Volume 31 Number 3
Assessment, management and prevention of chronic wounds in the Australian context: a scoping review
Ut T Bui, Peta E Tehan, Michelle Barakat-Johnson, Keryln Carville, Emily Haesler,
Peter A Lazzarini, Stephen M Twigg, Christina Parker, Kathleen Finlayson
Keywords pressure injury, Diabetes-related foot ulcers, Chronic wounds, Australia, leg ulcers
For referencing Bui UT et al. Assessment, management and prevention of chronic wounds in the Australian context: a scoping review. Wound Practice and Research 2023; 31(3):120-145.
DOI
https://doi.org/10.33235/wpr.31.3.120-145
Submitted 17 June 2023
Accepted 25 July 2023
Abstract
Aims To identify the current research on assessment, management and prevention of chronic wounds in Australia and within the global context.
Methods Electronic databases, trial registries and professional organisation websites were searched from 1 January 2010 to 31 May 2022. All original human research studies on chronic wounds conducted in Australia and reviews (systematic reviews (SRs), evidence-based guidelines (EBGs), evidence summaries, consensus documents) conducted worldwide were included. Results were tabulated and synthesised in a narrative review.
Results Overall, 365 Australian studies and 569 worldwide reviews were included. The designs of Australian studies were mostly cohort (31%) or cross-sectional (20%), with a few randomised trials (10%). Australian studies were mostly concentrated on wound management (43%) or assessment (40%), and only 17% on prevention; this profile was similar for worldwide reviews. The chronic wound types focused on in Australian studies were 43% pressure injuries (PIs), 27% diabetes-related foot ulcers (DFUs), 16% venous leg ulcers (VLUs), 8% mixed chronic wounds, 6% mixed leg/foot ulcers, <1% fungating wounds.
Conclusions This review found Australian chronic wound research focused on PIs and DFUs, with few randomised trials (10%), which is likely related to the lack of national competitive funding and difficulties in infrastructure support for adequately powered trials.
Introduction
Chronic wounds have protracted progression in healing, often taking months or years to heal, and are typically linked to underlying health conditions1–3. Any wound may become chronic, however, frequently found chronic wound types include venous leg ulcers (VLU), arterial leg ulcers (ALU), pressure injuries (PI) and diabetes-related foot ulcers (DFU)4. It can be anticipated that with an ageing population, chronic diseases such as diabetes, venous insufficiency, peripheral arterial disease and malignant disorders will increase in prevalence, with a corresponding increase in the number of chronic wounds4,5. Thus, there is a significant need for research to identify effective strategies to assess, manage and prevent chronic wounds across all health settings.
In Australia, there is clear evidence that chronic wounds significantly impact healthcare expenditure and health-related quality of life (HRQoL) for persons with wounds2,6–8.To optimise effective care of chronic wounds, it is important to have a sound understanding of, and access to, available research evidence.
In 2020, the Australian Health Research Alliance Wound Care Initiative was established to develop strategies to optimise wound management in Australia. The Wound Care Initiative was divided into four streams, investigating the cost of wound care, wound care practice standards, education and research. The research stream included reviews of current research evidence for chronic wounds, acute wounds and fundamental wound science, to identify gaps and form the basis for consensus research on priorities for wound research in Australia.
This scoping review aimed to identify the existing research on assessment, management and prevention of chronic wounds to detect gaps in chronic wound research relevant to Australia. The review encompassed two arms: firstly original research studies conducted in Australia (termed Australian studies); and secondly worldwide reviews of research, i.e., systematic reviews (SRs), evidence-based guidelines (EBGs), evidence summaries and consensus documents, which involved review and synthesis of global research led by either Australian and/or international authors (termed worldwide reviews) to provide a global context.
Methods
The reporting of this review was guided by the standards of the Preferred Reporting Items for Systematic reviews and Meta-Analyses extension for Scoping Review (PRISMA-ScR) framework9. The full protocol is described in an earlier paper10 and registered with Open Science Framework Registries Network.
Eligibility criteria
In this scoping review chronic wounds are defined as wounds that “do not to proceed through the normal phases of wound healing in an orderly and timely manner”11(p56). A specific timeframe to healing or non-healing was not required for inclusion.
Inclusion criteria:
- Original quantitative or qualitative research focused on prevention, assessment and/or management of chronic wounds conducted in Australia;
- EBGs, evidence summaries, consensus statements and SRs on prevention, assessment and/or management of chronic wounds conducted worldwide (including Australia);
- Published between 1 January 2010 and 31 May 2022;
- Published in English; and
- Human studies.
Exclusion criteria:
- Case studies, case series, case reports, opinions, editorials, conference abstracts, general narrative literature reviews.
- Fundamental science articles related to wound healing, e.g., in vitro laboratory-based studies and animal studies.
Information sources
Information sources included academic databases (Medical Literature Analysis and Retrieval System Online, Excerpta Medica Database (Embase), Cumulative Index to Nursing and Allied Health Literature, Joanna Briggs Institute Library, Cochrane Library, PschINFO), clinical trial registries and professional wound organisation sites. Detailed information is published elsewhere10.
