Volume 30 Number 3
Managing a malignant wound in palliative care
David White and Sampath Kondasinghe
Keywords multidisciplinary team, hospice care, malignant wounds, palliative care
For referencing White D & Kondasinghe S. Managing a malignant wound in palliative care. Wound Practice and Research 2022; 30(3):150-157
DOI
https://doi.org/10.33235/wpr.30.3.150-157
Submitted 9 June 2022
Accepted 26 July 2022
Abstract
Malignant wounds cause significant suffering for patients with advanced cancer. These wounds are often non-healing, and the burden of wound-related symptoms is associated with declining physical function, social isolation and a sense of loss of control among patients. Holistic multidisciplinary support and specialised wound care are key to caring for patients with malignant wounds; however, this area remains under-researched and evidence-based management remains challenging. This paper reviews the existing evidence for management of malignant wounds and highlights the role of palliative care in supporting patients and caregivers.
Introduction
Malignant wounds commonly occur among patients with advanced cancer receiving palliative care and can be challenging to manage. Pain, bleeding, exudate, malodour and the psychological distress associated with non-healing malignant wounds cause significant suffering. These patients benefit from a palliative care approach which aims to optimise quality of life and function in the face of a terminal illness. A holistic approach in addressing these issues involves assessing physical, psychosocial and spiritual domains of a patient’s experience. Management plans should involve a patient-centred therapeutic relationship with the aim of relieving suffering. The body of evidence behind management strategies for non-healing malignant wounds in palliative care is generally of low quality. This review aims to summarise current practice in the management of malignant wounds and highlight the need for further research. The following case identifies common issues in managing malignant wounds in a palliative care setting.
Case history
A 35-year-old man with metastatic anal adenocarcinoma was admitted to an inpatient palliative care unit for symptom management. Two years following diagnosis and treatment of his primary cancer, including chemoradiotherapy, an abdominoperineal resection and formation of an end-colostomy, metastatic disease involving the perineum and scrotum developed. This lead to a malignant genital and perineal wound involving the corpora of the penis extending to inguinal and suprapubic regions. The wound was complex with nodular and ulcerated components, with pain, malodour, bleeding and purulent discharge causing significant distress. Prominent psychosocial issues included a lack of social support at home, marriage breakdown while raising a school-aged child, inability to work, financial stress, disruption of body image, and cultural attitudes limiting the provision of nursing care.
The patient rarely allowed medical staff to review the wound and desired to privately manage his own dressings without nursing input; however, exudate would soak through the dressings into his clothes, requiring him to shower multiple times a day, and pain was poorly controlled. As his social situation was better understood, trust developed through the therapeutic relationship and an acceptable dressing plan was established which focused on maintaining his independence. He would wash the wound twice daily with normal saline or with showering, the surrounding skin was then cleaned with cleansing wipes and a barrier cream applied. A silver impregnated carboxymethylcellulose dressing was secured with a low-profile continence pad supported by his underwear and, if bleeding was present, an absorbent calcium alginate dressing with silver could be used. His pain management regimen was optimised including background analgesia with methadone, neuropathic adjuvant agents including pregabalin, and immediate release hydromorphone prior to dressing changes. The patient was discharged home, supported by a community palliative care service with this plan. He was able to spend quality time with his daughter and was re-admitted to the unit 2 months later for end-of-life care.
This case highlights the complex and interacting issues encountered in managing patients with malignant wounds in palliative care which are discussed in the following review.
Malignant wounds in palliative care
Fungating malignant wounds arise when ulceration and necrosis occur in cancer-related skin lesions, either from a primary tumour or cutaneous metastasis1,2. The rate of malignant wounds among patients with cancer is not well established, as incidence is not recorded in cancer registries, but likely lies between 5–10% and may be as high as 15% among those receiving palliative care3–5. Cancers most commonly associated with malignant wounds include breast, head and neck and primary skin cancers where their presence may reflect advanced disease indicating a poor prognosis4,6,7. Metastatic lung, renal and colorectal cancers are also associated with the development of malignant wounds4,6. The pathogenesis of malignant wounds involves destruction of lymphatic and vascular structures leading to oedema, impaired perfusion, necrosis, proliferative growth, ulceration and polymicrobial colonisation, impairing the ability of the wound to heal8. Symptoms, including pain, bleeding, malodour and exudate, are associated with decreased self-esteem, impaired functioning and poorer quality of life among patients with malignant wounds9,10.
Systemic treatments targeting the underlying malignancy – including chemotherapy, immunotherapy, hormonal treatments and local therapies including radiotherapy or surgery – can provide disease control and improve wound symptoms. Unfortunately, malignant wounds are often not amenable to surgery due to the extent of the wound, and systemic anti-cancer treatments may not be indicated due to poor patient performance status. When these strategies are not appropriate, a malignant wound is unlikely to heal and the focus of care should shift to identifying what is important to the patient, establishing realistic expectations, and the provision of education and psychosocial support to patients and their caregivers11. When wound healing is not achievable, management goals will include preventing further wound deterioration and breakdown, managing symptoms and promoting quality of life, comfort and dignity12. Comprehensive wound and patient assessment is foundational to the holistic management of patients with malignant wounds.
