Volume 30 Number 1
Hidradenitis suppurativa: an up-to-date review of clinical features, pathogenesis and therapeutic approaches
Anthony Moussa, Anneliese Willems and Rodney D Sinclair
Keywords chronic inflammation, follicular occlusion, hidradenitis suppurativa
For referencing Moussa A et al. Hidradenitis suppurativa: an up-to-date review of clinical features, pathogenesis and therapeutic approaches. Wound Practice and Research 2022; 30(1):40-49.
DOI
https://doi.org/10.33235/wpr.30.1.40-49
Submitted 18 January 2022
Accepted 1 February 2022
Abstract
Hidradenitis suppurativa (HS) is a chronic autoinflammatory disease characterised by recurrent painful nodules, abscesses, sinus tracts and scars at apocrine gland-bearing sites. Treatment is universally challenging and sufferers may live with chronic and recurrent draining wounds. This review provides an up-to-date, evidence-based summary of HS, including clinical features, severity assessment, disease pathogenesis and current and emerging therapeutic approaches.
Introduction
Hidradenitis suppurativa (HS), also known as acne inversa, is a chronic and debilitating autoinflammatory disease typified by painful nodules, abscesses, sinus tracts and scars in predominantly intertriginous, apocrine gland-bearing sites1. Estimates of HS prevalence are highly variable, ranging from 0.03–4.1% globally2–4. Health-related quality of life may be profoundly impacted, with HS sufferers at an increased risk of anxiety, depression and suicide5,6. HS exhibits a diverse clinical phenotype and management of disease remains universally challenging7–9. In this review article, we summarise clinical features of HS, explore disease pathogenesis and associations, and discuss current and emerging medical therapies.
Epidemiology
Onset of HS typically occurs after puberty in the second decade of life at a mean age of 24.6±10.5 years10,11. Onset before puberty or after menopause is rarely reported11. A 2018 cross-sectional study estimated a HS prevalence of 0.67% in Australia4. Larger European studies have reported a prevalence of 1%2, while a point prevalence of 4.1% has also been described in an adult population undergoing screening for sexually transmitted infections3. Several factors are likely to account for under-recognition of HS, including misdiagnosis, under-reporting of symptoms by patients and decentralisation of care. Females are more commonly affected than males (ratio 2.8:1)12,13.
Clinical features and diagnosis
HS is diagnosed clinically using the Dessau criteria as modified and adopted at the 2nd International Conference on hidradenitis suppurativa in San Francisco 20091. In order to establish a diagnosis, all three criteria must be fulfilled:
- Typical lesions – deep-seated painful nodules: ‘blind boils’ in early lesions, and abscesses, draining sinus, bridged scars and ‘tombstone’ double-ended pseudo-comedones in secondary lesions.
- Typical topography – axillae, groins, perineal and perianal regions, buttocks, infra- and inter-mammary folds.
- Chronicity and recurrence1.
Clinical presentations of HS, including the anatomical distribution, number and types of lesions, can vary widely between patients. Unfortunately, the average diagnostic delay of HS is 7.2±8.7 years14. Diagnostic delay is associated with more severe disease, a higher burden of concomitant disease and higher rates of surgical treatment10,14.
Typical lesions
HS lesions include dermal and subcutaneous nodules (inflamed and non-inflamed), rounded abscesses, double-ended pseudo-comedones in secondary lesions, sinus tracts (draining or non-draining) and scarring (bridged or rope-like, hypertrophic or atrophic, and contractures)1,15 (Figures 1–3). Painful inflammatory nodules and abscesses generally persist for a mean of 7–15 days, after which complete spontaneous regression or partial regression into a painless inflammatory nodule occurs. Inflammatory nodules or abscesses that fail to regress may instead progress in size, with subsequent rupture and release of purulent discharge16,17. Some patients may experience prodromal symptoms 12–48 hours before developing a HS lesion, including warmth, burning, pain, pruritus or hyperhidrosis16,17.
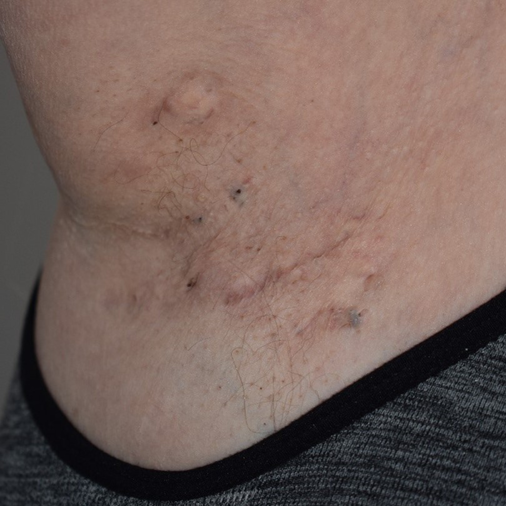
Figure 1. Right axillary comedones in a patient with HS
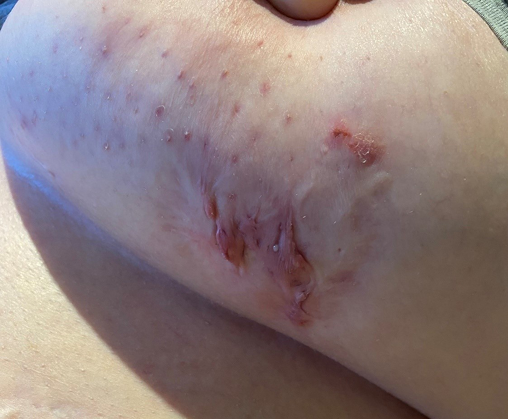
Figure 2. Left mammary inflammatory nodules, cicatricial scarring and draining sinuses in a patient with HS
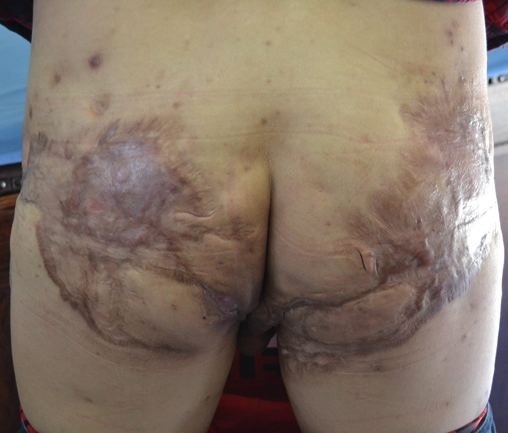
Figure 3. Severe scarring of bilateral gluteal regions secondary to HS
Typical topography
HS lesions are typically localised to intertriginous, apocrine gland-bearing sites including the axillae, sub-mammary, inter-mammary, inguino-genital, perineal and perianal areas. Less commonly affected areas include the trunk, neck, pre-auricular and retro-auricular areas1,17. Males are more prone to gluteal and perianal disease while females are more likely to experience genitofemoral involvement17.