Search strategy
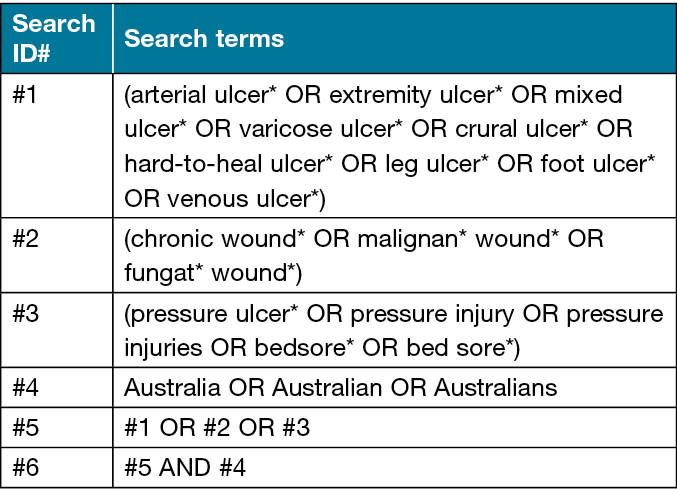
We used search strings for each of the databases that included keywords of chronic, hard-to-heal, wound, ulcer, leg ulcer, malignant or fungating wound, venous or varicose or stasis, arterial, pressure injury/ulcer/sore, decubitus ulcer, neuropathic, ischaemic, neuro-ischaemic, diabetes-related foot ulcers/diabetic foot ulcer. Three primary search strategies were used (published elsewhere10) and refined as needed to identify all eligible articles (see an example search string in Table 1).
Table 1. Search strategy example for Australian original research studies
Selection of documents
Identified records were uploaded to Covidence®. Titles and abstracts were screened and those that met eligibility criteria during screening had their full texts retrieved and were further assessed for eligibility – the process can be seen in Figure 1. All screening and assessments were conducted independently by two authors. Any disagreements were resolved by a third author.
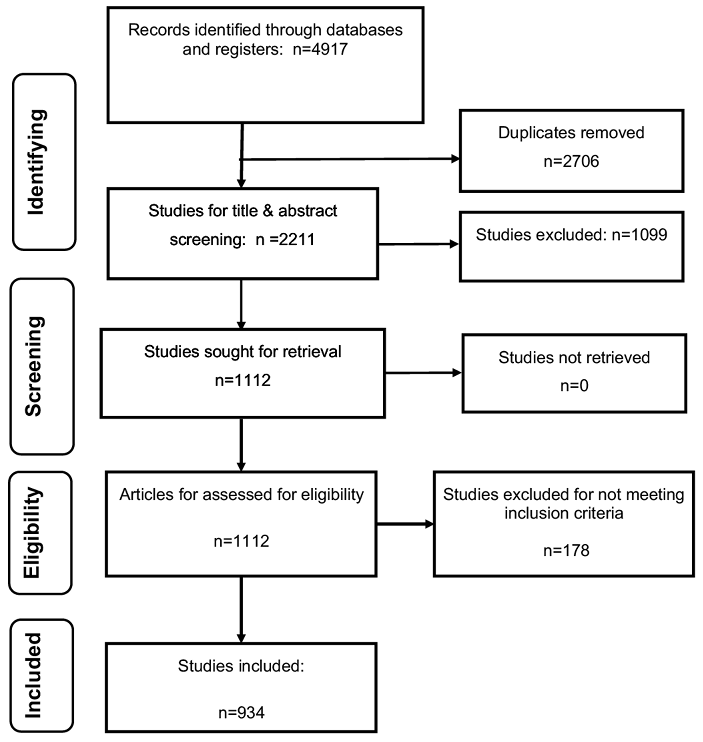
Figure 1. PRISMA flow chart
Data extraction and charting process
Data were extracted from included papers by one author and were independently checked by a second author. Key items extracted included study design, document type, aims, setting, population, outcomes and the chronic wound type focused on in the study.
Synthesis of results
Results were grouped and reported according to the chronic wound type the study or review focused on i.e., PIs, DFUs, VLUs, ALUs, studies with leg/foot ulcers of multiple aetiologies, malignant/fungating wounds, mixed chronic wounds (studies including chronic wounds of multiple aetiologies), and ‘other’ rarer wound types (e.g., Buruli ulcers, tophaceous ulcers). A narrative synthesis of findings and study outcomes was then undertaken according to the area of primary investigation – assessment, management or prevention. The topics of research studies were grouped into broad categories based on content.
