Volume 30 Number 4
Moisture accumulation detection technologies for identifying pressure injuries: a literature review
Madeline A Bone, Sharon Latimer, Rachel M Walker and Brigid M Gillespie
Keywords prevention, pressure ulcer, early wound detection, point of care technologies
For referencing Bone MA et al. Moisture accumulation detection technologies for identifying pressure injuries: a literature review. Wound Practice and Research 2022; 30(4):207-215.
DOI
https://doi.org/10.33235/wpr.30.4.207-215
Submitted 25 February 2022
Accepted 7 June 2022
Abstract
Background Recent prevalence rates of 9% in Australia indicate that pressure injury (PI) remains a significant problem. The current methods used to detect PI are limited and can be imprecise and subjective. More recently, technology has been used in clinical practice to aid in detecting PIs.
Aim The purpose of this literature review was to describe the effectiveness of two moisture accumulation detection technologies – ultrasound imaging and subepidermal moisture (SEM) – for identifying the early development of PI in comparison to the standard visual skin assessment (VSA).
Methods A systematic search of MEDLINE, CINAHL and Embase databases was undertaken using MeSH terms. The quality of the research was evaluated using a Mixed Method Appraisal Tool (MMAT).
Results We identified five SEM and two ultrasound studies. Our findings suggest that both bedside technologies can be effective for identifying and preventing PI. However, the SEM scanner identified abnormal tissue pathology 2 days before ultrasound indicated signs of PI.
Conclusion The evidence suggests that the use of the SEM scanner may lead to a reduction of PI and a decrease in PI progression. However, there is a need for wider testing of the SEM scanner to establish optimal protocols for use in practice.
Introduction
Pressure injuries (PI) carry a heavy burden on the healthcare system and on the psychological wellbeing of the patients who experience them1. The direct costs of treating PI in the Australian public hospital system in 2020 was reported at A$9.11 billion per annum2 and, for the individual, the development of a PI can contribute to significant psychological stress and lower health-related quality of life (HRQoL)3. Highlighting the severity of this issue, the most recent worldwide prevalence rates of hospital-acquired PIs ranges from 6.0–18.5%1. Of these, the sacrum (37.3%) was the most common anatomical location for PI development, followed by heels (29.5%) and hips (7.8%)4.
There are four classifications for PIs: Stage I (non-blanchable erythema of the skin), Stage II (partial thickness loss of skin), Stage III (full thickness loss of skin) and Stage IV (full thickness skin and tissue loss with exposure of bone, tendons and muscles)5,6. Overall, there is no single risk factor that can explain why some individuals are at higher risk of PI development. Rather, there are a combination of potential aspects of an individual’s context that can result in the development of PI7. Some of these include mobility, perfusion status, malnutrition, BMI and changes in skin characteristics and cellular regeneration that occur more commonly in older patients7.
PI are the result of external mechanical loading leading to cellular hypoxia and eventually tissue death8. The aetiology of PI development has become better understood in recent years. As a cell becomes deformed, intracellular fluid leaks into the interstitial space, changing the pH of the tissue, leading to tissue death9. This physiological process occurs often before visual or tactile changes are visible on the skin10. In clinical practice, the current ‘gold-standard’ for detecting tissue damage relies on visual skin assessments (VSA), a method that is criticised for being subjective and qualitative, adding to its lack of precision11. Technologies that can identify tissue damage under the skin prior to visual changes may potentially fill this gap in practice12. Over the past decade, there is an emerging market for bedside technology which offers point of care clinicians a quantitative biomarker for potential tissue damage13. These technologies could be used as an adjunct tool that provides clinicians with a quantitative value to prospectively assess the potential of PI development12. This review of the published literature examines two non-invasive bedside technological methods for identifying impending tissue damage – SEM and ultrasound imaging.
The Bruin Biometrics subepidermal moisture (SEM) scanner device – the Provizio® SEM Scanner – is the first Food and Drug Administration (FDA)-authorised risk assessment device for PI prevention14. The SEM scanner utilises electromagnetism to quantitatively measure the biocapacitance of tissue in the subepidermal layer10. Biocapacitance refers to the level of ease an electrical current can pass through the cell membrane and into the intracellular space15. The SEM scanner reports the individual biocapacitance of the subepidermal tissue as a unitless value16. The SEM value is a quantitative reading that indicates the degree of localised oedema and potential tissue damage expected at the scanned site in comparison to healthy tissue13. While the SEM scanner utilises biocapacitance to detect localised oedema in the subepidermal tissue17, ultrasound imaging uses high frequency sound waves to identify abnormalities in the soft tissue12. High frequency ultrasound imaging refers to ultrasound probe frequency above 10 MHz12. Ultrasound imaging of this frequency is used to identify abnormalities in the underlying dermal structures12. The review aims to evaluate ultrasound imaging and SEM for identifying the incidence of PI in comparison to the standard VSA.