Chronicity and recurrence
Patients with HS may experience a variable trajectory of continuous disease, acute intermittent flares and periods of prolonged remission (weeks to months). Following resolution of lesions, HS recurrence may occur at new sites or via pre-existing non-inflammatory nodules. Long-standing active disease may result in the coalescing of nodules with sinus tract formation; these patients can experience chronic pain with daily purulent discharge17,18.
Complications
HS may be complicated by acute secondary infection, resulting in disease exacerbation. Long-standing HS may result in lymphatic obstruction and consequent lymphoedema19,20. Scrotal elephantiasis secondary to HS associated lymphatic obstruction in the ano-genital area has also been described19,20. Long-standing HS in the ano-genital areas has also, in rare instances, been associated with fistula formation into the urethra, bladder, rectum and peritoneum, and requires an exclusion of Crohn’s disease19. Furthermore, chronic ano-genital involvement carries an increased risk of cutaneous squamous cell carcinoma, typically in males and less commonly in females; the prognosis is usually poor due to diagnostic delay19,20. A recent, population-based cohort study of patients with HS reported an increased risk of several specific cancers, including oral cavity and pharyngeal cancer, Hodgkin lymphoma, central nervous system cancer, non-melanoma skin cancer, prostate cancer and colorectal cancer21. Other potential complications of HS include anaemia, hypoproteinaemia and amyloidosis19.
Severity staging
Several severity staging tools have been developed to assess HS severity and response to treatment in clinical practice and research trials22–29.
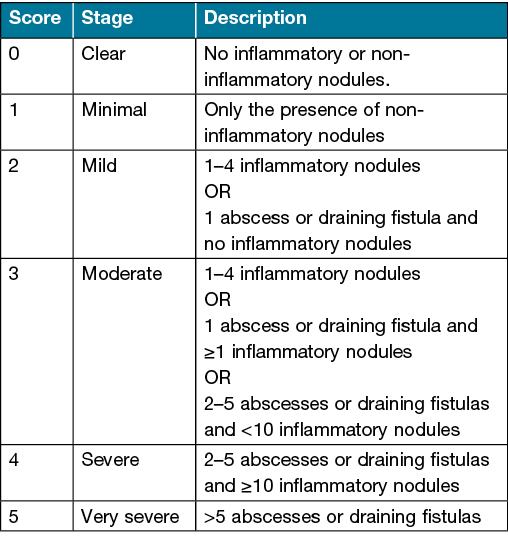
Hurley staging
First described in 1989 by Dr Harry James Hurley, the Hurley staging system remains one of the most widely used HS severity assessment tools. Patients are stratified into three stages based on the extent and type of HS lesions (Table 1)22 (Figures 4–6). Hurley staging was originally developed as a tool to help select the suitability of HS-affected areas for medical management (Hurley stage I) or surgical management (Hurley stage II and III). It is commonly used because of its speed and simplicity but has several limitations. Notably, it does not take into account the specific number of lesions or sites affected, and so is limited in assessing treatment response20,22.
Table 1. Description of Hurley stages in the assessment of HS22
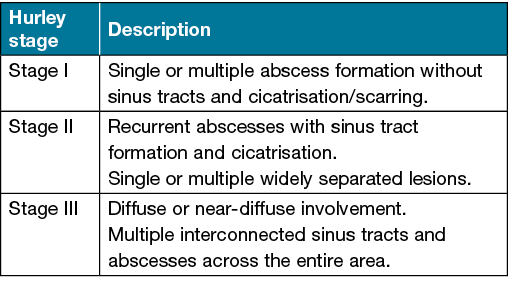
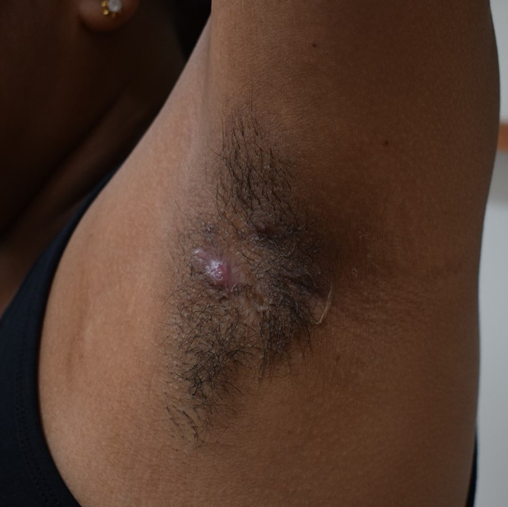
Figure 4. Hurley stage I HS of the left axilla
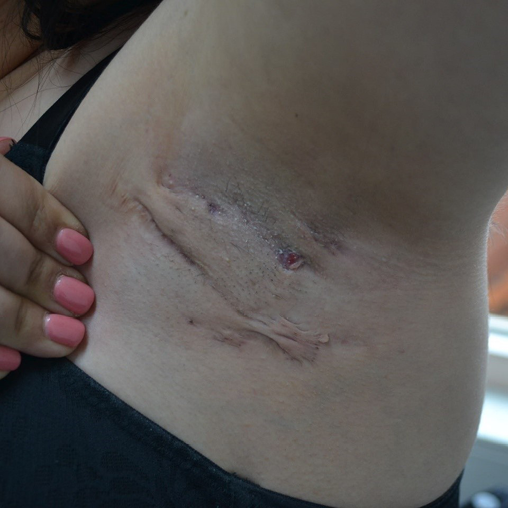
Figure 5. Hurley stage II HS of the left axilla
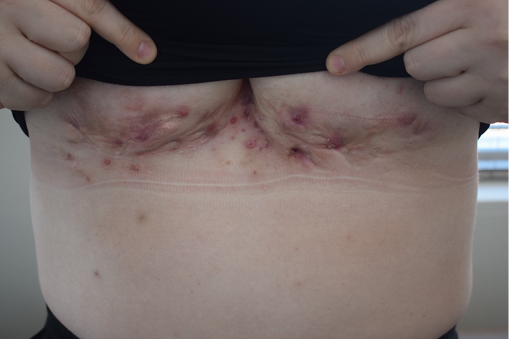
Figure 6. Hurley stage III HS of the inter-mammary and bilateral sub-mammary regions
Modified Sartorius Score
The Modified Sartorius Score is a more detailed assessment tool than Hurley staging and is principally used for research purposes. It was originally designed by Sartorius et al. (2003) but later modified to a simpler version that placed greater emphasis on inflammatory lesions23,24. It is calculated by separately measuring the number and type of lesions at each HS-affected site and the longest distance between two relevant lesions. Different points are awarded per parameter to produce a score per body area and an overall score24. The Modified Sartorius Score has a low interobserver variability, strongly correlates to a patient’s Dermatology Life Quality Index (DLQI) and is useful in assessing response to treatment in research studies. However, it is more time consuming than other tools as a precise lesion count is required. Furthermore, the applicability of this tool may be limited in patients with severe disease where separate lesions become confluent25.
