Volume 30 Number 4
Clinical and economic analysis on compression treatment of venous leg ulcers: clinical trial protocol VENOS
Fernanda P Cordova, Ana C Fuhrmann, Diani de Oliveira Machado, Duane Mocellin, Bárbara Uuritz da Silva, Amália de Fátima Lucena, Lisiane M G Paskulin
Keywords Venous ulcer, compression bandage, clinical trial, cost-benefit analysis, primary healthcare
For referencing Cordova FP et al. Clinical and economic analysis on compression treatment of venous leg ulcers: clinical trial protocol VENOS. Wound Practice and Research 2022; 30(4):216-222
DOI
https://doi.org/10.33235/wpr.30.4.216-222
Submitted 9 June 2022
Accepted 17 October 2022
Abstract
Background Compression therapy has been considered as the gold standard in the treatment for venous ulcer healing. However, there is not sufficient evidence about the effectiveness and economic analysis of different compression therapies.
Aim To compare the effects and perform an economic analysis of the monolayer high-compression elastic bandage and Unna’s boot on venous ulcer healing in patients treated at primary healthcare services.
Design and methods A blinded randomised clinical trial with a sample consisting in 100 venous ulcers in patients monitored at primary healthcare services assessing the effect of compression therapies on VEnous ulcer and Nursing OutcomeS (VENOS). Group A will receive a monolayer high-compression elastic bandage and Group B Unna’s boot. The compression therapies will be applied weekly by nurses until venous ulcer healing is completed or until the 26th week. A blinded evaluator will assess the wounds by an instrument based on the Nursing Outcomes Classification (NOC) and planimetry on the first day and in the following weeks. The Short Form 6 Dimension (SF‑6D) questionnaire will be applied in the first and last assessment.
Discussion This study will provide knowledge about the effectiveness and economic analysis of using compression therapies in the management of venous ulcers in order to expand evidence that can exert an impact on the patients’ quality of life, improving the quality of professional practice, and help managers direct resources.
Trial registration NCT04703569 (ClinicalTrials.gov).
Introduction
Venous ulcers are one of the most severe complications of chronic venous insufficiency. They are characterised as an open skin lesion of the lower limbs, caused by venous hypertension and deficient venous return, resulting from venous reflux or obstruction1. The prevalence of venous ulcers in the world’s population varies from 1.5–3% and, in adults aged 80 years old or over, the variation is from 4–5%2. In addition, venous ulcers represent 70–90% of the cases of chronic ulcers in lower limbs2.
Chronicity is a major problem in the emergence of venous ulcers, as 60% of them last more than 6 months, and 20% more than 5 years3. Therefore, these wounds require daily care for a long period of time and exert a negative impact on patients’ quality of life, as they generate physical, social, economic and psychological complications such as pain, limitations in mobility, restraint in the performance of work-related activities, and disability in daily living activities, as well as social exclusion and symptoms of depression3.
Venous ulcer healing occurs by secondary intention and management includes the application of dressings and compression therapy, which is considered the gold standard in the management of venous ulcers1,4. The use of compression therapy aims at improving venous return, decreasing oedema and venous pressure, assisting in faster healing and reducing recurrence of the ulcer1.
Long-term treatment also exerts an impact on the health system in regard to expenses relating to the professionals involved, the dressings and medication3. The decision of which dressing and compression therapy to be used must be based on guidelines and care protocols from scientific communities. Moreover, the choice will depend on which products and compression therapy are available to use in the healthcare service2.
An effective and accessible treatment for patients with venous ulcers can exert an impact on their quality of life, as well as reduce healthcare costs1. Internationally, different types of compression therapies are available; these are classified into elastic and inelastic bandages and according to their number of layers1. Compression variation depends on the material, the number of layers, and the way in which the bandage is applied1,2.