Results
Overall selection results
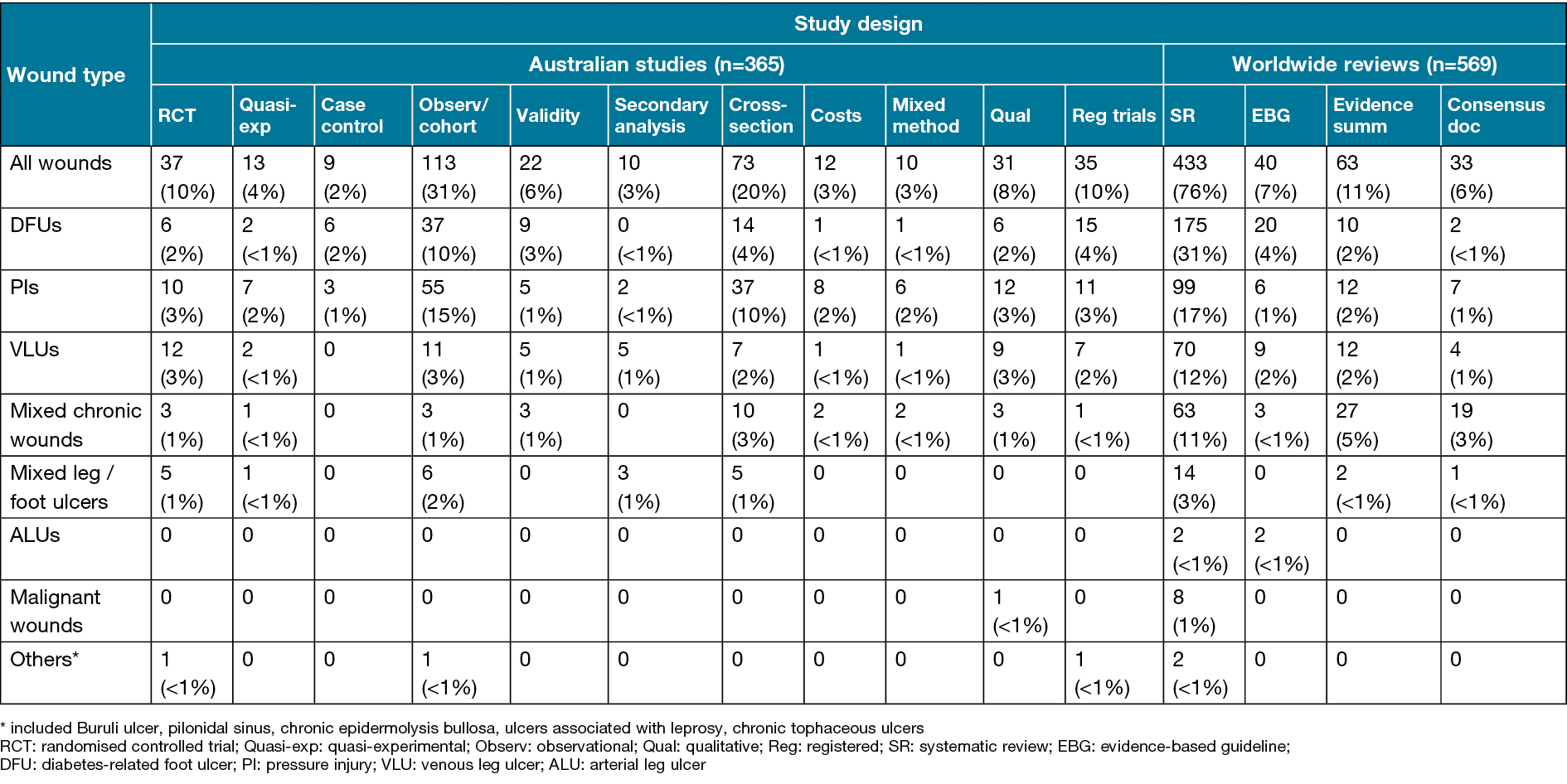
In total, 4,917 records were identified using the search strategy, of which 2,211 unique records remained after removing duplicates, 1,112 remained after title and abstract screening, and 934 remained after full text assessment and were included in this review (Figure 1). In total there were 365 original Australian studies and 569 worldwide reviews. Of the original Australian studies, the most frequently used designs were cohort studies (31%, n=113) and cross-sectional studies (20%, n=73). Specific study designs are displayed in Table 2. Most Australian studies were primarily concentrated on wound management (43%, n=157), with 40% (n=146) on assessment and 17% (n=64) on prevention of wounds.
Table 2. Study designs of included articles (n=934)
The largest number of documents overall (of the 934 Australian studies and worldwide reviews) were on DFUs (n=304, 33%) and PIs (n=280, 30%), followed by VLUs (n=155, 17%), mixed chronic wounds, e.g., samples combining VLUs, mixed ALUs/VLUs, PIs, DFUs (n=140, 15%), mixed types of leg/foot ulcers (n=37, 4%), malignant fungating wounds (n=9, 1%), ALUs (n=4, <1%), and ‘others’ (n=5, <1%), which included Buruli ulcers, tophaceous ulcers, ulcers associated with Hansen’s disease, pilonidal sinus and chronic epidermolysis bullosa wounds. Original Australian studies were focused on PIs (43%, n=156), DFUs (27%, n=97) and VLUs (16%, n=60), with fewer studies in populations with mixed types of chronic wounds (8%, n=28), mixed leg/foot ulcers (6%, n=20), only one study on fungating wounds and no studies specifically on ALUs. In contrast, the worldwide reviews focused on DFUs (37%, n=207), PIs (21%, n=122), mixed chronic wounds (19%, n=112), VLUs (17%, n=95), mixed leg/foot ulcers (3%, n=17) and 1% (n=8) on malignant wounds.