Wound and patient assessment
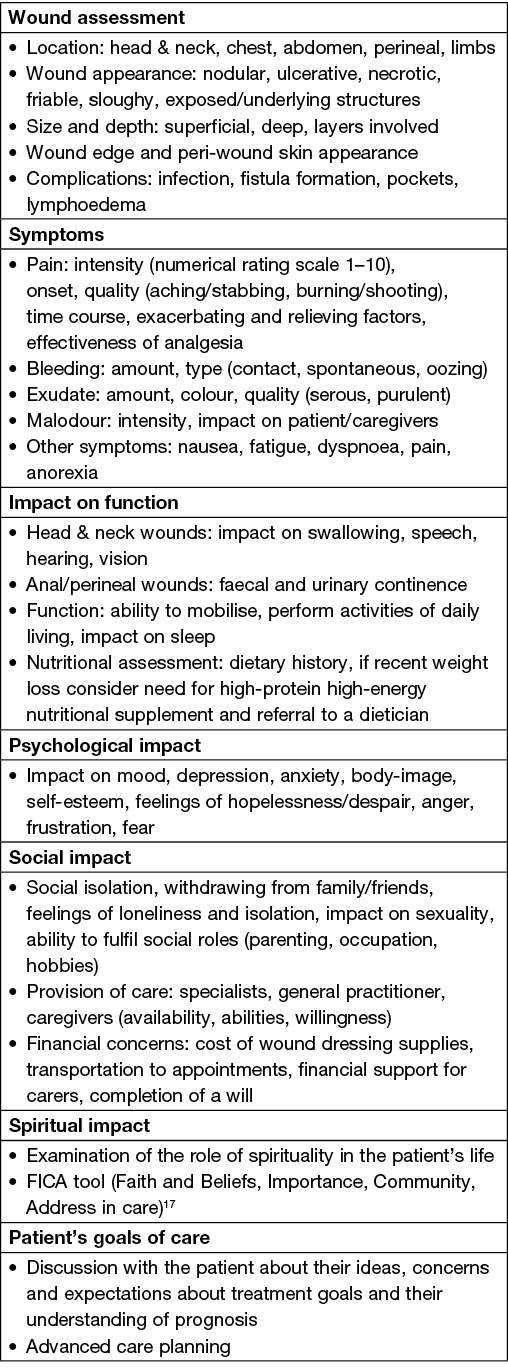
Wound assessment includes a detailed inspection of the wound, a review of symptoms, the impact of the wound on functioning, including psychosocial and spiritual domains, and the patient’s goals of care (Box 1)13–16. When evaluating pain, patients should be specifically asked about exacerbating and relieving factors, level of discomfort with dressing changes and the degree of relief from analgesia. Malignant wounds can develop and transform rapidly and require frequent re-evaluation. Given the differences in wound location, features, presence of bleeding, exudate and pain, local knowledge and nursing expertise in wound assessment is required to develop an individualised dressing plan. The Treatment Evaluation by Le Roux’s method (TELER) and the Wound and Symptoms Self-Assessment Chart (WoSSAC) have been validated to guide malignant wound assessment, measuring symptoms and outcomes among patients with malignant wounds, though the impact of these tools in clinical practice have not been evaluated14.
Box 1. Comprehensive assessment of patients with malignant wounds14,15
Wound cleansing and dressings
The evidence base for the topical management of malignant wounds is mostly based on case reports and expert opinion3,5. A Cochrane review of dressings for fungating wounds found there was insufficient evidence to give clear directives for clinical practice18. Dressing changes can cause pain through local trauma to the friable wound surface, making an appropriate cleansing and dressing regimen crucial19. Among wounds that are not expected to heal, consideration for patient comfort, convenience and cost should be a priority when selecting the type of dressing, frequency and timing of dressing changes11. The cost and financial burden of dressings may also be a concern for patients and should be discussed when deciding on a dressing regimen. More expensive specialty dressings can be cost-effective if they lead to a reduction in frequency of dressing changes compared to simple cotton or gauze dressings20.