HS Physician Global Assessment (HS-PGA)
The HS Physician Global Assessment (HS-PGA) is a tool which classifies disease severity as one of six categories based on the number of HS lesions (Table 2)26. It is simple and quick to perform and has been widely used to date in clinical trials20. However, while the HS-PGA enables monitoring of HS severity and response to treatment, it considers the global severity of HS, and not individually affected areas. Furthermore, it does not account for marked heterogeneity which may exist in patients with severe disease, and so improvements of disease in this group may not be reflected in the HS-PGA score.
Table 2. HS-PGA scale20,26
Hidradenitis Suppurativa Severity Index (HSSI)
The Hidradenitis Suppurativa Severity Index (HSSI) is a scoring tool that incorporates categorical objective parameters with categorical subjective parameters20,27. Features considered include the number of HS lesions, the sites involved, lesion drainage and pain assessment via a visual analogue scale. The HSSI score ranges from 0–19. A score of ≥13 denotes severe disease, a score of 8–12 indicates moderate disease and a score of 0–7 indicates mild disease27.
Hidradenitis Suppurativa Severity Score System (IHS4)
The Hidradenitis Suppurativa Severity Score System (IHS4) is a validated tool used in clinical practice and research settings to assess disease severity and monitor response to treatment28. It is calculated by adding the number of inflammatory nodules (multiplied by 1), the number of abscesses (multiplied by 2) and the number of draining tunnels (multiplied by 4). A score of ≥11 denotes severe HS, a score of 4–10 denotes moderate HS and a score of ≤3 denotes mild HS28.
Hidradenitis Suppurativa Area and Severity Index (HASI)
The Hidradenitis Suppurativa Area and Severity Index (HASI) is a severity assessment tool used in clinical trials. It combines signs of HS-related inflammation with the affected body surface area (BSA)29. To calculate the regional HASI score, each sign of HS-related inflammation (erythema, thickness, drainage, tenderness) is scored on a Likert scale from 0–3 for each affected region. The sum of the four variable scores is multiplied by the area score of each involved region and then by the proportion of the BSA of that region. Regional HASI scores are added together to give a cumulative HASI score, ranging from 0–7229.
Pathogenesis
HS has a complex multifactorial pathogenesis that is not yet fully understood. Traditionally, hair follicle occlusion has been regarded as the main driver of disease activity in HS. In this pathogenic model, follicular hyperkeratinisation is followed by occlusion, dilatation, rupture and resultant lympho-histiocytic inflammation20,30,31. Recently, this model has been challenged. Current observational, experimental and therapeutic evidence supports the concept of inflammation being the primary driver of disease activity in HS31. Despite its use, the term hidradenitis suppurativa is a historical misnomer as the central pathogenic event is not a suppurative inflammation of the apocrine glands30.
Inflammation
Immune dysregulation with overexpression of pro-inflammatory cytokines has been demonstrated in HS pathogenesis. Elevated levels of interleukin (IL)‑1β, IL‑12, IL‑17, IL‑23 and tissue necrosis factor alpha (TNF)‑α are seen in HS-affected tissue32,33. IL‑23 is involved in the induction of a distinct IL‑17 producing T helper (Th) cell subset, called Th17. Th17 cells have been found to infiltrate the dermis in chronic HS lesions. Several inflammatory cytokines that are upregulated in HS are produced by Type 1 Th (Th1) cells20,34.
Genetics
Approximately 30–40% of HS patients report a family history of disease in a first-degree relative, suggesting a hereditary component with a mostly autosomal dominant transmission pattern20,35. Mutations in the gamma (γ)-secretase Notch signalling pathway have been implicated in a subset of patients with severe HS phenotypes. γ-secretase is an intramembranous endoprotease complex of four hydrophobic proteins. It is involved in the cleavage of multiple type 1 transmembrane proteins including Notch receptors. Disruption of γ-secretase or Notch signalling in mice results in skin changes that are histopathologically similar to HS20,35.
Causative microorganisms
The role of bacteria in HS pathogenesis remains cryptogenic. While a polymorphic flora may be observed at HS-affected sites, superficial and deep bacterial cultures from early unruptured lesions are often sterile. Cultures of older lesions that have ruptured may demonstrate a wide range of bacteria, often contaminants of the normal skin flora20. In rare cases, a positive bacterial culture may represent secondary infection of an initially sterile process. It has been theorised that bacteria are implicated in HS pathogenesis by promoting an inflammatory response. Furthermore, biofilm formation is associated with chronic HS lesion, which likely aggregates as a secondary event and contributes to the persistence of inflammation seen in HS36.
Comorbidities and associations
Obesity
Obesity is observed in over 75% of patients with HS20. Furthermore, severity of HS appears to positively correlate with an increase in one’s body mass index. Several factors may account for this, including increased epidermal barrier stress and increased physical occlusion at affected intertriginous sites37. Increased visceral fat may also contribute to a chronic, low-grade inflammation and increased release of pro-inflammatory cytokines (IL‑1β, IL‑6 and TNF‑α) that are implicated in disease pathogenesis32. Weight loss, as later described, can lead to significant HS improvement in overweight and obese patients18,37.
Smoking
Several studies have established an association between smoking and HS. Up to 88.9% of patients with HS are active smokers. Despite this, studies assessing the effects of smoking on HS severity have produced conflicting results, ranging from slight associations to no associations20,38. Nevertheless, improvement of HS severity has been reported following smoking cessation38. There is evidence that smoking may also negatively affect treatment response in HS patients18. The mechanisms underlying the interplay between smoking and HS are likely multifactorial. Nicotine promotes follicular plugging by inducing infundibular epithelial hyperplasia in vitro39. It may exert these actions via the nicotinic acetylcholine receptors (nAChR) found on HS lesion immune cells. Other immunological effects of smoking include altered function of regulatory T cells, an increase in the number and responsiveness of dendritic cells and Th17 pathway activation32.
Mechanical stress
Mechanical stress refers to the direct pressure or friction at an affected HS site and may be seen in situations of obesity or when tight clothes are applied. Reports of HS lesions induced by mechanical stress and improved by avoidance of tight-fitting clothing are described in the literature; however, a direct causal relationship has yet to be proven. Mechanical stress-induced epidermal hyperplasia may be implicated20,40.
Sex hormones
Although the mechanisms underlying the association between HS and sex hormones are not completely understood, several observations provide evidence of a link. Notably, HS has a female predilection, rarely occurs prior to puberty and after menopause, is seen at a higher incidence in patients with polycystic ovarian syndrome, may flare during perimenstrual periods, typically improves with pregnancy, and may be responsive to anti-androgen therapy18,20. However, in most patients, testosterone, dehydroepiandrosterone sulfate, oestrogens and progesterone are not significantly elevated compared to controls, suggesting that end-organ sensitivity to normal levels and/or in situ production may be a factor in HS41.
