to 2012–13. Canberra: Centre for Aboriginal Economic Policy Research; 2017.
- Anderson I, Baum F, Bentley M. Beyond Bandaids: exploring the underlying social determinants of Aboriginal health. Cooperative Research Centre for Aboriginal Health; 2007.
- Australian Institute of Health and Welfare. Deaths in Australia. Canberra: AIHW; 2021.
- Kaufman BP, Guttman-Yassky E, Alexis AF. Atopic dermatitis in diverse racial and ethnic groups: variations in epidemiology, genetics, clinical presentation and treatment. Exp Dermat 2018;27(4):340–57.
- McMeniman E, Holden L, Kearns T, Clucas DB, Carapetis JR, Currie BJ, et al. Skin disease in the first two years of life in Aboriginal children in East Arnhem Land. Australas J Dermatol 2011;52(4):270–3.
- Flegg K, Phillips C, Collins A, Sharp P, Kanagasundaram M, Lovett R. Health service attendance patterns in an urban Aboriginal health service. Med J Australia 2010;193:146–8.
- Hengge UR, Currie BJ, Jäger G, Lupi O, Schwartz RA. Scabies: a ubiquitous neglected skin disease. Lancet Infect Dis 2006;6(12):769–79.
- Currie BJ, Carapetis JR. Skin infections and infestations in Aboriginal communities in northern Australia. Australas J Dermatol 2000;41(3):139–43; quiz 44–5.
- Davidson L, Knight J, Bowen AC. Skin infections in Australian Aboriginal children: a narrative review. Med J Aust 2020;212(5):231–7.
- Gosbell IB. Epidemiology, clinical features and management of infections due to community methicillin-resistant Staphylococcus aureus (cMRSA). Intern Med J 2005;35 Suppl 2:S120–35.
- Green BN, Johnson CD, Egan JT, Rosenthal M, Griffith EA, Evans MW. Methicillin-resistant Staphylococcus aureus: an overview for manual therapists. J Chiro Med 2012;11(1):64–76.
- Engelman D, Hofer A, Davis JS, Carapetis JR, Baird RW, Giffard PM, et al. Invasive Staphylococcus aureus infections in children in tropical northern Australia. J Ped Infect Dis Soc 2014;3(4):304–11.
- Streeton C, Hanna J, Messer R, Merianos A. An epidemic of acute post-streptococcal glomerulonephritis among Aboriginal children. J Paed Child Hlth 1995;31(3):245–8.
- Thomas L, Bowen AC, Tong SY. Complicated skin and soft tissue infections in remote Indigenous communities. Int Med J 2020;50(6):752–4.
- Kearns T, Evans C, Krause V. Outbreak of acute post streptococcal glomerulonephritis in the Northern Territory, 2000. NT Dis Control Bull 2001;8:6–14.
- Boon V. Factors responsible for the high rate of kidney disease in Indigenous Australians: a multifaceted approach focusing on streptococcal disease. James Cook University; 2007.
- Hoy WE, Mott SA, McDonald SP. An update on chronic kidney disease in Aboriginal Australians. Clinical Nephrol Supp Jan 2020;93(1):124–128.
- Australian Indigenous HealthInfoNet. Summary of kidney health among Aboriginal and Torres Strait Islander people. Australian Indigenous HealthInfoNet; 2020.
- Jeffries-Stokes C, Stokes A. Place or race? Findings from the Western Desert Kidney Health Project [conference abstract]. J Paed Child Hlth 2017;53:13-.
- Calma T, Dick D, editors. Social determinants and the health of Indigenous peoples in Australia: a human rights based approach. International Symposium on the Social Determinants of Indigenous Health; 2007.
- Hulshof L, Van’t Land B, Sprikkelman AB, Garssen J. Role of microbial modulation in management of atopic dermatitis in children. Nutrients 2017;9(8):854.
- Chung J, Simpson EL. The socioeconomics of atopic dermatitis. Annal Allergy, Asthma Immunol 2019;122(4):360–6.