Moistening dressings with saline, warm water or use of an adhesive remover assist in minimising wound trauma13. If immediate release opioid analgesia is required it should be administered a suitable period of time prior to removal of dressings. Gentle irrigation with 0.9% saline or tap water is preferred when cleaning malignant wounds rather than scrubbing which can cause bleeding. Patients may prefer to cleanse a wound when showering or bathing before applying a new dressing13. Irrigation may not be appropriate for wounds with deep tunnels or where the irrigant will accumulate in the wound19. Protecting surrounding intact skin should be a priority, using barrier creams and ostomy skin barriers for exudative wounds. Sharp debridement is usually avoided due to the risk of precipitating bleeding, especially when there are underlying vascular structures21–23. Autolytic debridement of necrotic tissue can help to reduce infection and malodour but needs to be balanced against increasing the amount of exudate and may not be appropriate for non-healing wounds13. Negative pressure wound therapy is generally avoided in malignancy due to the potential risk of increasing tumour growth and bleeding, though case reports of use in selected patients have been published24,25.
Wound dressings aim to assist in managing symptoms of a malignant wound, promoting comfort and facilitating patients to maintain social engagement26. The visual aspect of malignant wounds is often distressing to patients and carers, and dressing changes can be an unpleasant experience27. Patients and carers will find creative ways to cope in managing a wound, which can be facilitated by health professionals responding to psychosocial and emotional needs and providing education and empathetic care27. Consideration of the cosmetic appearance of dressings is important for patients’ quality of life, with a preference for less bulky dressings and those that do not impede mobility. Practical methods for securing dressings such as use of sports bras or mesh underwear are useful15. A process of trial and error of available dressings and regimens may be required before finding a plan that meets a patient’s needs.
Pain
Malignant wounds are often painful and pain control is frequently a main concern of patients and caregivers6,14. Patients should have a pain management plan with adequate systemic analgesia provided prior to dressing changes. The WHO analgesic ladder and other guidelines (ESMO28 and EAPC29) provide instruction on the management of cancer pain. For pain not managed with simple analgesics (paracetamol and non-steroidal anti-inflammatory drugs), a combination of immediate and long-acting opioids to address incident and background pain may be necessary11. Other adjuvant therapies that target neuropathic pain may also be used, including antidepressants (amitriptyline and duloxetine) and calcium channel ligands (gabapentin and pregabalin). There is some evidence that gabapentin is effective as an adjuvant analgesic for dressing changes due to its anxiolytic effects; however, this was not supported by a subsequent meta-analysis among patients with cancer pain30,31.
When providing analgesia prior to dressing changes the time to onset of action of systemic opioids should be considered. Oral immediate release morphine should be taken 30 minutes prior, whereas rapid-onset opioids have faster onset of action (within 30 minutes) including transmucosal fentanyl which is used for breakthrough cancer pain among select patients32. Topical opioids in the form of morphine mixed with a hydrogel may have a role through its local analgesic effect5,33. An advantage of topical opioids over systemic opioids may include reduced side-effects, particularly constipation; however, the bioavailability of morphine when applied over broken skin is highly variable19. Morphine gel can be prepared by mixing 10mg morphine (ampoule for injection) with 10g of water-soluble carrier gel, applied to the wound using a gloved finger or to a non-absorbent dressing placed over the wound up to three times a day11. However, the evidence for topical morphine in malignant wounds remains weak and is limited to small studies at risk of bias; further evidence is required to guide practice5.
Malodour, exudate and infection
Odour and exudate associated with malignant wounds are often the symptoms most detrimental to quality of life reported by patients7,34. Malodour can impact patients’ relationships with others, particularly caregivers. Assessment of odour is subjective and includes patient self-assessment and reports from family, carers and clinicians. While desensitisation to offensive odours after prolonged exposure does occur, patients will usually remain aware of malodour and be conscious of the impact it has on others3. Consideration of the stigmatising impact of malodour and its effect on social functioning can provide insights that will influence the wound management plan.
Bacterial colonisation of malignant wounds contributes to malodour, particularly the presence of anaerobes producing fatty acid volatiles and the metabolites putrescine and cadaverine which are associated with wound odour35. Higher bacterial loads in a wound are associated with increased pain and exudate, thus strategies to reduce this load may assist in symptom control35. Topical antimicrobials have been used to manage wound odour with 0.75–0.8% metronidazole gel applied to the wound once or twice daily with dressing changes8. A systematic review in 2015 found there was a scarcity of clinical trials of the effectiveness of metronidazole gel limiting the interpretation of its effect, with only one randomised control trial reporting an improvement in odour36. A 2018 randomised trial which compared polyhexamethyl biguanide and 0.8% metronidazole applied twice daily over 4 days with dressing changes found that both treatments improved malignant wound odour with marginal improvement in quality of life measures; however, there was no difference between groups and the study was limited by a small sample size37. Further evidence is necessary to guide routine practice in this area, with the cost of metronidazole gel also limiting its use.
Medical-grade honey applied topically to malignant wounds has been used to manage odour and promote wound healing among burns, diabetic and pressure ulcers; however, its benefit is yet to be confirmed in randomised trials for malignant wounds12,38,39. Topical antiseptic dressings incorporating iodine, medical-grade honey, silver or polyhexamethyl biguanide can assist in the control of infection; however, the evidence for their use in improving symptoms remains weak40.