Metabolic syndrome
Metabolic syndrome – obesity, dyslipidaemia, hypertension, hyperglycaemia and insulin resistance – is more prevalent among individuals with HS. Adipose tissue dysfunction and insulin resistance are the key mechanism underlying metabolic syndrome. The chronic inflammatory nature of HS is a likely driver of the increased risk of metabolic syndrome; however, the precise mechanisms remain under investigation42. Importantly, there is evidence that HS increases an individual’s risk of cardiovascular disease-associated death, myocardial infarction and ischaemic stroke independent of confounders18,20,42.
Medications
Drug-associated HS is rare, but has been reported following lithium, sirolimus, leflunomide, cyclosporin and certain formulations of the oral contraceptive pill43,44. However, a systematic review by Frew et al. (2018) noted the presence of alternative explanations in many cases of purported drug-associated HS43.
Autoinflammatory disease
A well-known association exists between HS and certain autoinflammatory conditions including Crohn’s disease, pyoderma gangrenosum, spondyloarthropathy and Familial Mediterranean Fever18,45.
Genodermatoses
Genodermatoses associated with HS include Dowling Degos disease (DDD), Keratisis-Ichthyosis-Deafness syndrome (KIDS), steatocystoma multiplex and pachyonychia congenita45.
Follicular occlusion syndrome
The follicular occlusion syndrome or tetrad describes a group of co-existing diseases characterised by blockage of the hair follicle with keratin and subsequent rupture. Acne conglobata, dissecting cellulitis of the scalp, HS and pilonidal sinus comprise the follicular occlusion tetrad18,20,45.
Other syndromic associations
Several syndromic associations of HS have been described, including:
- PASH: Pyoderma gangrenosum, Acne and Suppurative Hidradenitis.
- PASS: Pyoderma gangrenosum, Acne conglobata, Suppurative hidradenitis, axial Spondyloarthritis.
- PAPASH: Pyogenic Arthritis, Pyoderma gangrenosum, Acne, Suppurative Hidradenitis.
- PsAPASH: Psoriatic Arthritis, Pyoderma gangrenosum, Acne, Suppurative Hidradenitis.
- SAPHO: Synovitis, Acne, Pustulosis, Hyperostosis, Osteitis42,45.
Management
The multi-layered and variable clinical course of HS can make treatment challenging. A holistic, patient-centred approach is pivotal9. Several global treatment guidelines have been published by various expert groups, including the European Academy of Dermatology and Venerelogy, the Swiss consensus group, the Canadian Dermatology Association (CDA), the British Association of Dermatologists (BAD), the Brazilian Society of Dermatology (SBD) and the US and Canadian HS Foundations9,20,46.
Lifestyle measures
General, non-pharmacological adjuvant measures should be taken for management of HS in the relevant circumstances. Overweight or obese individuals should be encouraged and counselled on weight loss, and smokers should be encouraged to takes steps towards cessation8,9,20. Weight loss can lead to significant HS improvement, likely due to decreased epidermal barrier stress, reduced physical occlusion at affected sites and a reduction in generalised, chronic low-grade inflammation18,37. Furthermore, smoking cessation improves the long-term HS prognosis9,18.
Psychosocial support
The deleterious psychosocial impacts of HS are well documented. Health-related quality of life can be significantly compromised with patients at an increased risk of social isolation, anxiety, depression and suicide5,6. Sexual dysfunction is also described as a feature of disease, more commonly among females13,20. Furthermore, patients with HS are also more likely to be absent from work and experience slower income growth10. As part of the multidisciplinary management of patients with HS, referral for mental health care may be indicated.
Wound care and dressings
Proper wound care is an integral component of HS management. HS sufferers may live with chronic and recurrent draining wounds (Figure 7). No standard wound care protocol exists for managing HS lesions and indeed several factors must be considered when determining an appropriate dressings regimen, including the location of the lesions, the morphology of the lesions (i.e., draining tunnels, abscesses), the degree of exudate, wound odour and the cost and availability of dressings20,47. Wound care aims to improve patient quality of life by helping to manage pain and to mitigate staining of clothes and odour. Desirable dressing qualities include high absorption, comfort, easy accessibility and cost effectiveness. Furthermore, dressing shape is an important consideration given the dynamic contours of intertriginous areas. Selecting the right shape is necessary to ensure the dressing remains in place and avoids friction. Similarly, atraumatic adhesives are preferred as they reduce the risk of trauma and minimise pain during dressing changes47. Superficial HS lesions may be managed with plain absorptive dressings. The degree of exudation will guide the choice of dressing. HS lesions with cavities and tunnelling are best managed with dressings that can be packed to fill these spaces and adequately absorb fluid20,47.
The importance of a proper dressings plan cannot be overemphasised. A study by Schneider et al. (2021) demonstrated improvement in quality of life of HS patients who had access to a variety of dressings48. The European S1, SBD and Canadian consensus guidelines all highlight the need for further studies evaluating wound care and dressings in HS20,46.
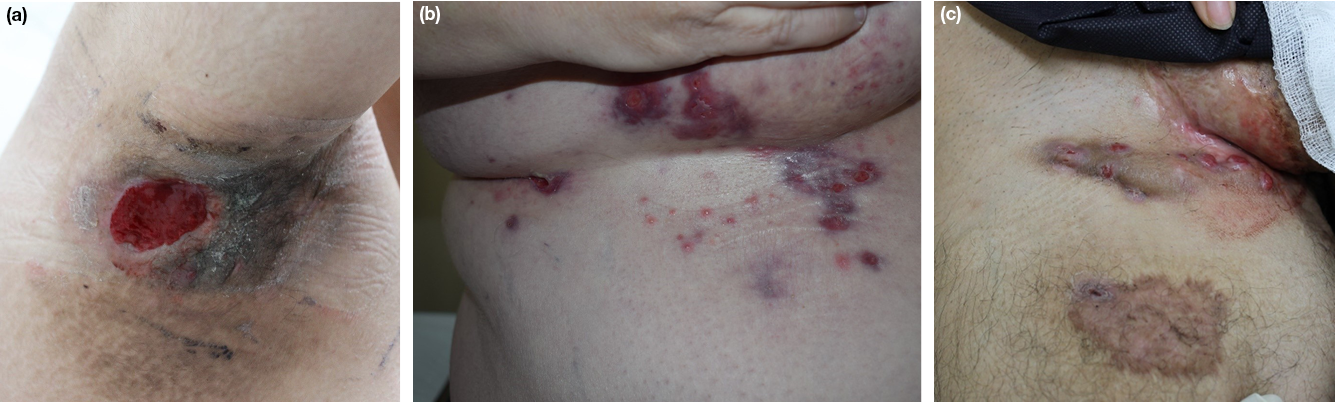
Figure 7. Chronic wounds secondary to HS of the (a) axilla; (b) mammary and sub-mammary regions;
(c) genitofemoral regions
Analgesia
The pain associated with HS may be significant and debilitating. Non-pharmacological measures to manage pain include minimising pressure and friction in areas of disease such as with loose fitting undergarments. However, analgesia is often required and prescription should follow the principles of the World Health Organization Analgesic Ladder9,20. Anti-inflammatory therapy, such as intralesional or oral steroids, may also be used to provide acute symptomatic relief9,20. These are later described in detail.