Methods
The quality of a literature review depends on the rigour and consistency of the systematic methods used to execute database searches, data extraction, synthesis and quality appraisal18. The 2020 Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guidelines were used to guide this review (Figure 1) and facilitated the transparent reporting of what and how the studies were identified and selected19. Full text articles were critically appraised with a structured approach using a Mixed Method Appraisal Tool (MMAT)20. We used a narrative approach to summarise the existing literature on two bedside technologies currently used to detect PI. The rigour of this review is reflected in the detailed research strategy and the transparency of the methodology.
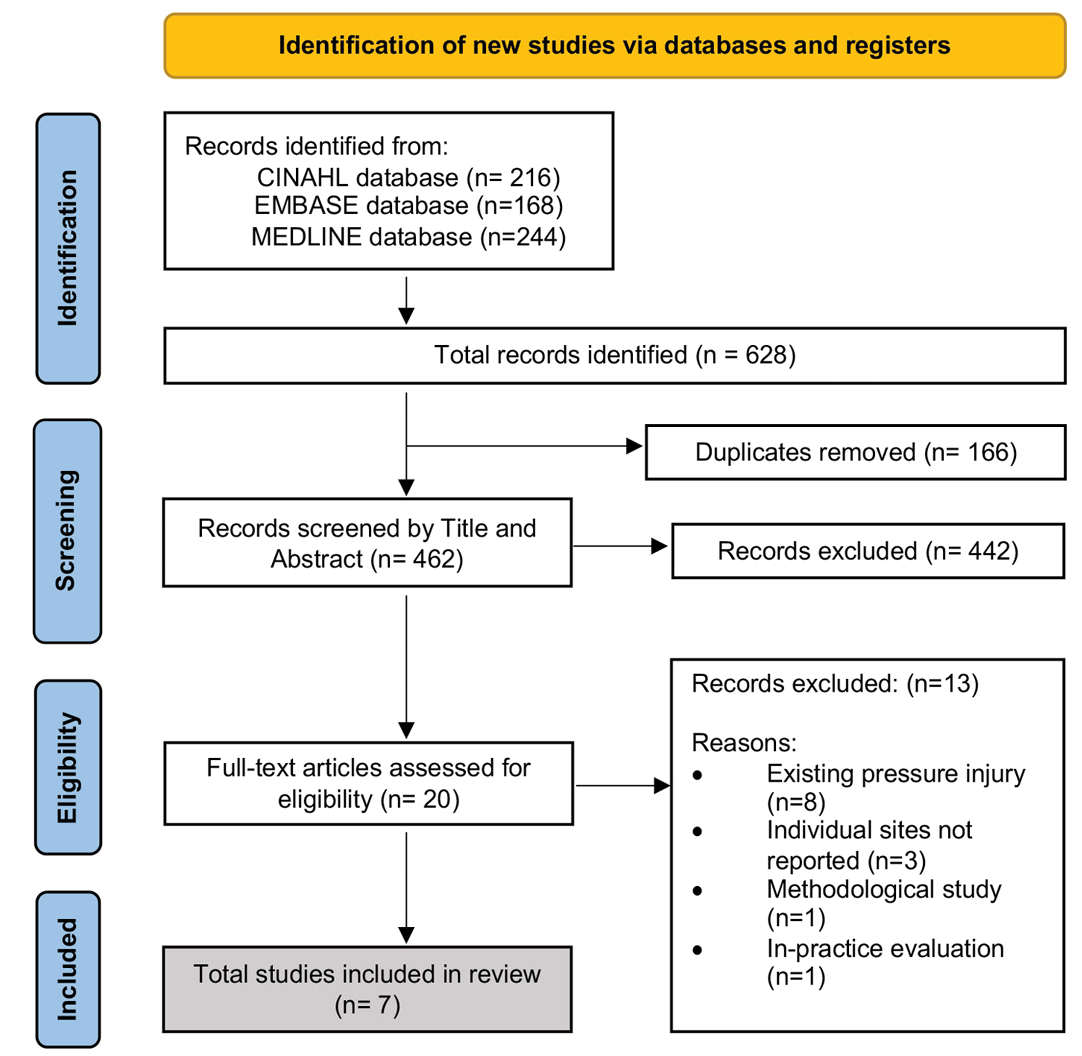
Figure 1. PRISMA flow diagram of search outcomes19
Problem identification
The clinical problems that guided this review include: (1) tissue damage, as a result of prolonged pressure, occurring on a cellular level prior to visible changes being seen on the skin, and (2) the subjectivity of VSA in recognising the early stages of a pressure injury. These problems shaped the following research question: What is the most accurate method for detecting incidence of PI in adults in comparison to visual skin assessments?