As for the availability and use frequency of compression therapies across different countries, it is verified that Unna’s boot is popularly applied in the United States of America5. In the United Kingdom, the four-layer bandage, which includes elastic components, is widely used5. In continental Europe and Australia, short-stretch bandaging is standard practice5. In Brazil, in the guidelines there are no specific recommendations regarding the type of compression therapy to be used; however, it is observed that the use of compression therapy is directly related to availability, that is, to the type of therapy existing in the healthcare services, both public and private6.
The healthcare to patients with venous ulcers, from an international perspective, frequently occurs in the community, mainly by teams that include district nurses, either in clinics or at home7. In Brazil, most of the patients with venous ulcers are treated in primary healthcare services. In these services, the population has access to promotive, preventive, curative, rehabilitative and supportive health services provided by nurses, physicians, nursing technicians and community health agents near patients’ houses. Unna’s boot is the most traditional compression therapy in Brazil; the use of monolayer high-compression elastic bandage was recently introduced. In these services, nurses indicate and apply the compression therapy; they also monitor evolution of these wounds. When it is hard to heal a wound or there is a lack of products, the patients are referred to specialised services, which are mostly located far from their residence, thus generating low adherence to the treatment8.
A systematic review published in 2012 with 59 randomised clinical trials (n=4,321) evaluated the effects of compression therapy on venous ulcer healing, and concluded that the use of some compression therapies increases venous ulcer healing rates. By comparing the type of compression applied, it was identified that compression systems with multicomponents are more effective than those with a single component, and that systems with multicomponents containing an elastic bandage seemed to be more effective than those composed mainly of inelastic bandages5.
The most recent systematic review with 14 randomised clinical trials (n=1,391) assessed the effects of using a short-stretch bandage, a four-layer compression bandage and Unna’s boot, compared with the absence of compression, on the venous ulcer healing. It was found that, when using any of the bandages within a 12-month period, complete healing of the ulcer occurred faster than when not using them. Diverse evidence also suggested that resorting to bandages probably reduced pain more than not using compression, and that it improved some aspects of people’s quality of life between 12 weeks and 12 months. However, no evidence was found whether the use of bandages results in health benefits that outweigh their costs9.
Other studies have been published on the effectiveness of different compression therapies. One study compared the low-stretch bandage and four-layer bandage in patients in community wound care services and determined the effectiveness of the compression therapies, recurrence rates, health-related quality of life, pain and cost10. Another study compared two-layer compression stockings and four-layer bandages in patients in community nurse services, family doctor practices and wound clinics11. Another study compared clinical effectiveness and cost-effectiveness of two-layer compression stockings with four-layer bandages in patients in community nurse services, family doctor practices and wound clinics11. The outcomes of this study were time to healing, health-related quality of life, resource use, treatment change, adverse events, and ulcer recurrence11. Only one study, a randomised clinical trial in Brazil, compared Unna’s boot and monolayer high-compression elastic bandages13. The outcomes were reduction of venous ulcer area (cm2), increased tissue granulation, and reduction in the amount of exudate, oedema and pain13. However, it was performed in a specialised service, with a small sample (n=18), and with a short follow-up period (13 weeks)13.
Analysing the different therapies utilised in the cited studies, and the guidelines of Australia, Canada and Brazil2,5,9, it is identified that there is no global and national consensus on the best compression therapy to be used with regard to the effect on healing, pain reduction, improvement in quality of life and other symptoms4,5,14. Thus, more studies are needed comparing different compression therapies than those studies so far, so that other countries with similar economic conditions to Brazil can also use the therapies that will be investigated in this study, whose outcomes have not yet been studied.
The publications on economic analysis of compression therapies are directed towards multilayer high-compression bandages11,15,16. Studies of economic analysis with Unna’s boot and monolayer high-compression bandages have not been identified up to now. A study carried out in England and Northern Ireland analysed two-layer and four-layer high-compression bandages11. Another research study from the United Kingdom performed an economic analysis of three compression systems – a two-layer cohesive compression bandage (TLCCB), a two-layer compression system (TLCS), and a four-layer compression system (FLCS)15. In another study, a Canadian randomised clinical trial evaluated the four-layer high-compression bandage and short-stretch multicomponent bandage therapies16.