Activated charcoal impregnated dressings can contain odour by adsorbing malodourous chemicals; however, their use is based on clinical experience alone18,21,41. There is evidence to support the use of foam dressings impregnated with silver, with a randomised control trial involving 26 patients with malignant fungating wounds finding a significant reduction in odour with silver foam dressings compared to those without42. A randomised trial of silver-coated bandages and honey-coated bandages found both improved malodour and exudate among malignant wounds but no significant difference between the two43.
Management of exudate assists in minimising odour, where highly absorbent gelling fibre (hydrofibre) dressings with water-proof backings, foam and alginate dressings may be of use20. A secondary adhesive dressing can be used to contain exudate and odour. Hydrocolloid dressings that retain moisture are generally avoided in malignant wounds as excessive moisture encourages bacterial growth and worsens odour, but may be used to protect wound edges from maceration22. Zinc oxide-based and petrolatum-based skin protectants and liquid polymer acrylates can also be used to protect wound edges15. Wound drainage bags and incontinence pads can be used to contain exudate, isolate odour and prevent leakage12. Home environment modifications, including keeping rooms well ventilated, dispersing odour absorbing products such as charcoal briquettes and use of deodorants and fragrances, can be also used to mask malodour12.
The use of systemic antibiotics should be limited to managing acute infection44. Bacterial colonisation of necrotic tissue leads to chronic infection which is best managed with an appropriate dressing regimen. Antibiotics can disrupt the normal skin flora contributing to fungal infection; repeated use may contribute to antibiotic resistance. A Cochrane review of systemic antibiotics to treat malodour among malignant wounds found only one study, with a small sample size, comparing systemic metronidazole to placebo which was inconclusive45.
Pruritus
Itching and pruritus related to malignant wounds is mediated by local inflammation activating primary afferent nerve endings; however, other systemic processes are also implicated among patients with advanced cancer (neuropathic, immune-mediated, allergic, opioid-induced, uraemic and cholestatic causes)46,47. The evidence base for treating localised pruritus is sparse and management frequently results in only partial improvement48. Appropriate wound dressing and cleansing regimens that reduce peri-wound dermatitis may help prevent pruritus as well as treating wound infection when present15. Topical agents applied to the peri-wound skin, including moisturisers, topical corticosteroids, menthol, capsaicin and lidocaine creams, may be of benefit47. Antihistamines generally do not improve wound-associated pruritus49,50. Medications used for neuropathic pain, including antidepressants (amitriptyline, mirtazapine) and antiepileptics (pregabalin, gabapentin, sodium valproate), may be trialled for pruritus not responsive to other treatments49. Transcutaneous electrical nerve stimulation (TENS) has also been used for refractory chronic pruritus in other skin conditions and reported among those with malignant wounds, but requires further evidence to support its use33,51.
Bleeding
Bleeding from malignant wounds can be persistent due to the presence of abundant friable vessels, and catastrophic haemorrhage may be a risk if the wound overlies major vascular structures22. Malignant wounds at risk of bleeding may benefit from interventions, including radiotherapy for tumour reduction, radiological embolisation, surgical cautery or ligation of vessels, to manage bleeding. If patients are taking anticoagulants or antiplatelet agents a medication review should occur by the patient’s prescriber considering the indication and risk–benefit profile. Patients at risk of major bleeding should receive education regarding a plan at home if major bleeding occurs. This usually includes having dark-coloured towels available, preparing carers and family to be able to provide a calming presence, and access to a crisis medical order (midazolam and/or an opioid) for comfort with an appropriate community palliative care service if available11.
Using non-adhesive dressings helps to minimise local trauma by reducing the risk of bleeding during dressing changes11. Calcium alginate impregnated dressings supported with gentle pressure from elastic bandages can assist in managing minor bleeding from wounds13. Topical haemostatic agents can be used for intermittent bleeding from a wound such as cautery with silver nitrate sticks. Focal bleeding can be managed by applying a compressive dressing with coagulants such as alginate, or application of vasoconstricting agents including 1:1000 adrenaline soaked gauze or oxymetazoline spray combined with external pressure for at least 10 minutes to control heavily bleeding areas; however, rebound bleeding can occur once the vasoconstricting effects wear off12,22,52. Topical tranexamic acid solution (500mg in 5ml of saline) soaked into gauze is an alternative or oral tranexamic acid among selected patients where bleeding is anticipated. These strategies are based on case reports only and there is a lack of high-quality evidence to the detriment of patients.