Topical antiseptic and antibacterial agents
Topical antiseptic and antibacterial agents, such as chlorhexidine 4%, benzoyl peroxide, sodium hypochlorite, triclosan and zinc pyrithione, have been used in the management of HS to reduce bacterial colonisation. Despite being recommended by experts, robust evidence to support their use in HS is lacking8,9,20. Topical antiseptics are supported by expert opinion in the North American and Swiss consensus group guidelines9,46.
Topical retinoids
Topical retinoids, such as adapalene and tazarotene, have been described for use in HS for their anti-inflammatory properties. However, evidence to support their use is lacking8,9.
Topical resorcinol
Topical resorcinol 15% has been used in the treatment of HS. It is a 1,3-isomer of benzenediol that promotes removal of follicular plugging and early abscess rupture via its keratolytic properties. It also has antipruritic and antiseptic effects8,9,20. Topical resorcinol is recommended as a first and second-line agent in several international HS treatment guidelines9,20,46.
Topical antibiotics
Topical clindamycin 1%, applied twice daily for 3 months, is used for the treatment of localised Hurley stage I or mild stage II disease, and is widely recommended. It is considered to be most beneficial in patients with superficial lesions (i.e., folliculitis, pustules, papules) or solitary lesions, and less beneficial for deeper lesions8,20,46.
Systemic antibiotics
Tetracycline monotherapy (i.e., tetracycline, minocycline, doxycycline) has been used to effectively treat more widely spread Hurley stage I or mild Hurley stage II HS. Regimens include oral tetracycline 500mg twice daily, doxycycline 100mg daily or minocycline 50mg twice daily. Treatment is often continued for 3 months. Despite their antibacterial properties (inhibition of the 30S ribosomal unit), the beneficial effects of tetracyclines in HS are likely achieved via their anti-inflammatory effects8,20,46.
Oral rifampicin (600mg daily or 300mg twice daily) and clindamycin (300mg twice daily) combination therapy may be used in patients who fail to respond to tetracycline monotherapy, or as first-line therapy in patients with any severity of active inflammatory HS. Treatment duration is typically 10–12 weeks8,20,46.
Other oral antibiotics, such as erythromycin, moxifloxacin and metronidazole, have been described in the literature for use in HS. Patients who respond to systemic antibiotics should only be selectively kept on long-term maintenance doses to reduce the risk of adverse events. Indeed, patients who achieve satisfactory control of their HS with systemic antibiotics may cease treatment and continue topical maintenance therapy8,9,20,46.
Anti-inflammatory therapy
Intralesional corticosteroids: intralesional triamcinolone acetonide (5–10mg/ml), either as monotherapy or in combination with systemic therapy, may be used directly on an inflammatory nodule for the rapid reduction of inflammation. Clinical improvement of pain and inflammation may occur within 48–72 hours. Intralesional triamcinolone acetonide should not be used if bacterial infection is suspected due to the rare risk of superinfection. Complications may include atrophy and dyspigmentation at the site of injection9,20.
Oral corticosteroids: oral corticosteroids (i.e., prednisolone 0.5–0.7mg/kg) may be used as short-term rescue therapy to effectively treat acute flares. Withdrawal of short-term or long-term oral corticosteroids can result in a rebound flare of HS, and so tapering to stop is important. Corticosteroids should be used with care as they may increase the risk of HS-related comorbidities such as weight gain, insulin resistance and dysplidaemia8,9,20.
Cyclosporine
Cyclosporine is a calcineurin inhibitor that has been used to treat cases of recalcitrant HS through its potent immunosuppressive activity. It has been used at doses of 2–6mg/kg for variable durations, ranging from 6 weeks to 7 months. Major adverse events may include nephrotoxicity, hypertension and an increased risk of malignancy, especially lymphoma and skin cancer8,9,20. In the CDA, SBD, European S1 and Swiss consensus treatment guidelines, cyclosporine is reserved as a third-line agent in refractory HS20,46.
Dapsone
Dapsone is a sulphone drug that has be used to treat Hurley stage I and II HS. Its effects are likely achieved via its anti-inflammatory and antibacterial properties. For the treatment of HS, dapsone has been used at doses of 25–200mg daily. It is contraindicated in patients with G6PD deficiency, severe anaemia, acute porphyria and sulphonamide allergy8,9,20.
Oral retinoids
Isotretinoin has been used to treat HS off-label with mixed results. Its benefits, if any, appear to be restricted to patients with mild HS9,20. The European S1 guidelines do not currently recommend the use of isotretinoin in the treatment of HS20. Other international guidelines describe its use as a third-line agent in patients with concomitant acne46.
Acitretin, a metabolite of etretinate, has been used to treat HS with conflicting results of efficacy. It appears to have a high response rate in a reasonable proportion of patients. It is indicated for usage in early Hurley stage I and II HS as second-line agent. The optimal dosing for acitretin for treatment of HS has yet to be established, with doses ranging from 0.25–0.88mg/kg in the literature8,9,20,46.
Biologics
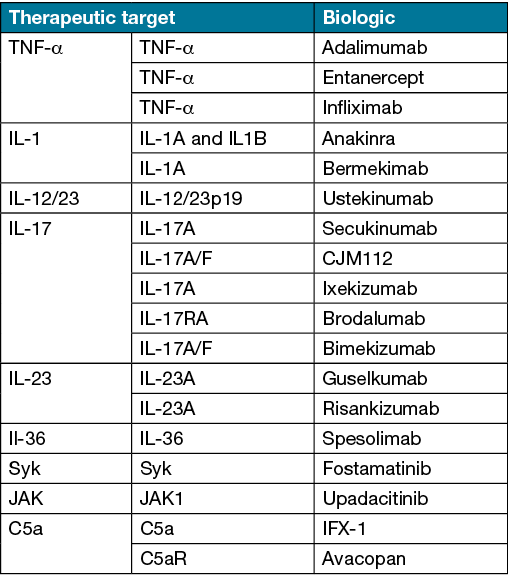
Biologics have revolutionised the treatment of several chronic inflammatory disorders. Advances in the understanding of HS pathogenesis has led to the recognition of several potential therapeutic targets9,20,33. The TNF‑α inhibitors adalimumab and infliximab have demonstrated efficacy in the management of moderate to severe HS. In Australia, adalimumab is the only approved and PBS-subsidised biologic for severe HS. Potential therapeutic targets in HS, including biologics undergoing investigation, are presented in Table 3.