Therefore, this study aims to compare the effects and perform an economic analysis of the monolayer high-compression elastic bandage and Unna’s boot on venous ulcer healing in patients treated at primary healthcare services. As such, this study will contribute to the expansion of knowledge on the theme and cooperate in the development of guidelines for the management of venous ulcers. Thus, it will inform nurses’ actions in the care of venous ulcers, especially in primary healthcare services, and improve the quality of life of patients with venous ulcers.
Methods
This is a randomised, blinded, equivalence clinical trial with 26-week monitoring assessing the effect of compression therapies on VEnous ulcer and Nursing OutcomeS (VENOS).
Sample size
The sample size will be 100 venous ulcers, 50 in each group. This was based on the study by Abreu and Oliveira13, using healing as the primary outcome, with the aid of the G*power program version 3.1.9.2; 80% power, 5% significance level and an effect size of 0.1 were considered, lower than what was observed in the aforementioned study.
Participants
The study will be performed with patients with active venous ulcers with a physician’s diagnosis of chronic venous insufficiency followed-up in primary healthcare services from the city of Porto Alegre, the capital of the southernmost Brazilian state.
Inclusion criteria
Patients, aged 18 years old or over; who are not using compression therapies when recruited to participate in the study; with an Ankle-Brachial Index (ABI) >0.8 and <1.2 and pulses present on palpation in the lower limbs (pedal and posterior tibial), verified at the time of the initial evaluation; who walk with or without the aid of devices; and with an ankle circumference greater than 18cm (required to apply one of the therapies).
If the recruited patient has more than one ulcer, all will be included in the study and they will be monitored individually, but randomisation will be per patient. If new wounds appear during monitoring, they will not be included in the research, but they will be treated.
Exclusion criteria
Pregnant patients; those with mixed ulcers; who are in antibiotic therapy for infected ulcers, in the epithelialisation phase (presence of epithelial tissue in 90% of the ulcer area), or girdle ulcer, that is, an ulcer that covers the entire circumference of the leg; who report allergy to any of the components of the therapies used in the study; with rheumatological and oncological diseases in the same limb of the ulcer; or using immunosuppressants and/or corticosteroids, systemic or topical, for more than 21 days.
Monitoring of patients will be terminated if there is discontinuation in the use of the compression therapy or death. If any patients present an allergic reaction to any of the compression therapy components, they will be changed to the other therapy in the study.
Recruitment and data collection
Eligible patients will be identified daily by the nurses from the research team in the health services where they are treated. The patients who meet the inclusion criteria will be invited to participate in the study. After acceptance, the instrument of socioeconomic characterisation, clinical conditions will be applied through a face-to-face interview. Subsequently, a blinded evaluator will carry out the initial assessment of the venous ulcer through an evaluation instrument based on the Nursing Outcomes Classification (NOC)17, filling out one instrument to each of the patient’s venous ulcers, and will apply the Short Form 6 Dimension (SF‑6D) questionnaire to identify reported quality of life18.
After completing these procedures, the evaluator will leave the dressing room and an interventionist nurse will perform venous ulcer care, according to the institutional protocol, and will apply the compression therapy, according to randomisation. This nurse will fill out a cost instrument indicating the products used, the time to perform the dressing, and if there was a need for evaluation by a physician.
The dressing of venous ulcers will be weekly, on the seventh day, with tolerance of one day. In the even weeks, the blinded examiner will evaluate the venous ulcer and fill out the assessment instrument. In the first and in the last assessment, the blinded evaluator will fill out the SF‑6D questionnaire18. The participants will be monitored until complete wound healing or for 26 weeks.
The evaluators were trained regarding the instruments and the aspects of the healing process. In addition, in order to increase accuracy of the assessments, the evaluators will take a photographic record of the wounds every evaluation week and will discuss it with a stomal therapist nurse.
The diagram corresponding to the study logistics and to operationalisation of data collection is presented in Figure 1.