- Williams C, Hunt J, Kern JS, Dunn R. A casemix study of patients seen within an urban Aboriginal Health Service dermatology clinic over a five-year period. Aust J Dermatol 2021 Aug;62(3):331–335.
- Heyes C, Chan J, Halbert A, Clay C, Buettner P, Gebauer K. Dermatology outpatient population profiling: Indigenous and non-Indigenous dermatoepidemiology. Aust J Dermatol 2011;52(3):202–6.
- Glasgow N, Goodchild E, Yates R, Ponsonby AL. Respiratory health in Aboriginal and Torres Strait Islander children in the Australian Capital Territory. J Paed Child Hlth 2003;39(7):534–9.
- Ramirez FD, Chen S, Langan SM, Prather AA, McCulloch CE, Kidd SA, et al. Association of atopic dermatitis with sleep quality in children. JAMA Pediatric 2019;173(5):e190025-e.
- Wan J, Shin DB, Gelfand JM. Association between atopic dermatitis and learning disability in children. J Allergy Clin Immunol 2020;8(8):2808–10.
- Smirnova J, Von Kobyletzki L, Lindberg M, Svensson Å, Langan SM, Montgomery S. Atopic dermatitis, educational attainment and psychological functioning: a national cohort study. Br J Dermatol 2019;180(3):559–64.
- Heede NG, Thyssen JP, Thuesen BH, Linneberg A, Szecsi PB, Stender S, et al. Health-related quality of life in adult dermatitis patients stratified by filaggrin genotype. Contact Dermatitis 2017;76(3):167–77.
- Kage P, Simon JC, Treudler R. Atopic dermatitis and psychosocial comorbidities. JDDG: Journal der Deutschen Dermatologischen Gesellschaft 2020;18(2):93–102.
- Einsiedel LJ, Pham H, Woodman RJ, Pepperill C, Taylor KA. The prevalence and clinical associations of HTLV‑1 infection in a remote Indigenous community. Med J Aust 2016;205(7):305–9.
- Bravo FG, editor. Infective dermatitis: a purely cutaneous manifestation of HTLV‑1 infection. Semin Diagn Pathol 2020;Mar;37(2):92–97.
- Davari DR, Nieman EL, McShane DB, Morrell DS. Current perspectives on the systemic management of atopic dermatitis. J Asthma Allergy 2021;14:595–607.
- Le M, Berman-Rosa M, Ghazawi FM, Bourcier M, Fiorillo L, Gooderham M, et al. Systematic review on the efficacy and safety of oral Janus Kinase inhibitors for the treatment of atopic dermatitis. Frontiers Med 2021;8(1102).
- Gupta G, Mallefet P, Kress DW, Sergeant A. Adherence to topical dermatological therapy: lessons from oral drug treatment. Br J Dermatol 2009;161(2):221–7.
- Hoy WE, Kondalsamy-Chennakesavan SN, Nicol JL. Clinical outcomes associated with changes in a chronic disease treatment program in an Australian Aboriginal community. Med J Aust 2005;183(6):305–9.
- Jayasinghe UW, Harris MF, Parker SM, Litt J, van Driel M, Mazza D, et al. The impact of health literacy and life style risk factors on health-related quality of life of Australian patients. Health Qual Life Outcome 2016;14:68.
- de Dassel JL, Ralph AP, Cass A. A systematic review of adherence in Indigenous Australians: an opportunity to improve chronic condition management. BMC Hlth Serv Res 2017;17(1):845.
- Gibson M, Lowe PM. Considerations in pre-treatment testing for Strongyloides stercoralis in an Australian cohort of 159 patients receiving biological therapies. Aust J Dermatol 2020;61(4):378–9.
- Kearns TM, Currie BJ, Cheng AC, McCarthy J, Carapetis JR, Holt DC, et al. Strongyloides seroprevalence before and after an ivermectin mass drug administration in a remote Australian Aboriginal community. PLoS Negl Trop Dis 2017;11(5):e0005607.
- Remote Primary Health Care Manuals. CARPA standard treatment manual. 7th ed. Alice Springs: Northern Territory Centre for Remote Health; 2017. p. 490.