Search strategy
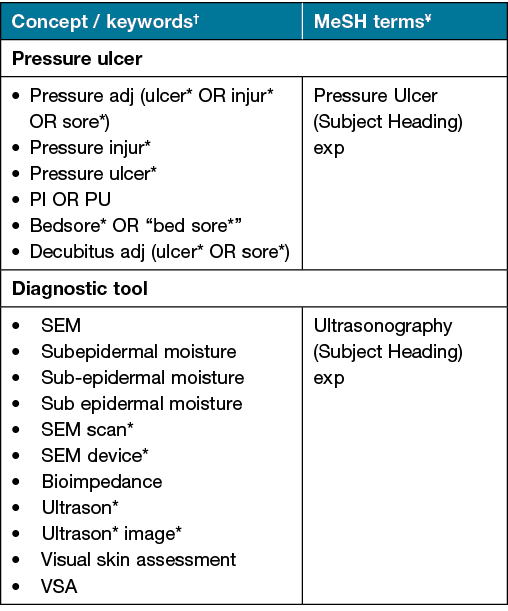
With the assistance of a health librarian, a search of the literature was carried out in September 2021 using the three following databases – Medical Literature Analysis and Retrieval System Online (MEDLINE), Cumulative Index of Nursing and Allied Health Literature (CINAHL) and Embase. Medical Subject Heading (MeSH) terms and key words were used in conjunction with Boolean operators (AND, OR) to identify relevant literature. The search terms used are presented in Table 1.
Table 1. Search strategy: keywords and MeSH terms used for literature review
Studies published from 2011 onwards were included if they satisfied the following inclusion criteria:
- Peer-reviewed research.
- Primary quantitative studies, including pilot studies.
- Primary research published in English.
- Published between 2011 and 2021.
- Adult patients ≥18 years in acute medical/surgical and aged care facilities.
- Used bedside PI detection technologies based on quantification of moisture accumulation or oedema, i.e., SEM and ultrasound, any brand or device.
Studies were excluded based on the following criteria:
- Reviews, editorials, letters, posters, conference presentations and clinical practice guidelines.
- Patients with existing PI.
- Community settings.
- Pregnant women.
- Alternate bedside PI detection technologies (i.e., thermography, alternative light sources, spectrophotometry) were excluded because they do not detect PI using moisture accumulation or oedema.
Selection of studies
Using Rayyan, a reference management tool, the primary author (MB) imported all references and removed duplicates. Following this process, two researchers (MB, BG) independently screened titles and, where available, abstracts, and excluded articles if they did not contain relevant data. A third researcher (SL) was available to assist adjudicating any differences of opinion. Full text versions of potentially relevant articles were screened against the inclusion criteria.
Data extraction and presentation
Data extraction of eligible studies was first undertaken independently by two researchers (MB, SL), with the information compared to ensure accuracy and completeness. A third researcher (BG) reviewed the data extraction for accuracy. Included studies were classified into two categories based on the method of PI detection: (1) Ultrasound imaging and (2) SEM scanner. Key items for data extraction were guided by the proposed research question and presented in tabular form. A data extraction table collated the date of publication, authors’ names, study aim(s), design, setting, sample size, PI identification procedure, and main findings.
Quality appraisal
A structured Mixed Methods Appraisal Tool (MMAT)20 was used to assess the methodological quality of the included studies. The MMAT checklist was chosen to evaluate the methodological strengths and limitations of the individual studies20. The MMAT allows qualitative, quantitative and mixed methods studies to be appraised with one checklist20. Each study was rated ‘Yes’ or ‘No’ using the questions in the appropriate study design category. If information was not reported, the ‘Can’t tell’ response was given20. As with any appraisal tool, the subjective nature of decision-making can affect validity21. Therefore, to improve the objectivity in assessment and inter-rater reliability of the MMAT checklist, two researchers independently rated the studies (MB, SL).
Results
The initial database search identified 628 articles (Figure 1). After removing duplicates (n=166) and assessing records for eligibility, seven articles met the inclusion criteria (Figure 1)22–27. These studies compared the use of the SEM scanner (n=5)23–25,27,28 and/or ultrasound devices (n=2)22,26 to standard VSA for identifying early signs of tissue damage and were accessible for bedside use. Figure 1 presents the PRISMA flow diagram.
Study characteristics
Of the included studies, six were observational22–24,26–28 and one was a non-randomised controlled study25. Most studies were conducted in long-term care facilities27,28 or acute hospital settings22–25. The mean sample size was 102 participants (range: 9–284)22–27. Most studies were conducted in Ireland23,28 and the United States of America22,24. In the six studies that reported patient socio-demographics, the average age was 76.7 years, with the majority of patients being female (n=291, 67%)22–24,26–28.
Category 1: ultrasound imaging
Ultrasound uses a transducer to emit and detect high frequency sound waves12. The sound waves can identify tissue boundaries and produce a two-dimensional image used for diagnosing soft tissue abnormalities12. Of the two studies evaluating the effectiveness of ultrasound imaging for detecting changes in the subepidermal layer in comparison to VSA (Table 2), both reported tissue property changes22,26. Ultrasound imaging was performed by a trained technician in both studies on the sacrum and left and right heels; Schäfer et al26 included scans from the upper back.