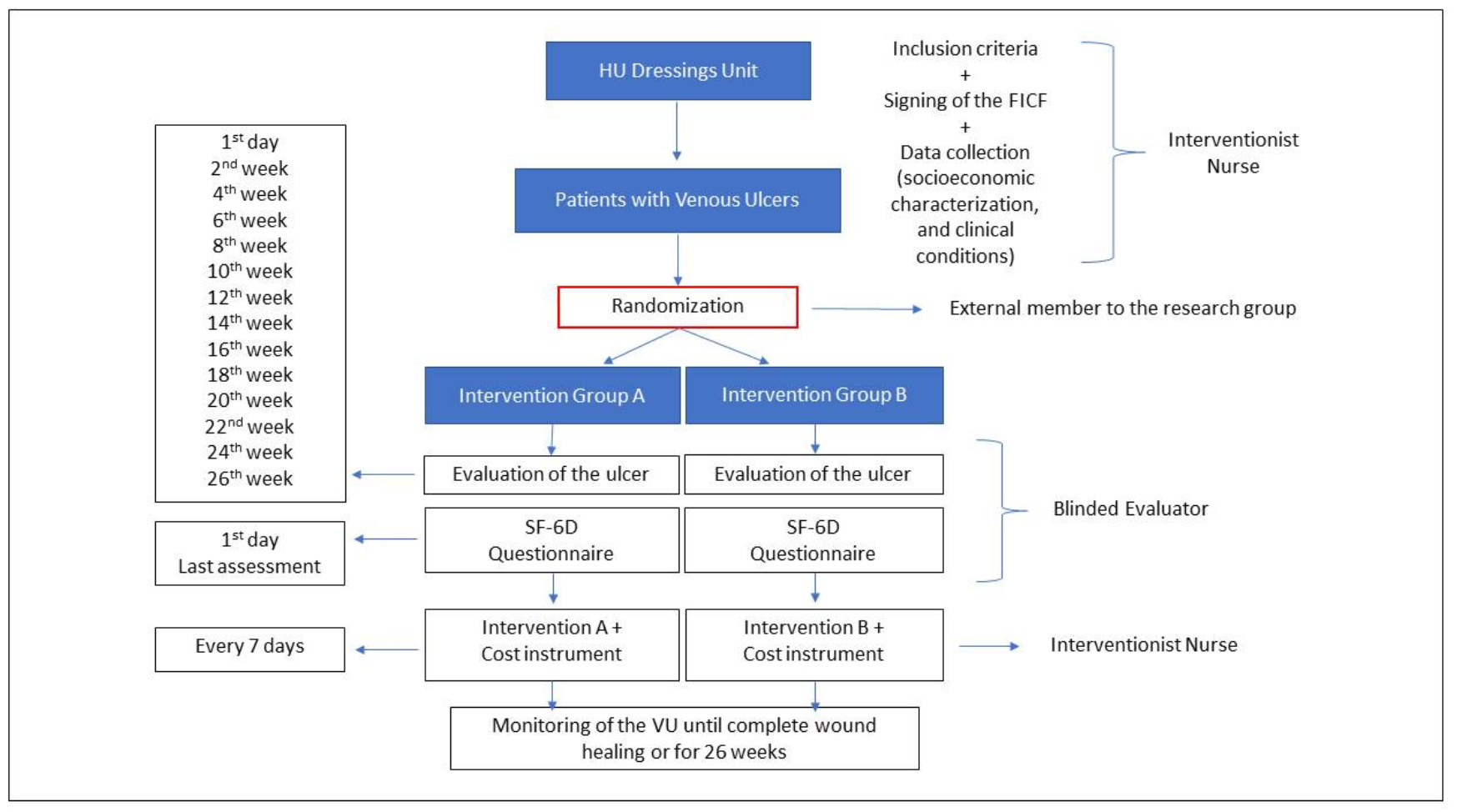
Figure 1. Study logistics note, prepared by the study researchers, 2020
Randomisation
The study participants will be randomly allocated to Intervention Group A, using monolayer high-compression elastic bandage, or to Intervention Group B, using Unna’s boot. Randomisation will be performed in two lists generated by the randomization.com website, which will be under the responsibility of a professional from the research group, external to the study, who will inform the interventionist nurse by telephone contact to which group (A or B) the patient was allocated.