The Australian studies (n=365) were mostly conducted in inpatient hospital settings (39%, n=143; 22 of these were in Intensive Care Unit (ICU)), 28% (n=103) in community wound clinics (including hospital outpatient wound clinics, high risk foot clinics, community wound clinics), 12% (n=44) in general community settings (e.g., those receiving in-home services), 4% (n=15) in residential aged care facilities (RACFs), 3% (n=10) in general practice settings, and 10% (n=38) in combined healthcare settings. Most study samples were amongst adults with or at risk of wounds (79%), 9% with healthcare professionals, 5% with residents in RACFs, 3% with healthcare professionals and adults with or at risk of wounds, 2% with ‘older adults’ (defined ages of older adults ranged from 45 years and older to 70 years and older), 1% with neonatal and/or paediatrics and <1% with all ages.
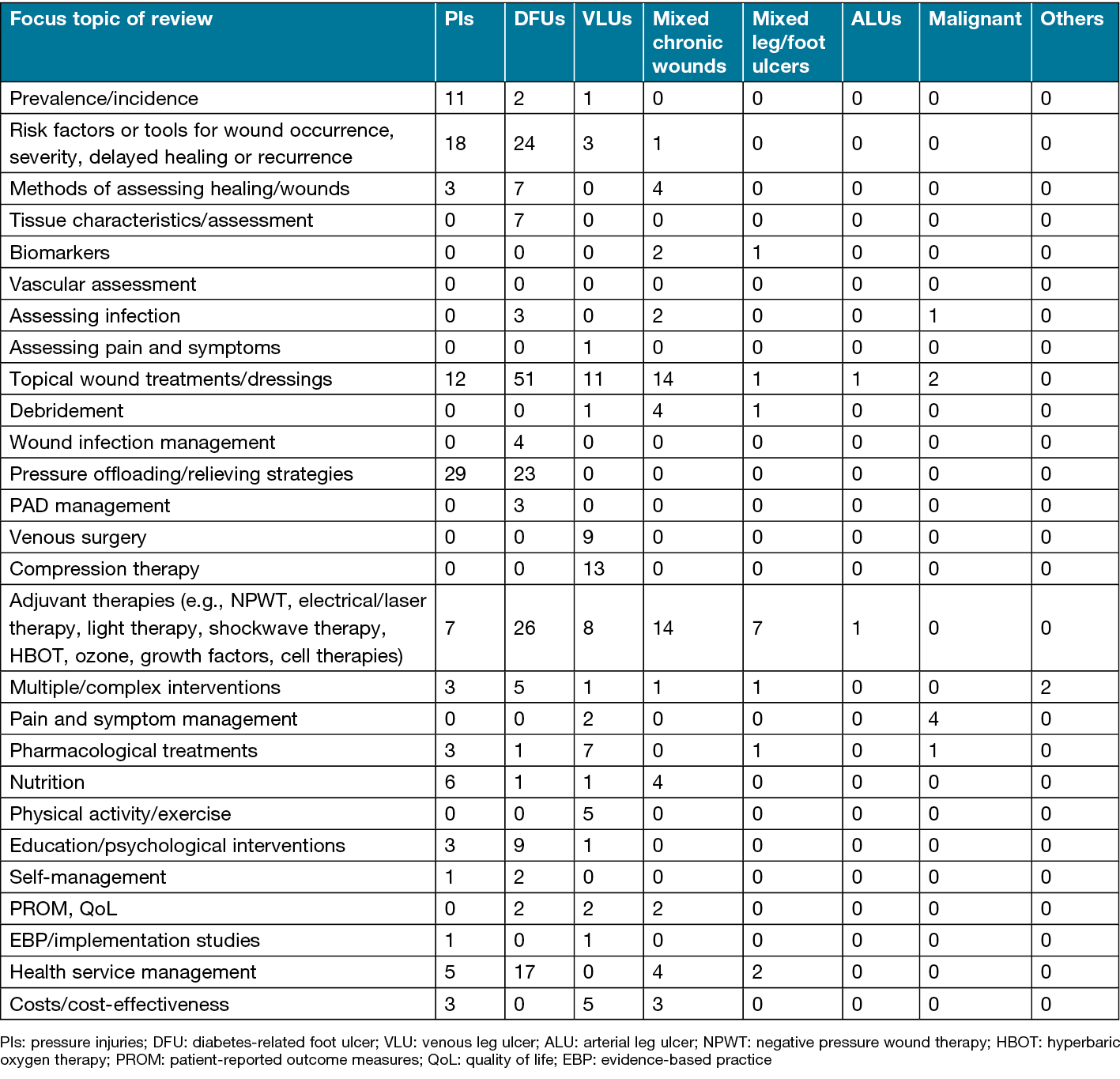
The most frequent topics of investigation by wound type were determined in both the Australian studies (Table 3) and the worldwide reviews (Table 4). An overview, by wound type and primary area of investigation (assessment, management or prevention), is outlined below.