Table 2. Included ultrasound studies (n=2)
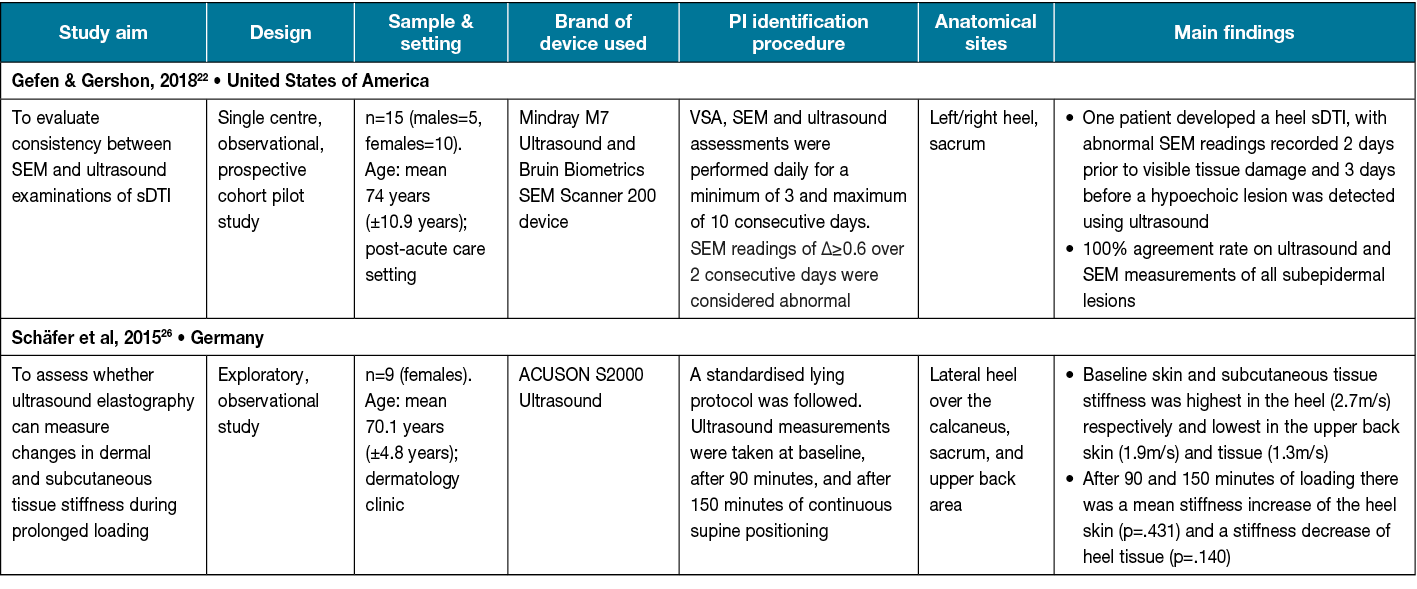
One study used a female-only sample. Sample sizes were small in both studies (range: 9–15 participants) and only the study by Gefen and Gershon22 collected participant demographics and reported findings based on ethnicity/skin tone. Schäfer et al26 noted statistically significant (p=.046) differences in the stiffness of the heel skin and subcutaneous tissues after a period of continuous loading (pressure). In the pilot study conducted by Gefen and Gershon22, ultrasound imaging identified hypoechoic lesions in the tissue, indicating suspected deep tissue injury (sDTI) prior to skin erythema. Additionally, the authors noted a 100% agreement rate on ultrasound imaging and SEM readings for identifying sDTI.
Category 2: SEM scanner
The SEM scanner uses electromagnetism to quantitatively measure the microscopic build-up of fluid in the interstitial spaces of the tissue10. Vascular permeability and interstitial fluid collection can immediately be detected through the measurement of biocapacitance13. As such, the initial phase of tissue damage can be identified at the microscopic level prior to the development of visible changes in the skin10.
As illustrated in Table 3, all five studies included in this category evaluated SEM scanner readings as an indicator of potential tissue damage and compared this to VSA23–25,27,28. Overall, findings suggest the SEM scanner was an effective adjunct tool for identifying PI earlier than VSA23,24,27,28. Four of the five studies used the Bruin Biometrics SEM Scanner 20023–25,28. Most studies collected SEM scanner readings on the sacral, and left and right heels23–25,28. The sample size was varied (range: 29–195 participants) and there were fewer than 50 participants in three out of five studies.