The randomisation will be stratified into two lists to ensure the same distribution between groups. Patients with obesity or uncontrolled diabetes (when the glycated haemoglobin is greater than or equal to 7%) will be randomised in one list. Patients without these conditions will be randomised in the other list. This is because these conditions frequently appear in primary healthcare services’s patients, and can delay the wound healing process.
Randomisation will occur as the participants are included in the study, and per patient, regardless of how many venous ulcers they have, until reaching the sample number. The patients who have more than one venous ulcer will receive the same therapy.
Blinding
As compression therapies are visible, the interventionist nurses and study participants will not be blind to the therapy applied. However, it is noteworthy that measures were adopted to minimise the biases that may result from non-blinding of participants and interventionists: blinded evaluator; standard approach; no access by the evaluator to the patient’s record; instructions to patients and professionals not to inform the blinded evaluator about the therapy used; blind statistical analysis. If the blinding is breached, the continuity of the assessments will be carried out by another evaluator.
Interventions
The interventionist nurses will provide standardised care for all the patients, which includes the following – wound cleaning, removal of devitalised tissues, application of primary dressing (according to the characteristics of the wound and to the institutional protocol), gauze, simple bandage and compression therapy according to randomisation, and home care guidelines regarding food and rest with their lower limbs elevated.
The monolayer high-compression elastic bandage consists of a single-layer made of cotton, viscose, nylon and polyurethane elastane. The Unna’s boot is composed of an inelastic component impregnated with zinc or calamine oxide, with or without glycerin, being a moist dressing that hardens after application. It is available in various commercial preparations. The Unna’s boot that will be used in the study is from the Casex Innovation in Healthcare brand. The technique for applying both therapies will be bandaging.
Study measures
Sociodemographic characteristics and clinical conditions
The patients’ sociodemographic data will be collected, as well as pre-existing clinical conditions. Regarding the health conditions, the data collected will be as following – height, weight, BMI, circumference and ABI of the limb with the venous ulcer, location and time since the ulcer appeared, and previous use of compression therapy, as well as the need for hospitalisation.
Evaluation of the venous ulcer
The size of the venous ulcer will be measured using mechanical planimetry to assess healing19. The characteristics and clinical evolution of the venous ulcer will be evaluated using an instrument based on the NOC. The NOC was developed based on research conducted by a large team at the University of Iowa College of Nursing faculty in conjunction with clinicians from a variety of settings. Methods included content validation analysis, concept analysis, survey of experts, and clinical field site testing. The outcomes were evaluated for inter-rater reliability, validity and usefulness in ten clinical sites17.
The results of wound healing are: Wound Healing: Secondary Intention, Pain Level and Tissue Integrity: Skin and Mucous Membranes17, with indicators for each result of the study. They are assessed using a Likert-type scale from 1–5 points, where 1 is the worst state and 5 is the most desirable. The indicators assessed for the Wound Healing: Secondary Intention (1103) outcome will be as follows: granulation, scar formation, reduced wound size, drainage, erythema, oedema, macerated skin and unpleasant odour. The indicators evaluated for the Pain Level (2102) outcome will be the following: reported pain, duration of the pain episodes and narrowing of the focus. The indicators evaluated for the Tissue Integrity: Skin and Mucous Membranes (1101) outcome will be hydration, scaling, abnormal pigmentation and necrosis. The pruritus indicator was added according to a previously developed study that used it20,21.
Economic assessment
The direct costs of the therapies under study will be measured, according to the analysis of the products used to perform the dressing each week, as well as the time spent by the nurse and, if needed, an evaluation by a physician. To estimate the cost of each of these items, the table adopted by the Brazilian Unified Health System will be used as a reference.