Table 3. Topics of investigation by wound type in Australian studies (n=365)
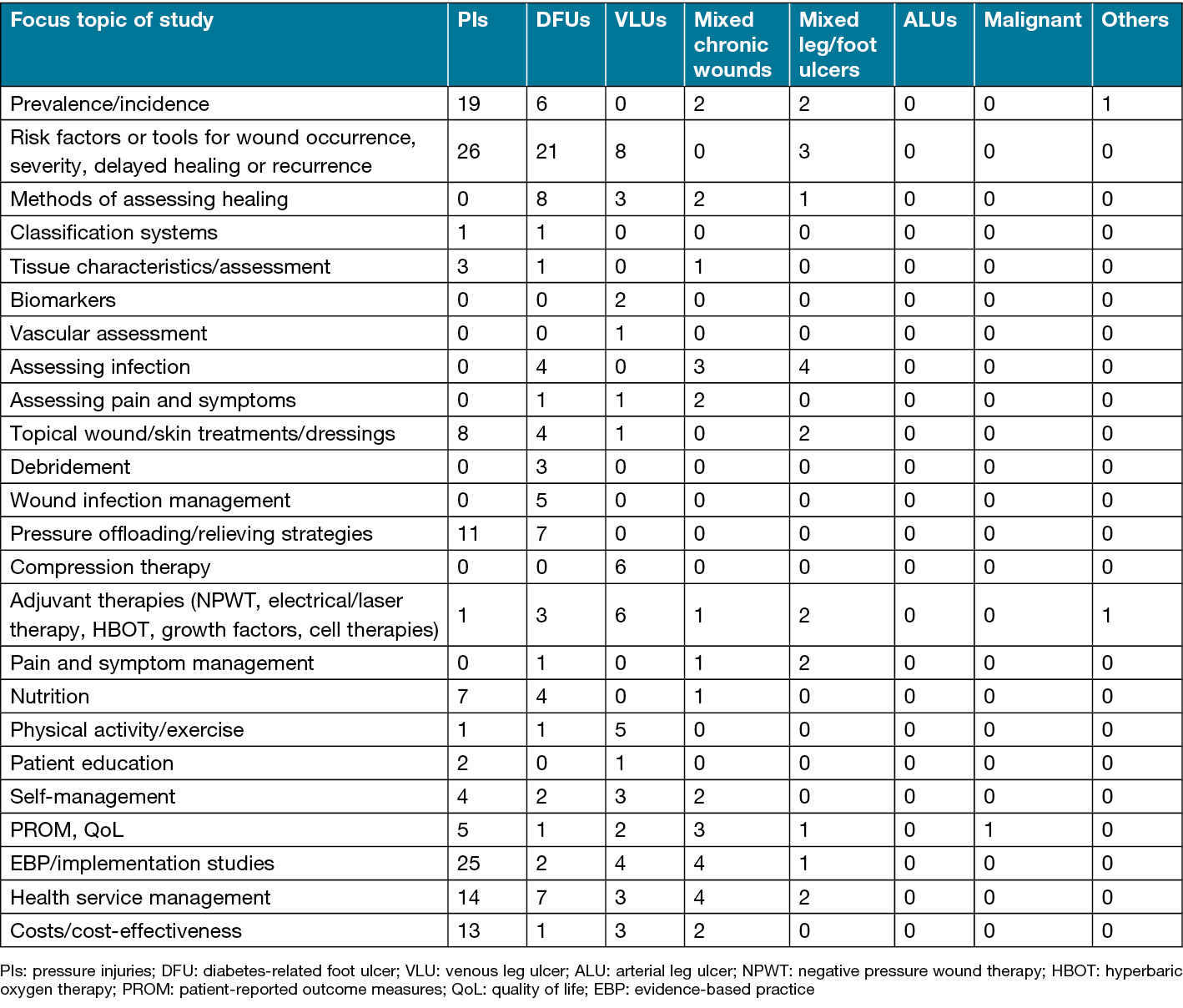
Table 4. Topics of investigation by wound type in worldwide reviews (n=569)
Diabetes-related foot ulcers (DFUs)
A total of 33% (n=304) of documents focussed on DFU, including 97 Australian studies and 207 worldwide reviews. Study designs and topics of research are shown in Tables 2–4.
Assessment
There were 29% (n=89) DFU documents covering assessment, comprised of 46 Australian studies and 43 worldwide reviews (27 SRs, three EBGs and three evidence summaries).
