Volume 31 Number 2
Local warming/heat therapy for chronic wounds: a WHAM evidence summary
Emily Haesler
For referencing Haesler E for Wound Healing and Management Collaborative. Local warming/heat therapy for chronic wounds: a WHAM evidence summary. Wound Practice and Research 2023; 31(2):90-92.
DOI https://doi.org/10.33235/wpr.31.2.90-92
Clinical question
What is the best available evidence for local warming/heat therapy for healing chronic wounds?
Summary
Local warming/heat therapy has historically been used to promote blood flow, tissue oxygenation and healthy granulation in chronic wounds. Application of radiant heat using non-contact heat sources has been demonstrated to effectively raise the temperature of the wound bed and peri-wound skin1. However, the available Level 1 evidence is insufficient to determine whether this translates to improved healing in chronic wounds2,3. Three small Level 1 studies4–6 conducted in pressure injuries (PIs), two small Level 1 studies7,8 in venous leg ulcers (VLUs) and one small Level 1 study9 in diabetic foot ulcers (DFUs) provided some evidence that a non-contact heated dressing might have an impact on healing, but this evidence was at high risk of bias, meaning no conclusions could be made on the role of local warming/heat therapy for chronic wound healing.
Clinical practice recommendations
All recommendations should be applied with consideration to the wound, the person, the health professional and the clinical context.
| The current evidence is insufficient to recommend the use of local warming/heat therapies to promote healing in chronic wounds. |
Sources of evidence
This summary was conducted using methods published by the Joanna Briggs Institute10–12. The summary is based on a systematic literature search combining search terms related to local heat therapy (e.g. warming, heat, normothermic) and chronic wounds. Searches were conducted in Embase, MEDLINE, CINAHL, the Joanna Briggs Institute database and Cochrane library for evidence published up to April 2023 in English. Levels of evidence for intervention studies are reported in the table below.
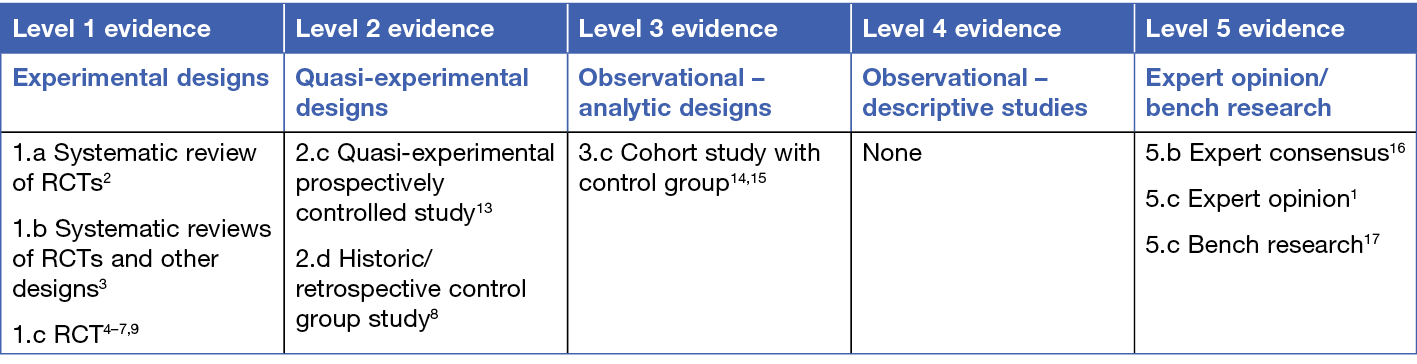
Background
Local warming therapy (also referred to as heat therapy or non-contact normothermic wound therapy) is a treatment by which heat is applied to a wound. Radiant heat can be applied using an infrared heating lamp, a non-contact warming unit or specially designed wound dressings that include a heating element2,3. Less often, conductive heat can be applied through moist compresses or heat packs applied directly to the wound14. The rationale for using local warming/heat is to increase local blood flow, thereby increasing delivery of oxygen to the wound bed, promoting collagen deposition and formation of granulating tissue1,15 and reducing microbial activity14. These theories require further investigation, particularly in people with peripheral arterial disease18. Observational studies14,15,17 have demonstrated that warming dressings6-8,13,15,17 and warm, moist towels14 can effectively increase the local skin-surface temperature14,15,17 and are associated with an increase in subcutaneous oxygen tension14,15.
Most of the identified research on local warming/heating for chronic wound healing focuses on non-contact normothermic wound dressing therapy (Warm Up® Active Wound Therapy [Augustine Medical, Inc., Eden Prairie, MN]). This dressing includes a bandage component consisting of a foam border dressing that creates a collar around the wound and a non-contact transparent film that sits over (but not touching) the wound bed. An infrared warming device is inserted into a pocket within the film that heats the wound according to the therapy parameters while maintaining 100% relative humidity5,7. The findings are likely to be transferable to other mechanisms of applying radiant heat whilst maintaining a moist healing environment because the evidence6,13 indicates that the dressing device effectively achieved the environmental conditions it claimed.
There was no evidence available on the effect of conductive heat (e.g., from a moist towel or heat pack) applied directly to a wound.