In addition, the SF‑6D questionnaire will be applied. The SF‑6D questionnaire was derived from the SF‑36 items, a generic instrument to measure health-related quality of life that is well known and used internationally18. The SF‑6D has six domains – functional capacity, global limitation, social aspects, pain, mental health and vitality. Each domain has from four to six possible answers and the final score of the questionnaire varies from 0–1, where 0 represents the worst health status and 1 the best. It is a questionnaire to describe health states and generate utility indices, which are used in the economic analysis18. The SF‑6D was chosen because it is derived from the SF‑36 questionnaire, which was adapted and validated for use in Brazil18. Based on the health results obtained in the questionnaire, the Quality-Adjusted Life Years (QALY) will be calculated, which will represent the effectiveness used in the economic analysis.
Data analysis
Data analysis will be performed by intention to treat. Normality of the quantitative variables will be verified by means of the Shapiro-Wilk Test. To compare the variables between the two groups, the Student’s t test, Mann-Whitney’s U test, Pearson’s chi-square test, or Fisher’s Exact test will be used, according to normality. Variation of the baseline and post-intervention scores, of the indicators and of the NOC outcomes in each group, obtained at all evaluation times, will be analysed by the General Estimation Equation (GEE) method or by Friedman’s Test.
For the quantitative outcomes, the Linear Regression model will be applied and, for the dichotomous outcomes, the Poisson Regression analysis will be used, considering a p value below 0.20 in the bivariate analysis. p<0.05 will be considered. The Statistical Package for Social Sciences (SPSS) version 21.0 will be used for the analysis.
The following formula will be used for the economic analysis – cost of the treatment / effectiveness, where effectiveness will be represented by the QALY outcome. This outcome is obtained by multiplying the utility score by the time spent in a given health status. The utility score is obtained through the SF‑6D questionnaire, as explained above.
The incremental cost-effectiveness ratio will also be calculated, obtained by dividing the difference in therapy costs by the difference in their effectiveness. A sensitivity analysis will also be performed.
Validity and reliability / rigour
Randomisation will be used to allocate the patients in the intervention groups, thus avoiding biases and minimising the influence of confounding variables. Blinding controls co-interventions and biases in the evaluation of the results. In addition, primary data of the cost of the therapies will be used, collected with the rigour of a controlled clinical trial, increasing validity when compared to secondary data.
Discussion
The occurrence of venous ulcers has been increasing, requiring more effective care, particularly in countries where an ageing population is more recent and the increase of chronic diseases is more prominent. As mentioned before, venous ulcers are a long-term condition, and their treatment is complex, involving, besides techniques and supplies, other aspects such as the people’s quality of life and individual characteristics14.
Studies for the treatment of venous ulcers with different compression therapies have been performed in several regions of the world, especially in developed countries with the most advanced technology treatments. Nonetheless, it is important to emphasise that there are differences in the countries’ health systems, as well as in the availability of products. Thus, it is important to show diverse evidence of available resources in various contexts. To date, there are no research studies regarding an economic analysis of the therapies proposed for this study.
Moreover, considering that most of the clinical studies with venous ulcer patients were performed in specialised services13,21,22, there is a need to conduct this type of study in primary healthcare services. These services represent the point of care capable of caring for individuals in their entirety, facilitated by the proximity to their residence, therefore increasing their access and adherence to the treatment. In addition, the diverse evidence found with this study will support a better organisation of the health systems of the countries that use the compression therapies analysed in this study.
Limitations
It is considered that a possible study limitation is the adherence to the treatment, with discontinuity in the use of the compression therapy. This can occur due to the patient’s preference for a type of treatment other than compression if there is discomfort in its use. Furthermore, as this is a controlled clinical trial, patients with some conditions that can interfere with the healing of wounds will not be included, although those with such health conditions are normally treated in primary healthcare services.
Conclusions
This study will contribute to and add knowledge about the effectiveness and economic analysis of the compression therapies studied, providing evidence for the implementation of the more cost-effective compression therapy in primary healthcare services by health systems managers in countries that use the compression therapies studied. Furthermore, it can promote improvement in the quality of life in patients with venous ulcers.