Table 3. Included SEM scanner studies (n=5)
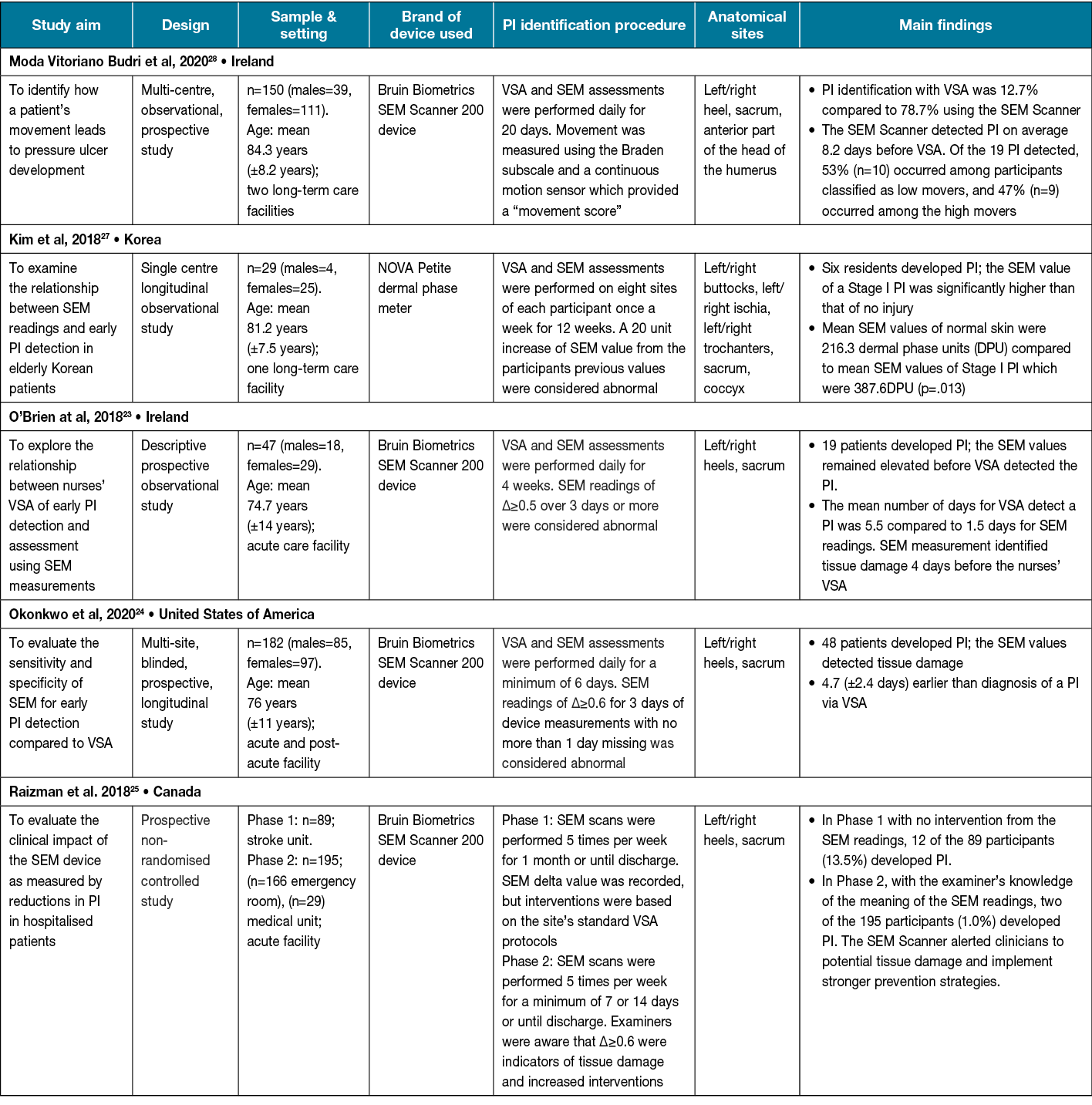
One non-randomised controlled study25 compared the effectiveness of the SEM Scanner compared to standard VSA using control and intervention groups. The study reported a 93% decrease in PI in the SEM Scanner intervention group25. The number of days SEM readings in this study were taken ranged from 7–30 days. Most studies conducted SEM scanner data collection within 30 days of study commencement. One study extended the length of daily SEM scanner assessments to 12 weeks27. In two studies comparing SEM readings to VSA for identifying signs of tissue damage, findings suggest SEM readings indicated a PI on average 4 days earlier than VSA23,24. However, the study by Moda Vitoriano Budri et al28 found that PI detection using the SEM Scanner was on average 8.2 days before VSA.