Australian studies
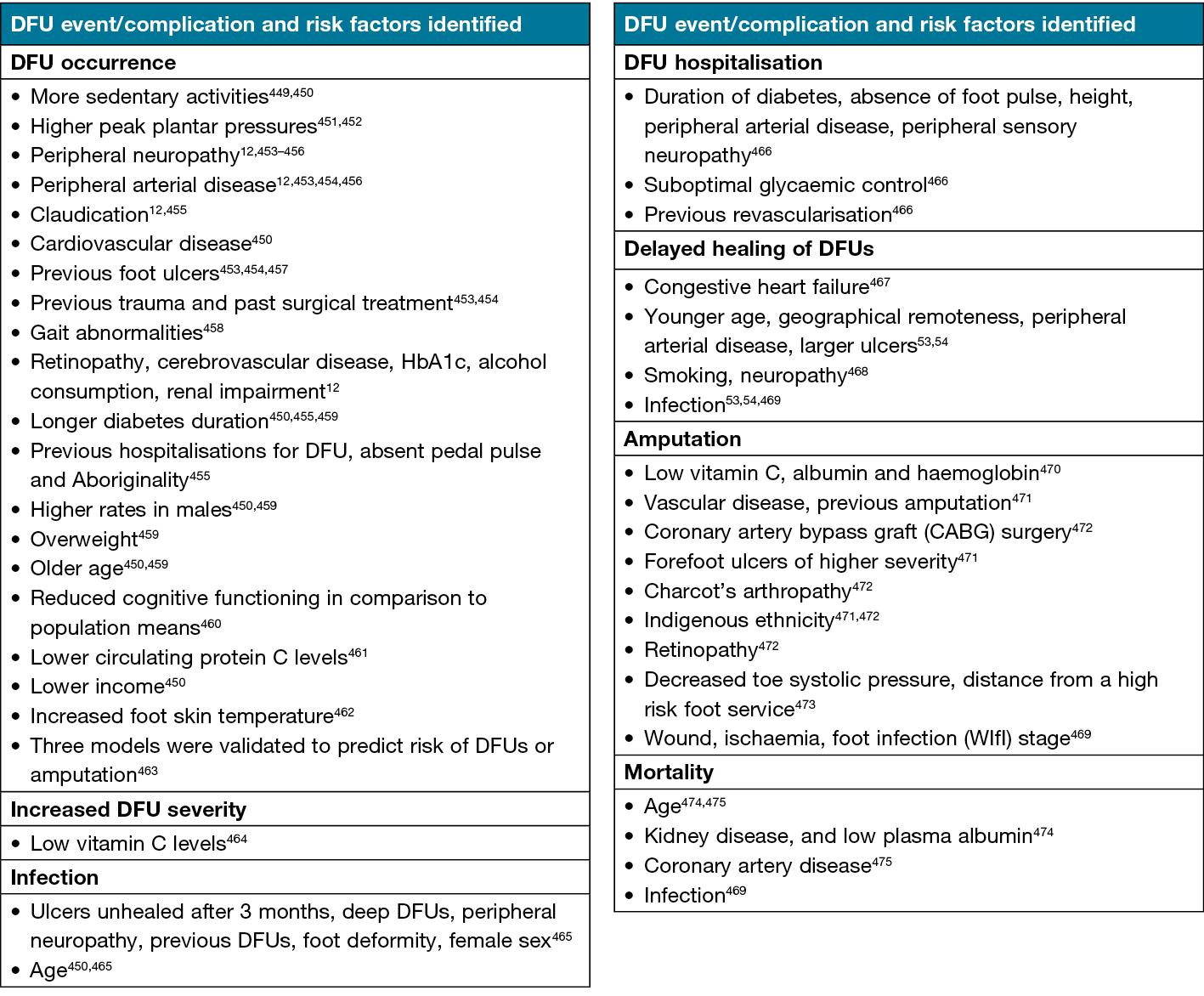
A DFU incidence of 1.2% was reported in community-based adults12, there was a prevalence of 5.4% in adults with diabetes aged 45 years and older13,14, while point prevalence in acute hospital populations was 6.7%15,16. Risk factors for developing DFUs or for complications from DFUs are shown in Table 5.
Table 5. Risk factors identified for developing DFUs or complications of DFUs
Studies assessing healing reported that: surface area change per day was superior to wound edge linear advancement17; changes in ulcer area measured by thermal imaging correlated with healing by 12 weeks18; and ulcer surface area, planimetry area and planar volume and curved volume were useful prognostic markers19. Excellent inter-rater agreement was found for the Wound Ischemia foot Infection (WIfI) score, and fair/moderate agreement for SINBAD classification score (site, ischaemia, neuropathy, bacterial infection and depth) and the Wagner and University of Texas Wound Classification System (UTWCS) scales20. There was also good agreement between: a digital program and clinicians21; 3-D cameras22,23; and reproducibility of gait and plantar pressures24. However, inconsistent agreement between visual and objective periwound assessment was determined25. One study found good agreement between a mobile phone application and Visitrak© wound grid and WoundVue© ulcer area measures26. However, another study found mobile phone assessment images had low validity and reliability for remote assessment27.