Clinical evidence
Two reviews indicated were unable to identify sufficient evidence to recommend the use of local warming therapies across different types of chronic wounds2,16 (Levels 1 and 5).
Local heat therapy for healing pressure injuries
Evidence for local warming/heat therapy in PIs comes from three small Level 1 studies4–6 and one Level 2 study13. All but one study had a high risk of bias. In all the studies, participants with full thickness PIs (Stage 3 or 4) received standard PI care, including pressure relieving surfaces and repositioning, in conjunction with their wound treatment. All the studies explored local warming/heath therapy using the same non-contact heated dressing that provides radiant heat in a high humidity micro-environment. Given the small study sizes, the methodological concerns and the inconsistent findings, this evidence is insufficient to recommend radiant heating of PIs to increase healing. The evidence included:
- An RCT5 at low risk of bias comparing hydrocolloid dressing (n=20) with the heated dressing (n=21). Warming was administered every 8 hours in 1-hour sessions for 12 weeks. There was no significant difference in rates of complete healing (57% in experimental group versus 44% in the control group, p=0.46)5 (Level 1).
- An RCT4 at high risk of bias comparing alginate dressing (n=25) with the heated dressing (n=25). Warming was administered twice daily in 1-hour sessions for up to 6 weeks. PIs that completely healed within 6 weeks were not different between groups (p>0.05). Nor was there any difference in time taken to achieve 75% or 50% reduction in wound surface area (p>0.05)4 (Level 1).
- An RCT6 at high risk of bias comparing standard wound care (n=14) with the heated dressing (n=15). Warming was administered via the dressing 3 times daily until 38˚C was reached, for up to 8 weeks. Complete healing rate was 53% in the warming therapy group and 43% in the standard care group (p = not reported)6 (Level 1).
- A non-randomised study13 at high risk of bias comparing standard wound (n=6) to the heated dressing (n=20). Heat was applied 4.5 hours per day, 5 days per week for 4 weeks. Mean reduction in wound surface area at 4 weeks favoured the treatment group (60.73% versus 19.24%, p<0.05)13 (Level 2).
Local heat therapy for healing venous and other lower leg ulcers
Evidence for warming/heat therapy in hard-to-heal VLUs comes from two small studies7,8 at high risk of bias. In both studies, participants received concurrent management of their venous disease. The evidence was insufficient to recommend radiant heating of VLUs to increase healing. The evidence included:
- An RCT7 at high risk of bias comparing heated dressing (n=8) with daily calcium-alginate dressing (n=5). Warming was delivered for 1 hour on/1 hour off cycles over 8 hours/day for 2 weeks. VLUs treated with heat showed a mean reduction in surface are of 32% and people reported a 39% reduction in pain. VLUs receiving the control treatment had a mean 25% reduction in surface area and pain decreased by 27% (p = not reported)7 (Level 1).
- A small study8 in which people with VLUs (n=17) acted as their own historic control. The heated dressing was applied for 1 hour on/1 hour off cycles, 5 hours/day for 2 weeks (Level 2). No VLUs completely healed, but a statistically significant reduction in wound size (mean 21.6±45.8cm2 versus 12.1±27.3cm2, p=0.0024) and a reduction in pain scores (3.2±2.6 versus 0.9±3.2, p=0.005) was observed8 (Level 2).
Local heat therapy for healing diabetic foot and neurological ulcers
Evidence for warming/heat therapy in DFUs comes from two studies3,9. The evidence included:
- An Australian evidence-based guideline3 which was unable to identify adequate evidence to recommend the use of local warming/heat therapy for DFUs (Level 1).
- One small RCT9 at high risk of bias which provided evidence on local warming/heat therapy for healing DFUs. People with DFUs received either daily moisture retentive dressing (n=18) or a heated dressing changed daily (n=18). Warming was delivered for 3 hours (1 hour heat cycle – 1 hour off – 1 hour heat cycle), 5 days/week for 2 months. Both groups delivered their own treatment at home, and wound assessments were conducted weekly at a wound clinic. Complete healing occurred more frequently in the heated dressing group (72% versus 28%, p=0.0003). The participants were described as having poor diabetes control9 (Level 1).
Considerations for patients who choose to use local warming/heat therapy
The heated dressing device reported in the included studies was considered experimental when the research was published in the 1990s and early 2000s, and is not currently registered as a medical device in Australia and New Zealand. Wound clinicians should check local licensing/regulation before using medical devices for applying local heat to a wound.
The evidence on whether heat reduces pain was mixed and at high risk of bias. In two studies4,7 there was no difference in the experience of wound pain in people with chronic wounds treated with radiant heat, but a third study showed clinically and statistically significant pain reduction in people with VLUs8.
In a small study in VLUs, there was no increase in skin irritation associated with a radiant heat7. In another small study6, a person using a heated dressing experienced peri-wound skin maceration necessitating cessation of treatment. None of the studies reported on the potential for local warming/heat therapy to increase wound bleeding or inflammation, or the risk of burns, particularly in people with impaired neurological function.
Limitations of a non-contact heated dressing are the inability to ambulate during treatment9.