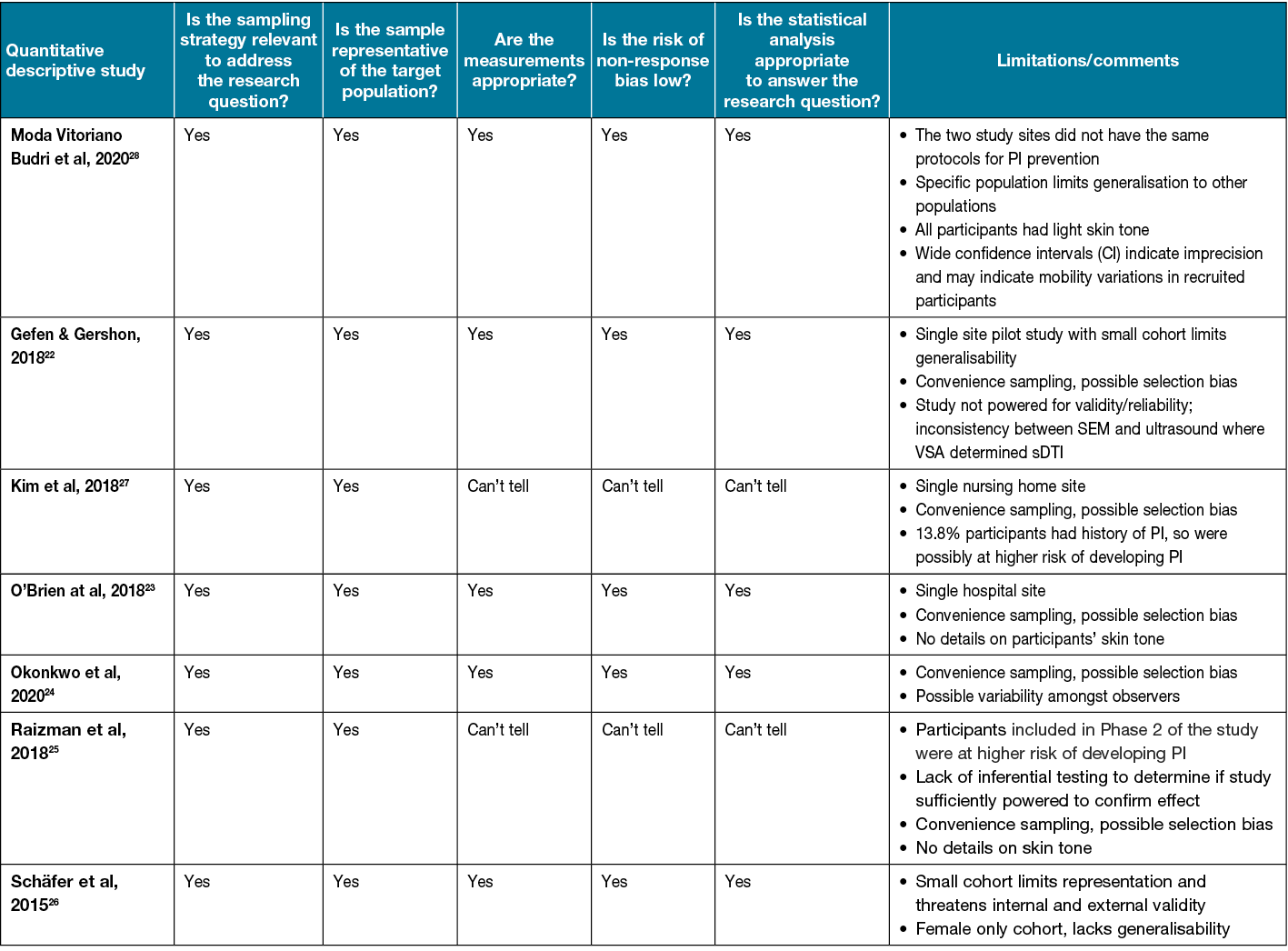
Assessment of methodological quality
All studies included in the review were quantitative studies and were assessed using the quantitative descriptive category of the MMAT20 (Table 4). Overall, the methodological quality of included studies varied. Most studies were observational in design. More than half of the studies provided sufficient methodological details to meet all five of the criteria in the MMAT checklist20. Due to limited methodological details reported in some studies, the ‘Can’t tell’ response was used. Only three studies reported blinding of the clinician performing data collection22–24. Most studies used convenience sampling, subjecting them to possible selection bias. Additionally, all but two of the studies were single site, inherently limiting generalisability.
Table 4. Results of the MMAT for the included studies
Discussion
Oedema accumulation in the subepidermal layer is known to be a prognostic factor in PI development29, which highlights the imperative for early implementation of preventative strategies30. This review synthesised evidence from five studies relating to the SEM scanner and two studies using ultrasound imaging.
There is a consensus that early PI detection technologies are beneficial for patients at risk of developing PI, in comparison to using standard VSA alone23–25,27,28. The review findings suggest the use of the SEM scanner is a more accurate and consistent method for detecting early signs of tissue damage in patients compared to standard VSA. Findings are similar to previous systematic reviews12,29 which support the use of SEM scanner measurements for the early identification of PI. Previous research by Oomens and Bader9 suggests the first event of cellular deformation occurs at the molecular level, when accumulation of interstitial fluid is irreversible and initiates cell death. The rise in interstitial fluid volume presents as localised oedema within the tissue which is not yet visible on the skin13. Using biocapacitance, the SEM scanner can detect microscopic pockets of fluid within the tissue and reflect this inflammation as an elevated SEM reading10. As the inflammatory process cascades, the injury progresses from the microscopic level to the macroscopic level10. It is only at this macroscopic level of fluid collection that ultrasound imaging can detect abnormal tissue pathology22.