Four studies on assessing infection found: minimal correlation between the Levine swabbing technique and tissue biopsy and culture28; no correlations between clinical signs of infection and the presence of wound biofilm29; differing microbiomes in cases of severe and mild diabetic foot infections30; and no association between skin and wound microbiomes and healing31. Two studies that investigated pain assessment found observational and non-verbal cues were preferred assessment methods32; and formal assessment tools reported higher reported pain scores as compared to a single question (e.g., how much pain do you have on a scale of 0 to 10?)33.
Worldwide reviews
Three EBGs were reviewed and covered the assessment of DFU infection34 or DFU classification35,36. Two evidence summaries focused on DFU assessment37,38, and one on evidence mapping39. Topics and number of SRs are shown in Table 4.
Management
There were 196 (64%) DFU documents covering management, comprised of 49 Australian studies and 147 worldwide reviews (123 SRs, 15 EBGs, seven evidence summaries, and two consensus documents).
Australian studies
Studies on topical wound treatments found: cadexomer iodine40,41 and a surfactant gel42 both reduced microbial load; improved healing from topical propolis43; and honey wound gel and alginate were easy to use and patients rated comfort as high44. Small trials of ‘spray on skin’ (ReCell®)45, ultrasonic debridement46 and topical activated protein C47 reported improved healing, while a larger randomised controlled trial (RCT) of ReCell® versus standard care found no difference in healing rates48. A qualitative study found logistical and communication issues influenced podiatrists’ perceptions of hyperbaric oxygen therapy (HBOT)49.
Research on offloading interventions found:
- a cushion-modified total contact cast (TCC) offloaded significantly more pressure than a conventional TCC50;
- higher healing rates were associated with TCCs compared to removable non-TCCs51;
- evaluation of a DH Pressure Relief Shoe™ found significantly lower peak pressures compared to a control shoe and participants’ standard shoes52;
- treatment with knee-high offloading was associated with faster healingg53,54;
- new felt padding offloaded half the pressure of plantar DFUs55; and
- the device side walls of TCCs were found to bear considerable load56.
Infection research indicated: an outpatient antimicrobial therapy service was as effective as inpatient services57; overuse of anti-pseudomonal therapy58; and heterogeneity in antimicrobial treatments59.
One study reported factors influencing pain management32 and one trial found improved healing after a vitamin C supplement60. Three studies investigated self-care, describing emotional isolation61, motivations to perform self-care62 and experience with phone apps to monitor DFUs63.
Research on health services included: a model for rural settings64; evaluation of a multidisciplinary team65; evaluation of an Aboriginal and Torres Strait Islander foot care service66; increased healing rates reported from telehealth67; improved access to services68; general adherences to EBGs with exception of non-removable offloading devices69; and foot care management was a low priority in primary healthcare70. Significant savings and health benefits were found when implementing EBG care71, and staff resources and regional/remote geography influenced frequency of evidence-based practice (EBP) in debridement72.
Worldwide reviews
A total of 15 EBGs discussed DFU management34,73–86. Evidence summary topics included debridement87,88, dressings89,90, offloading91, combined treatments37, and evidence mapping39. Consensus documents focused on DFU management92 and topical oxygen therapy93. Topics of SRs are shown in Table 4.
Prevention
There were 36 (12%) DFU documents covering prevention of DFUs, including five Australian studies and 31 worldwide reviews (21 SRs, eight EBGs and two evidence summaries).
Australian studies
Studies on offloading reported no benefit from silicone gel sheeting to prevent ulcer recurrence94, and significant differences in plantar pressures between a specialised shoe compared to canvas or participants’ own shoes52. One study found only 45% of health professionals reported removing shoes and socks of their patients for foot assessment95, and two protocols were registered on preventive interventions96,97.
Worldwide reviews
Eight EBGs73–75,85,86,98–100 and two evidence summaries101,102 focused on prevention of DFUs. Topics of SRs are shown in Table 4.
Pressure injuries (PIs)
Overall, 30% (n=280) of documents focused on PIs, including 156 Australian studies and 124 worldwide reviews. The study designs and topics are shown in Tables 2–4.
Assessment
Assessment was addressed in 98 documents, including 58 Australian studies, five EBGs, 29 SRs, four evidence summaries and two consensus documents.