Acknowledgements
EV Nunes, GG Medeiros, LR Martinelli and nursing students at the Nursing School, Federal University of Rio Grande do Sul, Porto Alegre, Brazil.
Conflict of interest
The authors declare no conflicts of interest.
Ethics statement
The study was approved by the Research Ethics Committee (CAAE No. 16087119.2.0000.5327) and registered at the Clinical Trials platform (NCT04703569). A Free and Informed Consent Form (FICF) will be provided to the participants. In case of intolerance to the therapy, the patients will receive the other therapy proposed in this study during the study period. In addition, the researchers will be available to assist the participants in case of any discomfort. Any adverse effects will be monitored and reported to the coordinator. If injuries occur, these will be registered and reported in the results presentation.
Funding
The study received funding from the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and the Unna’s Boot compression therapy was donated by the Casex Innovation in Healthcare company. Nevertheless, this company did not exert any influence on this study design and will not participate in data collection, analysis or interpretation, nor in the writing and publication of the results. The project received two scientific research scholarships from the Fundação de Amparo à Pesquisa do Rio Grande do Sul (FAPERGS). The project received a scholarship called Doctoral Sandwich from the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES)
Author contribution
All the authors participated in the conception of this article, revising it critically and approving the final version to be published, agreeing to be accountable for all aspects of the work, and ensuring that questions related to the accuracy and integrity of any part of this work are appropriately investigated and resolved.
Author(s)
Fernanda P Cordova1*, Ana C Fuhrmann1, Diani de Oliveira Machado2, Duane Mocellin1, Bárbara Uuritz da Silva3, Amália de Fátima Lucena1,4,5, Lisiane M G Paskulin1,4
1Nursing School, Federal University of Rio Grande do Sul, Porto Alegre, Brazil
2Nossa Senhora da Conceição Hospital, Porto Alegre, Brazil
3Ritter dos Reis University Center, Porto Alegre, Brazil
4Nursing Direction, Hospital de Clínicas de Porto Alegre, Porto Alegre, Brazil
5College of Nursing, University of Iowa, USA
*Corresponding author Email fernanda.peixoto.cordova@gmail.com
References
- Millan SB, Gan R, Townsend PE. Venous ulcers: diagnosis and treatment. Am Fam Physician 2019;100(5):298–305. https://pubmed.ncbi.nlm.nih.gov/31478635/
- Neumann HAM, Cornu-Thénard A, Jünger, M. Evidence-based (S3) guidelines for diagnostics and treatment of venous leg ulcers. JJ Eur Acad Dermatol Venereol 2016;30(11):1843–1875. doi:10.1111/jdv.13848
- Phillips P, Lumley E, Duncan R, Aber A, Woods HB, Jones GL, Michaels J. A systematic review of qualitative research into people’s experiences of living with venous leg ulcers. J Adv Nurs 2018;74(3):550–563. doi:10.1111/jan.13465
- Nelson EA, Adderley U. Venous leg ulcers. BMJ Clin Evid 2016;2016(1):1902. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4714578/
- O’Meara S, Cullum N, Nelson EA, Dumville JC. Compression for venous leg ulcers. Cochrane Database Syst Rev 2012;11(11):CD000265. doi:10.1002/14651858.CD000265.pub3
- Cardoso LV, Godoy JMP, Guerreiro MF, Czorny RCN. Unna boot applied to venous injuries: an integrative review of the literature. Rev Esc Enferm USP 2018;52(e03394). doi:10.1590/S1980-220X2017047503394