The ultrasound imaging study by Gefen and Gershon22 reported meaningful results with ultrasound imaging; however, notably, the SEM Scanner identified abnormal tissue pathology 2 days before the ultrasound clearly indicated signs of a PI. Further, the ultrasound device is limited by its requirement to be operated and interpreted by a trained technician22. This restricts the convenience and cost-effectiveness of earlier PI detection due to the lack of accessibility of ultrasound technicians. Additionally, the small sample size of the included ultrasound studies suggests that the ultrasound device needs further evaluation. It is clear that the earlier the detection of tissue damage is identified, the increased likelihood of the body self-repairing the injury13. Thus, it is imperative to adopt a technology that can detect early tissue changes after death of the first cells9.
Evidence from the included studies suggest elevated SEM readings were able to identify areas of tissue damage up to 4.7 days earlier than VSA24. Study results indicate the high sensitivity of the SEM Scanner contributes to the high success rate of identifying PI23. The SEM sensitivity of >80% reported in the studies23,24,28 is in stark contrast to the reported 50% sensitivity of VSA for PI detection23,24. VSA relies heavily on the nurse’s clinical judgement which can have poor predictive validity and inter-rater reliability24. In addition, although the included studies did not calculate associated cost savings with the SEM scanner, the reduced rates of PIs and reduced need for physical resources may equate to direct cost savings. Further, evidence from this review found the implementation of the SEM scanner into standard of care PI prevention was well received by the nursing staff. There was a sense of increased confidence and skill acquisition for the nurses that incorporated the device into their standard PI prevention assessments25.
The results of our review suggest that bedside technologies can be effective for identifying and preventing PI in patients. As such, it may be appropriate to supplement VSA with the more objective approach of PI detection technologies. Further studies with larger samples are needed to improve the rigour and generalisability of this body of research. Strengthening the methodological rigour in future SEM research may inform its implementation as an evidence-based technology to real world practice. Lastly, although the current state of literature in the field of SEM is expanding, our results indicate that research examining longitudinal changes over a fixed period of time in SEM readings in healthy adults is lacking.
Limitations
We note several limitations to this systematic review. First, despite undertaking a comprehensive search across large healthcare-specific databases (CINAHL, Embase, MEDLINE), it is possible that we missed relevant published research. Secondly, as the review exclusively included studies published in English, we cannot rule out language bias. We also acknowledge that pragmatically restricting the search criteria to English-only studies limits exposure to potentially relevant non-English studies. Furthermore, five of the included studies were single-centre studies. Thus, the generalisability of the findings may be limited to the study settings. Finally, although a rigorous quality appraisal tool was used, some included studies were rated ‘Can’t tell’, indicating some ambiguity. We did not contact the authors to ask for more information, which may have allowed a more complete quality assessment of all aspects of the methodology.
Conclusions
There have been notable advancements in the field of early PI detection technologies. As the aetiology of PIs are better understood, the need for early detection is essential. The evidence available suggests that the use of the SEM scanner may lead to a reduction of PI and a decrease in PI progression. To date, it is not clear how this technology will be implemented in standard practice in the clinical setting. There is a need for well-designed studies to establish optimal protocols for implementing the SEM scanner.
Acknowledgements
A statement of acknowledgement is not applicable.
Conflict of interest
No conflicts of interest.
Ethics statement
An ethics statement is not applicable.
Funding
The authors received no funding for this study.
Author contribution
The guiding concepts of the review were framed by MB, BG, SL and RW, and MB analysed the literature under the regular supervision of all other co-authors. All authors read and approved the final manuscript.
Author(s)
Madeline A Bone*1, Sharon Latimer2, Rachel M Walker2,3 and Brigid M Gillespie2,4
1NHMRC Wiser Wounds Centre in Research Excellence, Griffith University, Gold Coast, QLD, Australia
2Menzies Health Institute Queensland, Griffith University, Gold Coast, QLD, Australia
3The Princess Alexandra Hospital, Brisbane, QLD, Australia
4Gold Coast University Hospital, Gold Coast, QLD, Australia
*Corresponding author Email madeline.bone@griffithuni.edu.au
References
- Team V, Tuck M, Reeves J, Way M, Enticott J, Evans S, et al. Pressure injury data in Australian acute care settings: a comparison of three data sets. Int Wound J 2020;17(3):578–86.
- Nghiem S, Campbell J, Walker RM, Byrnes J, Chaboyer W. Pressure injuries in Australian public hospitals: a cost of illness study. Int J Nurs Stud 2022:104191.
- Charalambous C, Vassilopoulos A, Koulouri A, Eleni S, Popi S, Antonis F, et al. The impact of stress on pressure ulcer wound healing process and on the psychophysiological environment of the individual suffering from them. Med Arch 2018;72(5):362–6.
- Li Z, Lin F, Thalib L, Chaboyer W. Global prevalence and incidence of pressure injuries in hospitalised adult patients: a systematic review and meta-analysis. Int J Nurs Stud 2020;105:103546.
- Hess CT. Classification of pressure injuries. Adv Skin Wound Care 2020;33(10):558–9.