Psychosocial care
Suffering is a dynamic experience of severe stress that manifests as a sense of reduced wellbeing and quality of life, with physical, psychological, socio-cultural and spiritual components53. It occurs when a threat to one’s sense of wholeness overcomes available coping mechanisms53. Malignant wounds are a visible reminder of cancer and disease progression and are often a source of suffering where patients live with a sense of having an unbounded body54. A strong therapeutic relationship between care providers, the patient and their social support network is key in delivering palliative care where, through this relationship, relief of suffering is achieved. Empathic listening skills and a sensitive exploration of what contributes to patients’ suffering and mechanisms for coping with distress will help to inform a management plan.
Patients with malignant wounds benefit from an assessment of psychosocial concerns and fortunately there are published explorations of patients’ experiences in living with malignant wounds. Care providers may identify patient’s feelings of shame and revulsion due to the presence of a malignant wound and the threat to body image, sexuality and self-identity it may cause7. These thoughts can lead to hopelessness, depression and social isolation, further contributing to suffering15. Cancer can also negatively impact patients’ perception of body image, causing a reluctance to seek help due to embarrassment and shame, perhaps leading patients to attempt to manage a malignant wound independently55. Managing a rapidly changing wound without expertise may also contribute to a sense of loss of control and distress56. Consequences can include withdrawal from family and friends and the inability to fulfil social roles7. Identifying these issues allows for engagement with appropriate services including social workers, psychologists and cancer support networks.
Declining physical functioning with disease progression may lead to increased dependence on carers for management of a malignant wound and other aspects of care. Patients may report feelings of being a burden and caregivers often desire information and education when providing care for their loved one7. Helping patients cope with dressing changes, as well as providing adequate information and knowledge, may empower patients to live positively with a malignant wound56,57. In addition, caregivers often have a significant burden of unmet needs and desire information regarding financial and practical support that is available58. Family functioning, cohesion and communication are associated with a patient’s psychological adjustment following a diagnosis of cancer, influencing rates of depression and anxiety59. Early access to palliative care has shown to have beneficial effects on relatives’ distress, with reduced symptoms of anxiety and depression among caregivers during the course of a patient’s illness60. Considering a patient’s family unit and how their illness impacts others may allow for provision of practical support to those family members.
Spirituality is commonly a source of hope and means for coping among patients with advanced cancer, with patients, nurses and physicians acknowledging that provision of spiritual care is important and appropriate61,62. Exploring patients’ spirituality can be a lens through which healthcare providers discuss issues of meaning, purpose, dignity and the changing relationship patients are having with their own body and others63. Training for healthcare professionals in providing spiritual care may improve comfort and skill in discussing spirituality with patients and encourage referral to pastoral care workers62.
Multidisciplinary care
Patients receive multidisciplinary integrated care involving medical oncology, radiation oncology, allied health as well as surgical and wound care specialists while receiving anticancer treatment but they can lose access to these supports when transitions in care occur. Clinicians acknowledge that collaboration is essential in optimising quality of life for patients with malignant wounds; however, fragmentation of care may arise when the intent of treatment changes and specialists step back from providing care to patients with non-curative goals64. Palliative care seeks to engage the multidisciplinary team in coordination of care, establishing clear communication between care providers, particularly partnering with medical oncologists and identifying the unmet needs of patients and caregivers64. Patients desire expert input in wound management, symptom control and navigating complex psychosocial issues around wound-related stigma, social isolation and wellbeing57. Engagement of the multidisciplinary team promotes holistic patient-centred care to address these needs.
Further areas of research
There is minimal evidence for the benefit of local and systemic therapies for improving quality of life, pain, bleeding, malodour, exudate and tumour containment in patients with non-healing malignant wounds45. Clinical guidelines lack sufficient evidence base for recommendations, relying on uncontrolled cohort studies, case reports and translated research from other wound conditions. Malignant wound management differs from that of diabetic ulcers, pressure sores and burns and requires its own research to better inform clinical practice. High-quality research into topical interventions which improve quality of life, symptoms and function among patients with malignant wounds is indicated. Studies should focus on improvements in these outcomes rather than wound healing, which often does not occur among those with advanced cancer. As outlined by Ramasubbu et al45, it is important that studies designed for patients with advanced cancer should provide useful and reliable information, while minimising the burden on patients recruited. Further exploration of the patient experience of living with a malignant wound and the benefits of addressing specific psychosocial and spiritual concerns will provide greater insight into how care should be delivered.
Electrochemotherapy (ECT) is an established palliative treatment option for cutaneous and subcutaneous primary and secondary tumours. ECT involves delivering chemotherapeutic drugs (bleomycin or cisplatin) intravenously or directly into a tumour, followed by the application of electric pulses which promote localised drug uptake by increasing the permeability of tumour cells65. Melanoma, basal cell carcinoma and metastatic breast cancer have been most studied, where treatment is associated with a reduction in tumour size and improvement in associated wound symptoms including bleeding, malodour and exudate65,66. Highest response rates are seen in smaller tumours (<3cm); however, dramatic responses can be achieved for larger tumours65,67. ECT has few side effects and may be an option particularly for frail older patients unable to have other systemic therapies or as an alternative to other local treatments (radiotherapy, surgical excision)68. Comparative studies of ECT and other local therapies will help clarify ECT’s role in treating superficial tumours and improve its availability for patients receiving palliative care.