Funding
The development of WHAM evidence summaries is supported by a grant from The Western Australian Nurses Memorial Charitable Trust.
Conflicts of interest
The author declares no conflicts of interest in accordance with International Committee of Medical Journal Editors (ICMJE) standards.
About WHAM evidence summaries
WHAM evidence summaries are consistent with methodology published in Munn et al19. Methods are provided in detail in resources published by the Joanna Briggs Institute10–12. WHAM evidence summaries are peer-reviewed by an International Expert Reference Group. For more information see: www.WHAMwounds.com
WHAM evidence summaries provide a summary of the best available evidence on specific topics and make suggestions that can be used to inform clinical practice. Evidence contained within should be evaluated by appropriately trained professionals with expertise in wound prevention and management, and the evidence should be considered in the context of the individual, the professional, the clinical setting and other relevant clinical information.
Copyright ©2023 Wound Healing and Management Collaborative, Curtin University and the authors.
![]()
Author(s)
Emily Haesler, PhD, P Grad Dip Adv Nurs (Gerontics), BN, FWA
Adjunct Professor, Curtin Health Innovation Research Institute, Wound Healing and Management (WHAM) Collaborative, Curtin University, Bentley WA, Australia
Email emily.haesler@curtin.edu.au
References
- MacFie CC, Melling AC, Leaper DJ. Effects of warming on healing. J Wound Care 2005;14(3):133–6.
- Yue JH, Zhang SJ, Sun Q, Sun ZR, Wang XX, Golianu B, Lu Y, Zhang Q. Local warming therapy for treating chronic wounds: a systematic review. Medicine (United States) 2018;97(12):e9931.
- Baker IDI Heart and Diabetes Institute, The George Institute for Global Health, Adelaide Health Technology Assessment (The University of Adelaide). National evidence-based guideline on prevention, identification and management of foot complications in diabetes (part of the guidelines on management of type 2 diabetes). Melbourne, Australia: Baker IDI Heart and Diabetes Institute; 2011.
- Price P, Bale S, Crook H, Harding KG. The effect of a radiant heat dressing on pressure ulcers. J Wound Care 2000;9(4):201–5.
- Thomas DR, Diebold MR, Eggemeyer LM. A controlled, randomized, comparative study of a radiant heat bandage on the healing of stage 3–4 pressure ulcers: a pilot study. J Am Med Dir Assoc 2005;6(1):46-9.
- Whitney JD, Salvadalena G, Higa L, Mich M. Treatment of pressure ulcers with noncontact normothermic wound therapy: healing and warming effects. J Wound Ostomy Continence Nurs 2001;28(5):244–52.
- Robinson C, Santilli SM. Warm-up active wound therapy: a novel approach to the management of chronic venous stasis ulcers. J Vasc Nurs 1998;16(2):38–42.
- Santilli SM, Valusek P, Robinson C. Use of a noncontact radiant heat bandage for the treatment of chronic venous stasis ulcers. Adv Wound Care 1999;12(2):89–93.
- McCulloch J, Knight CA. Noncontact normothermic wound therapy and offloading in the treatment of neuropathic foot ulcers in patients with diabetes. Ostomy Wound Manage 2002;48(3):38–44.
- Aromataris E, Munn Z, editors. Joanna Briggs Institute reviewer’s manual. The Joanna Briggs Institute; 2017. Available from: https://reviewersmanual.joannabriggs.org/
- Joanna Briggs Institute Levels of Evidence and Grades of Recommendation Working Party. New JBI grades of recommendation. Adelaide: Joanna Briggs Institute; 2013.
- The Joanna Briggs Institute Levels of Evidence and Grades of Recommendation Working Party. Supporting document for the Joanna Briggs Institute Levels of Evidence and Grades of Recommendation. The Joanna Briggs Institute; 2014.
- Kloth LC, Berman JE, Dumit-Minkel S, Sutton CH, Papanek PE, Wurzel J. Effects of a normothermic dressing on pressure ulcer healing. Adv Skin Wound Care 2000;13(2):69-74.
- Rabkin JM. Local heat increases blood flow and oxygen tension in wounds. Arch Surg 1987;122:221–5.
- Ikeda T, Tayefeh F, Sessler DI, Kurz A, Plattner O, Petschnigg B, Hopf HW, West J. Local radiant heating increases subcutaneous oxygen tension. Am J Surg 1998;175(1):33-7.
- Centers for Medicare & Medicaid Services. Warm-Up Wound Therapy® (Noncontact Normothermic Wound Therapy), CAG-00114N.
- Xia Z, Sato A, Hughes MA, Cherry GW. Stimulation of fibroblast growth in vitro by intermittent radiant warming. Wound Repair Regen 2000;8(2):138–44.
- Anderson CP, Pekas EJ, Park SY. Microvascular dysfunction in peripheral artery disease: is heat therapy a viable treatment? Int J Environ Res Public Hlth 2021;18(5):2384.
- Munn Z, Lockwood C, Moola S. The development and use of evidence summaries for point of care information systems: a streamlined rapid review approach. Worldviews Evid Based Nurs 2015;12(3):131–8.