- European Pressure Ulcer Advisory Panel (EPUAP), National Pressure Injury Advisory Panel (NPIAP), and Pan Pacific Pressure Injury Alliance (PPPIA). Prevention and treatment of pressure ulcers/injuries: clinical practice guidelines. EPUAP/NPIAP/PPPIA; 2019.
- Chung ML, Widdel M, Kirchhoff J, Sellin J, Jelali M, Geiser F, et al. Risk factors for pressure ulcers in adult patients: a meta-analysis on sociodemographic factors and the Braden scale. J Clin Nurs 2022.
- Mervis JS, Phillips TJ. Pressure ulcers: pathophysiology, epidemiology, risk factors, and presentation. J Am Acad Dermatol 2019;81(4):881–90.
- Oomens CW, Bader DL, Loerakker S, Baaijens F. Pressure induced deep tissue injury explained. Ann Biomed Eng 2015;43(2):297–305.
- Gefen A, Ross G. The subepidermal moisture scanner: the technology explained. J Wound Care 2020;29(Sup2c):S10–S6.
- Moore Z, Fletcher J, Milne J. Enhancing efficiency of pressure ulcer/pressure injury care and patient outcomes with the SEM Scanner. Wounds UK 2018;14(1):68–71.
- Scafide KN, Narayan MC, Arundel L. Bedside technologies to enhance the early detection of pressure injuries: a systematic review. J WOCN 2020;47(2):128–36.
- Gefen A. The sub-epidermal moisture scanner: the principles of pressure injury prevention using novel early detection technology. Wounds Int 2018;9(3):30–5.
- Ross G, Gefen A. Assessment of sub-epidermal moisture by direct measurement of tissue biocapacitance. Med Engineer & Physics 2019;73:92–9.
- Peko Cohen L, Gefen A. Phantom testing of the sensitivity and precision of a sub-epidermal moisture scanner. Int W J 2019;16(4):979–88.
- Oliveira AL, Moore Z, T OC, Patton D. Accuracy of ultrasound, thermography and subepidermal moisture in predicting pressure ulcers: a systematic review. J Wound Care 2017;26(5):199–215.
- Bates-Jensen BM, McCreath HE, Patlan A. Subepidermal moisture detection of pressure induced tissue damage on the trunk: the pressure ulcer detection study outcomes. Wound Repair Regen 2017;25(3):502–11.
- Maggio LA, Sewell JL, Artino AR, Jr. The literature review: a foundation for high-quality medical education research. J Grad Med Educ 2016;8(3):297–303.
- Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71.
- Hong QN, Fàbregues S, Bartlett G, Boardman F, Cargo M, Dagenais P, et al. The Mixed Methods Appraisal Tool (MMAT) version 2018 for information professionals and researchers. Education for Info 2018;34:285–91.
- Belur J, Tompson L, Thornton A, Simon M. Interrater reliability in systematic review methodology: exploring variation in coder decision-making. Sociolog Method & Res 2018;50(2):837–65.
- Gefen A, Gershon S. An observational, prospective cohort pilot study to compare the use of subepidermal moisture measurements versus ultrasound and visual skin assessments for early detection of pressure injury. Ostomy/Wound Manage 2018;64(9):12–27.
- O’Brien G, Moore Z, Patton D, O’Connor T. The relationship between nurses assessment of early pressure ulcer damage and sub epidermal moisture measurement: a prospective explorative study. J Tissue Viability 2018;27(4):232–7.
- Okonkwo H, Bryant R, Milne J, Molyneaux D, Sanders J, Cunningham G, et al. A blinded clinical study using a subepidermal moisture biocapacitance measurement device for early detection of pressure injuries. Wound Repair Regen 2020;28(3):364–74.
- Raizman R, MacNeil M, Rappl L. Utility of a sensor-based technology to assist in the prevention of pressure ulcers: a clinical comparison. Int Wound J 2018;15(6):1033–44.
- Schäfer G, Dobos G, Lünnemann L, Blume-Peytavi U, Fischer T, Kottner J. Using ultrasound elastography to monitor human soft tissue behaviour during prolonged loading: a clinical explorative study. J Tissue Viability 2015;24(4):165–72.
- Kim C-G, Park S, Ko JW, Jo S. The relationship of subepidermal moisture and early stage pressure injury by visual skin assessment. J Tissue Viability 2018;27(3):130–4.
- Moda Vitoriano Budri A, Moore Z, Patton D, O’Connor T, Nugent L, Mc Cann A, et al. Impaired mobility and pressure ulcer development in older adults: excess movement and too little movement — two sides of the one coin? J Clin Nurs 2020;29(15/16):2927–44.
- Chaboyer W, Coyer F, Harbeck E, Thalib L, Latimer S, Wan CS, et al. Oedema as a predictor of the incidence of new pressure injuries in adults in any care setting: a systematic review and meta-analysis. Int J Nurs Stud 2022;128:104189.
- Rock KL, Lai J-J, Kono H. Innate and adaptive immune responses to cell death. Immunol Rev 2011;243(1):191–205.