Conclusion
Malignant wounds can have devastating impacts on patients’ quality of life. The principles of individualised wound care plans, management of distressing symptoms and provision of psychosocial support are key to relieving suffering. Further research into topical and systemic treatments in managing odour, exudate, pruritus, bleeding and pain among patients with malignant wounds is necessary to guide clinical practice. The importance of understanding what causes a patient suffering and targeting interventions towards these issues is integral to palliative care.
Ethics statement
As the patient passed away, explicit consent was not able to be gathered for publication. Identifying information has been withheld to maintain the patient’s anonymity.
Conflict of interest
None declared.
Funding
The authors received no funding for this study.
Author contribution
DW: literature search, manuscript production. SK: manuscript revision.
Author(s)
David White* and Sampath Kondasinghe
Bethesda Palliative Care Unit, Perth, WA, Australia
*Corresponding author Email david.white2@health.wa.gov.au
References
- Goode ML. Psychological needs of patients when dressing a fungating wound: a literature review. J Wound Care 2004;13(9):380–2.
- Grocott P. The palliative management of fungating malignant wounds. J Wound Care 2000;9(1):4–9.
- Alexander S. Malignant fungating wounds: epidemiology, aetiology, presentation and assessment. J Wound Care 2009;18(7):273–4, 6–8, 80.
- Maida V, Corbo M, Dolzhykov M, Ennis M, Irani S, Trozzolo L. Wounds in advanced illness: a prevalence and incidence study based on a prospective case series. Int Wound J 2008;5(2):305–14.
- Finlayson K, Teleni L, McCarthy AL. Topical opioids and antimicrobials for the management of pain, infection, and infection-related odors in malignant wounds: a systematic review. Oncol Nurs Forum 2017;44(5):626–32.
- Maida V, Ennis M, Kuziemsky C, Trozzolo L. Symptoms associated with malignant wounds: a prospective case series. J Pain Symptom Manage 2009;37(2):206–11.
- Gibson S, Green J. Review of patients’ experiences with fungating wounds and associated quality of life. J Wound Care 2013;22(5):265–75.
- da Costa Santos CM, de Mattos Pimenta CA, Nobre MR. A systematic review of topical treatments to control the odor of malignant fungating wounds. J Pain Symptom Manage 2010;39(6):1065–76.
- Lo SF, Hayter M, Hu WY, Tai CY, Hsu MY, Li YF. Symptom burden and quality of life in patients with malignant fungating wounds. J Adv Nurs 2012;68(6):1312–21.
- Tilley CP, Fu MR, Van Cleeve J, Crocilla BL, Comfort CP. Symptoms of malignant fungating wounds and functional performance among patients with advanced cancer: an integrative review from 2000 to 2019. J Palliat Med 2020;23(6):848–62.
- Therapeutic Guidelines. Cutaneous malignant wounds in palliative care eTG complete. Therapeutic Guidelines Limited; 2021. Available from: https://tgldcdp.tg.org.au/cutaneous%20malignant%20wound
- Carville K, Silver Chain Nursing Association Incorporated. Wound care manual. Silver Chain Nursing Association Incorporated; 2012.
- Tandler S, Stephen-Haynes J. Fungating wounds: management and treatment options. Br J Nurs 2017;26(12 Suppl):S6–s14.
- Grocott P, Browne N, Cowley S. Quality of life: assessing the impact and benefits of care to patients with fungating wounds. Wounds 2005;17:8–15.
- Tilley C, Lipson J, Ramos M. Palliative wound care for malignant fungating wounds: holistic considerations at end-of-life. Nurs Clinics Nth Am 2016;51(3):513–31.
- Schulz V, Triska OH, Tonkin K. Malignant wounds: caregiver-determined clinical problems. J Pain Symptom Manage 2002;24(6):572–7.
- Borneman T, Ferrell B, Puchalski CM. Evaluation of the FICA tool for spiritual assessment. J Pain Symptom Manage 2010;40(2):163–73.
- Adderley UJ, Holt IG. Topical agents and dressings for fungating wounds. Cochrane Database Systematic Rev 2014;2014(5):Cd003948.
- Woo KY, Krasner DL, Kennedy B, Wardle D, Moir O. Palliative wound care management strategies for palliative patients and their circles of care. Adv Skin Wound Care 2015;28(3):130–40; quiz 40–2.
- Seaman S. Management of malignant fungating wounds in advanced cancer. Semin Oncol Nurs 2006;22(3):185–93.