Table 3. Potential therapeutic targets in HS and biologics (including those under investigation)8,33
Other treatments
In published cases or small case series, other treatments that have been reported to be effective in treating HS include botulinum toxin, intramuscular immunoglobulin, zinc gluconate, liraglutide, metformin, anti-androgen therapy and cryo-insufflation8,9,20,46.
Surgery
Surgical therapy remains a pillar of HS management. Lesion-directed therapies include incision and drainage, deroofing, local excision of individual lesions, and extensive, wide excision of affected areas9,20. Incision and drainage is useful for the management of abscesses; however, recurrence rates are high. Inflammatory nodules without fluctuance should not be incised20. Deroofing is used as a tissue-sparing technique to surgically remove the roof of an abscess or sinus tract, often with a blunt probe (via conventional or electrosurgery). Excision of single lesions results in lower recurrence compared to incision and drainage, and may be suitable for patients with few, non-inflamed nodules or sinus tracts9,20. Wide local excision may be indicated in extensive HS where multiple interconnecting sinus tracts are seen. As the resulting wounds are rarely amenable to primary closure, further reconstruction with grafting (immediate or delayed) or a flap may be necessary depending on the anatomic site. Post-operatively, sites managed with wide local excision may be prone to strictures. Indeed, such radical surgery may be disfiguring and require a prolonged recovery20. Ultrasound imaging may be used to pre-operatively map HS lesions, delineate lesional areas and aid in characterising different fistula tracts. Furthermore, the inflammatory activity of HS lesions may be assessed using colour doppler. Overall, ultrasound has been shown to enhance surgical management49.
Ablative laser therapy
Scanner-assisted carbon dioxide (CO2) laser may be used to remove all affected HS tissue. Treatment aims to achieve focal radical vaporisation of all HS lesions (i.e., nodules, abscesses, fistulas) while preserving healthy tissue between lesions9,20. Ablative CO2 lasers have demonstrated efficacy in the treatment of HS in RCTs20. Wounds produced from ablative CO2 laser are often left to heal by secondary intention; however, this can take many weeks9,20. Overall, CO2 laser treatment appears to be an effective treatment modality for recurrent HS lesions and is associated with high levels of self-reported patient satisfaction50.
Non-ablative laser therapy
Neodymium-doped yttrium aluminium garnet (Nd:YAG) laser and intense pulsed light (IPL) have been used for the treatment of HS, based on the premise that occlusion of the upper part of the hair follicle is the central pathogenic event. Nd:YAG appears to be an effective treatment modality for HS and, while evidence for IPL is less established, results are also promising9,20.
Conclusions
HS is a chronic and often debilitating condition. Management is frequently challenging and sufferers may live with chronic and recurrent draining wounds. Further research is required to identify and optimise management in those with severe disease.
Author contribution
We declare that all listed authors have made substantial contributions to the conception of this work, drafting of this work and final approval of the version to be published. Furthermore, all authors attest to the validity and integrity of this work.
Acknowledgements
None.
Conflict of interest
The authors declare no conflicts of interest.
Ethics statement
An ethics statement is not applicable.
Funding
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Author(s)
Anthony Moussa*1, Anneliese Willems1 and Rodney D Sinclair1,2
1Sinclair Dermatology, East Melbourne, VIC, Australia
2University of Melbourne, Melbourne, VIC, Australia
*Corresponding author Email anthonymoussa@outlook.com
References
- Fimmel S, Zouboulis CC. Comorbidities of hidradenitis suppurativa (acne inversa). Dermatoendocrinol 2010 Jan;2(1):9–16. doi:10.4161/derm.2.1.12490. PMID:21547142; PMCID:PMC3084959.
- Revuz JE, Canoui-Poitrine F, Wolkenstein P, Viallette C, Gabison G, Pouget F, et al. Prevalence and factors associated with hidradenitis suppurativa: results from two case-control studies. J Am Acad Dermatol 2008 Oct;59(4):596–601. doi:10.1016/j.jaad.2008.06.020. PMID:18674845.
- Jemec GB, Heidenheim M, Nielsen NH. The prevalence of hidradenitis suppurativa and its potential precursor lesions. J Am Acad Dermatol 1996 Aug;35(2 Pt 1):191–4. doi:10.1016/s0190-9622(96)90321-7. PMID:8708018.
- Calao M, Wilson JL, Spelman L, Billot L, Rubel D, Watts AD, et al. Hidradenitis suppurativa (HS) prevalence, demographics and management pathways in Australia: a population-based cross-sectional study. PLoS One 2018 Jul 24;13(7):e0200683. doi:10.1371/journal.pone.0200683. PMID:30040827; PMCID:PMC6057625.
- Machado MO, Stergiopoulos V, Maes M, Kurdyak PA, Lin PY, Wang LJ, et al. Depression and anxiety in adults with hidradenitis suppurativa: a systematic review and meta-analysis. JAMA Dermatol 2019 Aug 1;155(8):939–945. doi:10.1001/jamadermatol.2019.0759. PMID:31166590; PMCID:PMC6551580.
- Thorlacius L, Cohen AD, Gislason GH, Jemec GBE, Egeberg A. Increased suicide risk in patients with hidradenitis suppurativa. J Invest Dermatol 2018 Jan;138(1):52–57. doi:10.1016/j.jid.2017.09.008. Epub 2017 Sep 20. PMID:28942360.
- Cazzaniga S, Pezzolo E, Bettoli V, Abeni D, Marzano AV, Patrizi A, et al. Characterization of hidradenitis suppurativa phenotypes: a multidimensional latent class analysis of the National Italian Registry IRHIS. J Invest Dermatol 2021 May;141(5):1236–1242.e1. doi:10.1016/j.jid.2020.08.032. Epub 2020 Oct 21. PMID:33098826.
- Frew JW, Hawkes JE, Krueger JG. Topical, systemic and biologic therapies in hidradenitis suppurativa: pathogenic insights by examining therapeutic mechanisms. Ther Adv Chronic Dis 2019 Mar 1;10:2040622319830646. doi:10.1177/2040622319830646. PMID:30854183; PMCID:PMC6399757.
- Alikhan A, Sayed C, Alavi A, Alhusayen R, Brassard A, Burkhart C, et al. North American clinical management guidelines for hidradenitis suppurativa: a publication from the United States and Canadian Hidradenitis Suppurativa Foundations: Part I: diagnosis, evaluation, and the use of complementary and procedural management. J Am Acad Dermatol 2019 Jul;81(1):76–90. doi:10.1016/j.jaad.2019.02.067. Epub 2019 Mar 11. PMID:30872156.