- Green J, Jester R, McKinley R, Pooler A. The impact of chronic venous leg ulcers: a systematic review. J Wound Care 2014;23(12):601–612. doi:10.12968/jowc.2014.23.12.601
- Liberato SMD, Araújo RO, Souza AJG, Pergola-Marconato AM, Costa I, Torres GV. Adherence to Venous Ulcer Treatment among Patients in Primary Health Care. Aquichan 2017;17(2):128–139. doi:10.5294/aqui.2017.17.2.2
- Shi C, Dumville JC, Cullum N, Connaughton E, Norman G. Compression bandages or stockings versus no compression for treating venous leg ulcers. Cochrane Database Syst Rev 2021;7(7):CD013397. doi:10.1002/14651858.CD013397.pub2
- Harrison MB, VanDenKerkhof EG, Hopman WM, Grahan ID, Carley ME, Nelson A. The Canadian Bandaging Trial: evidence-informed leg ulcer care and the effectiveness of two compression technologies. BMC Nursing 2011;10(1):20. doi:10.1186/1472-6955-10-20
- Ashby RL, Gabe R, Ali S. Clinical and cost-effectiveness of compression hosiery versus compression bandages in treatment of venous leg ulcers (Venous leg Ulcer Study IV, VenUS IV): a randomised controlled trial. Lancet 2014;383(9920):871–879. doi:10.1016/S0140-6736(13)62368-5
- Harding KG, Vanscheidt W, Partsch H, Caprini JA, Comerota AJ. Adaptive compression therapy for venous leg ulcers: a clinically effective, patient-centred approach. Int Wound J 2016;13(3):317–325. doi:10.1111/iwj.12292
- Abreu AM, Oliveira BGRB. A study of the Unna Boot compared with the elastic bandage in venous ulcers: a randomized clinical trial. Rev Lat Am Enfermagem 2015;23(4):541–7. doi:10.1590/0104-1169.0373.2590
- Andriessen A, Apelqvist J, Mosti G, Partsch H, Gonska C, Abel M. Compression therapy for venous leg ulcers: risk factors for adverse events and complications, contraindications – a review of present guidelines. J Eur Acad Dermatol Venereol 2017;31(9):1562–8. doi:10.1111/jdv.14390
- Guest JF, Fuller GW, Vowden P. Clinical outcomes and cost-effectiveness of three different compression systems in newly-diagnosed venous leg ulcers in the UK. J Wound Care 2017;26(5):244–254. doi:10.12968/jowc.2017.26.5.244
- Pham B, Harrison MB, Chen MH, Carley ME, Canadian Bandaging Trial Group. Cost-effectiveness of compression technologies for evidence-informed leg ulcer care: results from the Canadian Bandaging Trial. BMC Health Serv Res 2012;12:346. doi:10.1186/1472-6963-12-346
- Moorhead S, Johnson M, Maas ML, Swanson E. Nursing Outcomes Classification (NOC): measurement of health outcomes. 6th ed. Elsevier; 2018.
- Campolina AG, Bortoluzzo AB, Ferraz MB, Ciconelli RM. Validation of the Brazilian version of the generic six-dimensional short form quality of life questionnaire (SF-6D Brazil) . Ciênc Saúde Xoletiva 2011;16(7). doi:10.1590/S1413-81232011000800010
- Öien RF, Håkansson A, Hansen BU, Bjellerup M. Measuring the size of ulcers by planimetry: a useful method in the clinical setting. J Wound Care 2013;11:5. doi:10.12968/jowc.2002.11.5.26399
- Bavaresco T, Pires A, Moraes VM, Osmarin VM, Silveira DT, Lucena AF. Low-level laser therapy for treatment of venous ulcers evaluated with the Nursing Outcome Classification: study protocol for a randomized controlled trial. Trials 2018;19(1):372. doi:10.1186/s13063-018-2729-x
- Osmarin VM, Bavaresco T, Hirakata VN, Lucena AF, Echer IC. Venous ulcer healing treated with conventional therapy and adjuvant laser: is there a difference?. Rev Bras Enferm 2021;74(3):e20201117. doi:10.1590/0034-7167-2020-1117
- Kaizer UOA, Domingues EAR, Paganelli ABTS. Quality of life in people with venous ulcers and the characteristics and symptoms associated with the wound. ESTIMA – Braz J Enterostomal Ther 2021;19(e0121). doi:10.30886/estima.v19.968_PT