- Beers EH. Palliative wound care: less is more. Surgical Clin Nth Am 2019;99(5):899–919.
- Woo KY, Sibbald RG. Local wound care for malignant and palliative wounds. Adv Skin Wound Care 2010;23(9):417–28; quiz 29–30.
- Young T. Wound debridement in the community setting. Br J Comm Nurs 2011;16(Sup6):S14–S20.
- Cai SS, Gowda AU, Alexander RH, Silverman RP, Goldberg NH, Rasko YM. Use of negative pressure wound therapy on malignant wounds: a case report and review of literature. Int Wound J 2017;14(4):661–5.
- Tsichlakidou A, Govina O, Vasilopoulos G, Kavga A, Vastardi M, Kalemikerakis I. Intervention for symptom management in patients with malignant fungating wounds: a systematic review. J BUON 2019;24(3):1301–8.
- Merz T, Klein C, Uebach B, Kern M, Ostgathe C, Bükki J. Fungating wounds: multidimensional challenge in palliative care. Breast Care 2011;6(1):21–4.
- Probst S, Arber A, Trojan A, Faithfull S. Caring for a loved one with a malignant fungating wound. Support Care Cancer 2012;20(12):3065–70.
- Fallon M, Giusti R, Aielli F, Hoskin P, Rolke R, Sharma M, et al. Management of cancer pain in adult patients: ESMO Clinical Practice Guidelines. Annals Oncol 2018;29 Suppl 4:iv166–iv91.
- Caraceni A, Hanks G, Kaasa S, Bennett MI, Brunelli C, Cherny N, et al. Use of opioid analgesics in the treatment of cancer pain: evidence-based recommendations from the EAPC. Lancet Oncol 2012;13(2):e58–68.
- Devulder J, Lambert J, Naeyaert JM. Gabapentin for pain control in cancer patients’ wound dressing care. J Pain Symptom Manage 2001;22(1):622–6.
- Kane CM, Mulvey MR, Wright S, Craigs C, Wright JM, Bennett MI. Opioids combined with antidepressants or antiepileptic drugs for cancer pain: systematic review and meta-analysis. Palliat Med 2018;32(1):276–86.
- Jandhyala R, Fullarton JR, Bennett MI. Efficacy of rapid-onset oral fentanyl formulations vs. oral morphine for cancer-related breakthrough pain: a meta-analysis of comparative trials. J Pain Symptom Manage 2013;46(4):573–80.
- Grocott P. Care of patients with fungating malignant wounds. Nurs Stand 2007;21(24):57–8, 60, 2 passim.
- Gethin G, Grocott P, Probst S, Clarke E. Current practice in the management of wound odour: an international survey. Int J Nurs Stud 2014;51(6):865–74.
- Vardhan M, Flaminio Z, Sapru S, Tilley CP, Fu MR, Comfort C, et al. The microbiome, malignant fungating wounds, and palliative care. Front Cell Infect Microbiol 2019;9:373.
- de Castro DLV, Santos VLCG. Odor management in fungating wounds with metronidazole: a systematic review. J Hospice Palliative Nurs 2015;17(1).
- Villela-Castro DL, Santos VLCdG, Woo K. Polyhexanide versus metronidazole for odor management in malignant (fungating) wounds: a double-blinded, randomized, clinical trial. J WOCN 2018;45(5).
- Pieper B. Honey-based dressings and wound care: an option for care in the United States. J WOCN 2009;36(1):60–6.
- Tashkandi H. Honey in wound healing: an updated review. Open Life Sci 2021;16(1):1091–100.
- Phillips PL, Yang Q, Davis S, Sampson EM, Azeke JI, Hamad A, et al. Antimicrobial dressing efficacy against mature Pseudomonas aeruginosa biofilm on porcine skin explants. Int Wound J 2015;12(4):469–83.
- Holloway S, Bale S, Harding K, Robinson B, Ballard K. Evaluating the effectiveness of a dressing for use in malodorous, exuding wounds. Ostomy Wound Manage 2002;48(5):22–8.
- Kalemikerakis J, Vardaki Z, Fouka G, Vlachou E, Gkovina U, Kosma E, et al. Comparison of foam dressings with silver versus foam dressings without silver in the care of malodorous malignant fungating wounds. J BUON 2012;17(3):560–4.
- Lund-Nielsen B, Adamsen L, Kolmos HJ, Rørth M, Tolver A, Gottrup F. The effect of honey-coated bandages compared with silver-coated bandages on treatment of malignant wounds: a randomized study. Wound Repair Regen 2011;19(6):664–70.
- O’Neill L, Nelson Z, Ahmad N, Fisher AH, Denton A, Renzi M, et al. Malignant Fungating wounds of the head and neck: management and antibiotic stewardship. OTO Open 2022;6(1).