- Kokolakis G, Wolk K, Schneider-Burrus S, Kalus S, Barbus S, Gomis-Kleindienst S, et al. Delayed diagnosis of hidradenitis suppurativa and its effect on patients and healthcare system. Dermatol 2020;236(5):421–430. doi:10.1159/000508787. Epub 2020 Jul 1. PMID:32610312; PMCID:PMC7592906.
- Naik HB, Paul M, Cohen SR, Alavi A, Suàrez-Fariñas M, Lowes MA. Distribution of self-reported hidradenitis suppurativa age at onset. JAMA Dermatol 2019 Aug 1;155(8):971–973. doi:10.1001/jamadermatol.2019.0478. PMID:31166574; PMCID:PMC6551578.
- Garg A, Kirby JS, Lavian J, Lin G, Strunk A. Sex- and age-adjusted population analysis of prevalence estimates for hidradenitis suppurativa in the United States. JAMA Dermatol 2017 Aug 1;153(8):760–764. doi:10.1001/jamadermatol.2017.0201. PMID:28492923; PMCID:PMC5710402.
- Yee D, Collier EK, Atluri S, Jaros J, Shi VY, Hsiao JL. Gender differences in sexual health impairment in hidradenitis suppurativa: a systematic review. Int J Womens Dermatol 2020 Nov 10;7(3):259–264. doi:10.1016/j.ijwd.2020.10.010. PMID:34222580; PMCID:PMC8243154.
- Saunte DM, Boer J, Stratigos A, Szepietowski JC, Hamzavi I, Kim KH, et al. Diagnostic delay in hidradenitis suppurativa is a global problem. Br J Dermatol 2015 Dec;173(6):1546–9. doi:10.1111/bjd.14038. Epub 2015 Nov 3. PMID:26198191.
- Daxhelet M, Suppa M, White J, Benhadou F, Thorlacius LR, Jemec GBE, et al. Proposed definitions of typical lesions in hidradenitis suppurativa. Dermatol 2020;236(5):431–438. doi:10.1159/000507348. Epub 2020 Jun 9. PMID:32516781.
- Ring HC, Theut Riis P, Zarchi K, Miller IM, Saunte DM, Jemec GB. Prodromal symptoms in hidradenitis suppurativa. Clin Exp Dermatol 2017 Apr;42(3):261–265. doi:10.1111/ced.13025. Epub 2017 Feb 14. PMID:28194809.
- Revuz J. Hidradenitis suppurativa. J Eur Acad Dermatol Venereol 2009 Sep;23(9):985–98. doi:10.1111/j.1468-3083.2009.03356.x. PMID:19682181.
- Kromann CB, Deckers IE, Esmann S, Boer J, Prens EP, Jemec GB. Risk factors, clinical course and long-term prognosis in hidradenitis suppurativa: a cross-sectional study. Br J Dermatol 2014 Oct;171(4):819–24. doi:10.1111/bjd.13090. Epub 2014 Sep 7. PMID:24804604.
- Alikhan A, Lynch PJ, Eisen DB. Hidradenitis suppurativa: a comprehensive review. J Am Acad Dermatol 2009 Apr;60(4):539–61; quiz 562–3. doi:10.1016/j.jaad.2008.11.911. PMID:19293006.
- Zouboulis CC, Desai N, Emtestam L, Hunger RE, Ioannides D, Juhász I, et al. European S1 guideline for the treatment of hidradenitis suppurativa/acne inversa. J Eur Acad Dermatol Venereol 2015 Apr;29(4):619–44. doi:10.1111/jdv.12966. Epub 2015 Jan 30. PMID:25640693.
- Jung JM, Lee KH, Kim YJ, Chang SE, Lee MW, Choi JH, et al. Assessment of overall and specific cancer risks in patients with hidradenitis suppurativa. JAMA Dermatol 2020 Aug 1;156(8):844–853. doi:10.1001/jamadermatol.2020.1422. PMID:32459291; PMCID:PMC7254443.
- Hurley HJ. Axillary hyperhidrosis, apocrine bromhidrosis, hidradenitis suppurativa, and familial benign pemphigus: surgical approach In: Roenigk RK, Roenigk HH, editors. Dermatologic surgery: principles and practice. New York: Marcel Dekker; 1989. p. 729–39.
- Sartorius K, Lapins J, Emtestam L, et al. Suggestions for uniform outcome variables when reporting treatment effects in hidradenitis suppurativa. Br J Dermatol 2003; 149: 211–213.
- Sartorius K, Emtestam L, Jemec GB, et al. Objective scoring of hidradenitis suppurativa reflecting the role of tobacco smoking and obesity. Br J Dermatol 2009; 161: 831–839.
- Sartorius K, Killasli H, Heilborn J, Jemec GB, Lapins J, Emtestam L. Interobserver variability of clinical scores in hidradenitis suppurativa is low. Br J Dermatol 2010 Jun;162(6):1261–8. doi:10.1111/j.1365-2133.2010.09715.x. Epub 2010 Feb 22. PMID:20184581.
- Kimball AB, Kerdel F, Adams D, Mrowietz U, Gelfand JM, Gniadecki R, et al. Adalimumab for the treatment of moderate to severe hidradenitis suppurativa: a parallel randomized trial. Ann Intern Med 2012 Dec 18;157(12):846–55. doi:10.7326/0003-4819-157-12-201212180-00004. PMID:23247938.
- Grant A, Gonzalez T, Montgomery MO, Cardenas V, Kerdel FA. Infliximab therapy for patients with moderate to severe hidradenitis suppurativa: a randomized, double-blind, placebo-controlled crossover trial. J Am Acad Dermatol 2010 Feb;62(2):205–17. doi:10.1016/j.jaad.2009.06.050. PMID:20115947.
- Zouboulis CC, Tzellos T, Kyrgidis A, Jemec GBE, Bechara FG, Giamarellos-Bourboulis EJ, et al.; European Hidradenitis Suppurativa Foundation Investigator Group. Development and validation of the International Hidradenitis Suppurativa Severity Score System (IHS4), a novel dynamic scoring system to assess HS severity. Br J Dermatol 2017 Nov;177(5):1401–1409. doi:10.1111/bjd.15748. Epub 2017 Oct 30. PMID:28636793.
- Goldfarb N, Ingram JR, Jemec GBE, Naik HB, Piguet V, Hyde MJ, et al. Hidradenitis Suppurativa Area and Severity Index (HASI): a pilot study to develop a novel instrument to measure the physical signs of hidradenitis suppurativa. Br J Dermatol 2020 Jan;182(1):240–242. doi:10.1111/bjd.18335. Epub 2019 Sep 17. PMID:31286486; PMCID:PMC7769071.
- von Laffert M, Helmbold P, Wohlrab J, Fiedler E, Stadie V, Marsch WC. Hidradenitis suppurativa (acne inversa): early inflammatory events at terminal follicles and at interfollicular epidermis. Exp Dermatol 2010 Jun;19(6):533–7. doi:10.1111/j.1600-0625.2009.00915.x. Epub 2009 Jul 29. PMID:19659829.
- Frew JW. Hidradenitis suppurativa is an autoinflammatory keratinization disease: a review of the clinical, histologic, and molecular evidence. JAAD Int 2020 Jun 27;1(1):62–72. doi:10.1016/j.jdin.2020.05.005. PMID:34409324; PMCID:PMC8361883.
- Frew JW, Hawkes JE, Krueger JG. A systematic review and critical evaluation of inflammatory cytokine associations in hidradenitis suppurativa. F1000Res 2018 Dec 13;7:1930. doi:10.12688/f1000research.17267.1. PMID:30828428; PMCID:PMC6392156.
- Frew JW, Marzano AV, Wolk K, Join-Lambert O, Alavi A, Lowes MA, et al. A systematic review of promising therapeutic targets in hidradenitis suppurativa: a critical evaluation of mechanistic and clinical relevance. J Invest Dermatol 2021 Feb;141(2):316–324.e2. doi:10.1016/j.jid.2020.06.019. Epub 2020 Sep 9. PMID:32919760.
- van der Zee HH, Laman JD, de Ruiter L, Dik WA, Prens EP. Adalimumab (antitumour necrosis factor-α) treatment of hidradenitis suppurativa ameliorates skin inflammation: an in situ and ex vivo study. Br J Dermatol 2012 Feb;166(2):298–305. doi:10.1111/j.1365-2133.2011.10698.x. PMID:22013960.
- Pink AE, Simpson MA, Desai N, Dafou D, Hills A, Mortimer P, et al. Mutations in the γ-secretase genes NCSTN, PSENEN, and PSEN1 underlie rare forms of hidradenitis suppurativa (acne inversa). J Invest Dermatol 2012 Oct;132(10):2459–2461. doi:10.1038/jid.2012.162. Epub 2012 May 24. PMID:22622421.
- Ring HC, Bay L, Nilsson M, Kallenbach K, Miller IM, Saunte DM, et al. Bacterial biofilm in chronic lesions of hidradenitis suppurativa. Br J Dermatol 2017 Apr;176(4):993–1000. doi:10.1111/bjd.15007. Epub 2017 Feb 19. PMID:27564400.
- Kromann CB, Ibler KS, Kristiansen VB, Jemec GB. The influence of body weight on the prevalence and severity of hidradenitis suppurativa. Acta Derm Venereol 2014 Sep;94(5):553–7. doi:10.2340/00015555-1800. PMID:24577555.
- Denny G, Anadkat MJ. The effect of smoking and age on the response to first-line therapy of hidradenitis suppurativa: an institutional retrospective cohort study. J Am Acad Dermatol 2017 Jan;76(1):54–59. doi:10.1016/j.jaad.2016.07.041. Epub 2016 Sep 28. PMID:27692736; PMCID:PMC5391674.
- Kurzen H, Kurzen M. Secondary prevention of hidradenitis suppurativa. Dermatol Rep 2019 Oct 25;11(2):8243. doi:10.4081/dr.2019.8243. PMID:31728176; PMCID:PMC6826242.
- Boer J, Nazary M, Riis PT. The role of mechanical stress in hidradenitis suppurativa. Dermatol Clin 2016 Jan;34(1):37–43. doi:10.1016/j.det.2015.08.011. PMID:26617356.
- Nikolakis G, Kyrgidis A, Zouboulis CC. Is there a role for antiandrogen therapy for hidradenitis suppurativa? A systematic review of published data. Am J Clin Dermatol 2019 Aug;20(4):503–513. doi:10.1007/s40257-019-00442-w. PMID:31073704.
- Ergun T. Hidradenitis suppurativa and the metabolic syndrome. Clin Dermatol 2018 Jan-Feb;36(1):41–47. doi:10.1016/j.clindermatol.2017.09.007. Epub 2017 Sep 12. PMID:29241751.
- Frew JW, Vekic DA, Woods JA, Cains GD. Drug-associated hidradenitis suppurativa: a systematic review of case reports. J Am Acad Dermatol 2018 Jan;78(1):217–219.e2. doi:10.1016/j.jaad.2017.08.046. PMID:29241794.
- Machan A, Azendour H, Toufik H, Achemlal L, Boui M, Hjira N. Leflunomide-induced hidradenitis suppurativa. Case Rep Rheumatol 2020 Feb 18;2020:3549491. doi:10.1155/2020/3549491. PMID:32148994; PMCID:PMC7049404.
- Garcovich S, Genovese G, Moltrasio C, Malvaso D, Marzano AV. PASH, PAPASH, PsAPASH, and PASS: the autoinflammatory syndromes of hidradenitis suppurativa. Clin Dermatol 2021 Mar-Apr;39(2):240–247. doi:10.1016/j.clindermatol.2020.10.016. Epub 2020 Oct 16. PMID:34272017.
- Hendricks AJ, Hsiao JL, Lowes MA, Shi VY. A comparison of international management guidelines for hidradenitis suppurativa. Dermatol 2021;237(1):81–96. doi:10.1159/000503605. Epub 2019 Oct 23. PMID:31645040.
- Kazemi A, Carnaggio K, Clark M, Shephard C, Okoye GA. Optimal wound care management in hidradenitis suppurativa. J Dermatolog Treat 2018 Mar;29(2):165–167. doi:10.1080/09546634.2017.1342759. Epub 2017 Jun 29. PMID:28609151.
- Schneider C, Sanchez DP, MacQuhae F, Stratman S, Lev-Tov H. Wound dressings improve quality of life for hidradenitis suppurativa patients. J Am Acad Dermatol 2021 Oct 2:S0190-9622(21)02573-1. doi:10.1016/j.jaad.2021.09.058. Epub ahead of print. PMID:34610381.
- Martorell A, Alfageme Roldán F, Vilarrasa Rull E, Ruiz-Villaverde R, Romaní De Gabriel J, García Martínez F, et al. Ultrasound as a diagnostic and management tool in hidradenitis suppurativa patients: a multicentre study. J Eur Acad Dermatol Venereol 2019 Nov;33(11):2137–2142. doi:10.1111/jdv.15710. Epub 2019 Jun 23. PMID:31124183.
- Mikkelsen PR, Dufour DN, Zarchi K, Jemec GB. Recurrence rate and patient satisfaction of CO2 laser evaporation of lesions in patients with hidradenitis suppurativa: a retrospective study. Dermatol Surg 2015 Feb;41(2):255–60. doi:10.1097/DSS.0000000000000264. PMID:25654196.