- Ramasubbu DA, Smith V, Hayden F, Cronin P. Systemic antibiotics for treating malignant wounds. Cochrane Database System Rev 2017;8(8):Cd011609.
- Cornish L. Holistic management of malignant wounds in palliative patients. Br J Community Nurs 2019;24(Sup9):S19–s23.
- Alshammary SA, Duraisamy BP, Alsuhail A. Review of management of pruritus in palliative care. J Health Specialties. 2016;4(1).
- Siemens W, Xander C, Meerpohl JJ, Buroh S, Antes G, Schwarzer G, et al. Pharmacological interventions for pruritus in adult palliative care patients. Cochrane Database System Rev 2016;11(11):Cd008320.
- Furka A, Simkó C, Kostyál L, Szabó I, Valikovics A, Fekete G, et al. Treatment algorithm for cancerous wounds: a systematic review. Cancers (Basel) 2022;14(5):1203.
- European Oncology Nursing Society (EONS). Recommendations for care of patients with malignant fungating wounds. EONS; 2015. Available from: https://www.nfnn.com.au/wp-content/uploads/2020/01/EONSMalignantFungatingWounds.pdf.
- Badwy M, Baart SJ, Thio HB, Huygen F, de Vos CC. Electrical neurostimulation for the treatment of chronic pruritus: a systematic review. Exp Dermatol 2022;31(3):280–9.
- Recka K, Montagnini M, Vitale CA. Management of bleeding associated with malignant wounds. J Palliat Med 2012;15(8):952–4.
- Krikorian A, Limonero JT. An integrated view of suffering in palliative care. J Palliat Care 2012;28(1):41–9.
- Probst S, Arber A, Faithfull S. Malignant fungating wounds: the meaning of living in an unbounded body. Eur J Oncol Nurs 2013;17(1):38–45.
- Dowsett C. Malignant fungating wounds: assessment and management. Br J Community Nurs 2002;7(8):394–400.
- Piggin C, Jones V. Malignant fungating wounds: an analysis of the lived experience. J Wound Care 2009;18(2):57–8, 60–4.
- Lo SF, Hu WY, Hayter M, Chang SC, Hsu MY, Wu LY. Experiences of living with a malignant fungating wound: a qualitative study. J Clin Nurs 2008;17(20):2699–708.
- Chua GP, Pang GSY, Yee ACP, Neo PSH, Zhou S, Lim C, et al. Supporting the patients with advanced cancer and their family caregivers: what are their palliative care needs? BMC Cancer 2020;20(1):768.
- Edwards B, Clarke V. The psychological impact of a cancer diagnosis on families: the influence of family functioning and patients’ illness characteristics on depression and anxiety. Psycho-Oncol 2004;13(8):562–76.
- von Heymann-Horan AB, Puggaard LB, Nissen KG, Benthien KS, Bidstrup P, Coyne J, et al. Dyadic psychological intervention for patients with cancer and caregivers in home-based specialized palliative care: the Domus model. Palliat Support Care 2018;16(2):189–97.
- Ferrell B, Otis-Green S, Economou D. Spirituality in cancer care at the end of life. Cancer J 2013;19(5).
- Balboni MJ, Sullivan A, Amobi A, Phelps AC, Gorman DP, Zollfrank A, et al. Why is spiritual care infrequent at the end of life? Spiritual care perceptions among patients, nurses, and physicians and the role of training. J Clin Oncol 2013;31(4):461–7.
- Piderman KM, Kung S, Jenkins SM, Euerle TT, Yoder TJ, Kwete GM, et al. Respecting the spiritual side of advanced cancer care: a systematic review. Curr Oncol Rep 2015;17(2):6.
- Qiu JM, DelVecchio Good MJ. Making the best of multidisciplinary care for patients with malignant fungating wounds: a qualitative study of clinicians’ narratives. Palliat Med 2021;35(1):179–87.
- Morley J, Grocott P, Purssell E, Murrells T. Electrochemotherapy for the palliative management of cutaneous metastases: a systematic review and meta-analysis. Eur J Surg Oncol 2019;45(12):2257–67.
- Grocott P, Gethin G, Probst S. Malignant wound management in advanced illness: new insights. Curr Opin Support Palliat Care 2013;7(1).
- Gallagher M, Chin KY, MacKenzie-Ross A. Bleomycin electrochemotherapy for the management of locally advanced metastatic melanoma: two notable clinical cases potentially indicating a greater therapeutic role in the era of targeted and immuno-therapy. JPRAS Open 2020;26:43–8.
- Sersa G, Mascherini M, Di Prata C, Odili J, de Terlizzi F, McKenzie GAG, et al. Outcomes of older adults aged 90 and over with cutaneous malignancies after electrochemotherapy with bleomycin: a matched cohort analysis from the InspECT registry. Eur J Surg Oncol 2021;47(4):902–12.