Australian studies
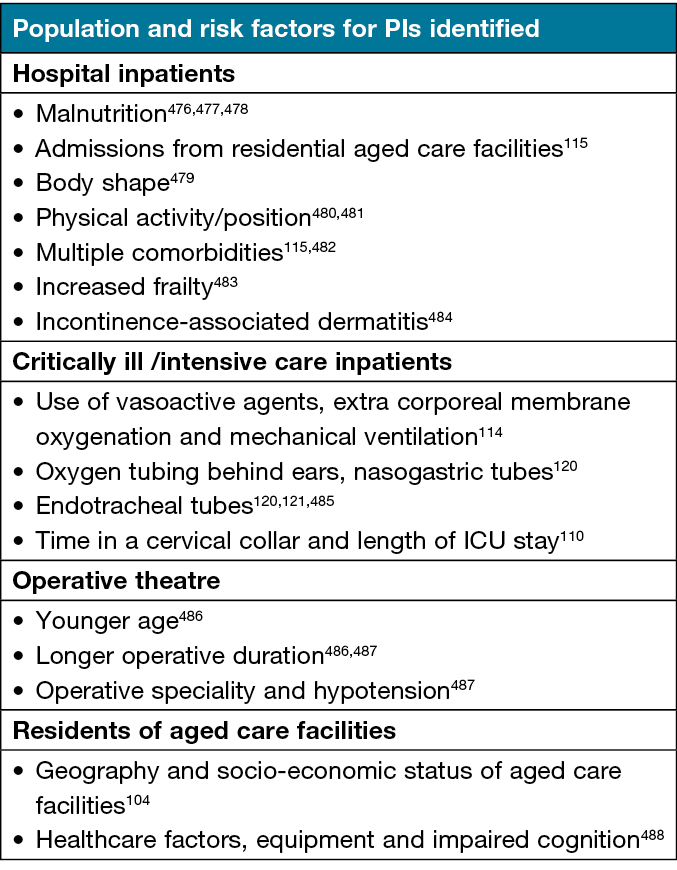
Prevalence and incidence research reported varied ranges and there was evidence of inaccurate reporting of hospital-acquired PIs (HAPIs) incidence103. Incidence of 1.33/1000 resident days was reported in RACFs104, with no difference between respite and permanent aged care105. Reported incidences of HAPIs among intensive care unit (ICU) patients varied from 6.8–16.9%106–110, noting that almost 60% of PIs in ICU adults were HAPIs111. The reported incidence of HAPIs in general acute hospital settings was lower than that observed in ICUs, and ranged from 3.3%109,112 to 12.8%113,114. In one study, almost 11% of people aged over 65 years developed a PI in the first 36 hours hospitalised115. Surgically-acquired PI incidence ranged from 0.7%116 to 1.3%116,117. The reported incidence of device-related PIs (DRPIs) ranged from 4.3%118–120 to 27.9%121. The PI incidence rate/1000 patient bed days in patients with contact precautions was 2.97122 and 0.5/1000 patient days in paediatrics123.
Prevalence of PIs varied across settings, from 4–13.7%124,125 in hospitals, 3.6% in a regional hospital126, 5.2% in adults transferred by ambulance127, and 8.9% in community services128. One study reported 137 PIs in 103 people in a district nursing service129, another examined location of heel PIs130, and one examined the costs of auditing PI prevalence131.
Several studies assessed validity and reliability of risk assessment tools, including Waterlow106,132, Ramstadius106, the Northern Hospital-Pressure Ulcer Prevention Plan (TNH-PUPP)133, Glamorgan Scale134, Conscious level, Mobility, Haemodynamics, Oxygen, Nutrition (COMHON) Index135, Reaper Oral Mucosa Pressure Injury Scale (ROMPIS)136, modified ROMPIS137, Braden Score138,139, and the interRAI scale112. Risk factors for developing PIs are shown in Table 6. One study found no difference in PI rates between using different PI risk assessment tools106, while another found an electronic checklist significantly improved screening rates140. In one study, visual and objective measures of epidermal hydration and colour were significantly correlated with Norton scores141.
Table 6. Risk factors for developing PIs, by population type
Studies on documentation found PI location influenced reporting142, and patient mobility influenced documented care143. Nurses and care workers reported that their beliefs related to risk of PIs influenced their care144.
Worldwide reviews
Five EBGs covered PI assessment145–148. Evidence summaries focused on PIs in burn patients149 and risk assessment tools150–152. Consensus documents were found on heel PIs153 and medical device-related PIs (MDRPIs)154. SR topics are shown in Table 4.
Management and/or prevention
Management and/or prevention of PIs was addressed in 106 Australian studies, in addition to 76 SRs, seven EBGs, eight evidence summaries and seven consensus documents.
Australian studies
Studies on pressure-relieving strategies found: repositioning was the most frequent treatment for MDRPIs120; increased repositioning frequency halved the incidence of PIs155; 2-hourly repositioning failed to prevent PIs in a third of at-risk residents156; and alternative seating support surfaces decreased interface pressures compared to standard chairs157. Other studies evaluated: a purpose-designed positioning device158; positioning in immobile critically ill patients159; changing device securement type160; and that a fluidised positioning device was found feasible and effective in reducing occipital PIs161. An observational study investigated peak interface pressures and pressure gradients162; a study of interface pressures in the operating theatre found difficulties in using a device to protect all heel/ankle sites163; and the association between positioning and tissue perfusion in ICU patients was investigated164.
Research on dressings found that two foam dressing types were equally effective for negative pressure wound therapy (NPWT) to treat PIs165 and that silicone foam dressings were effective in preventing sacral and/or heel PIs in critically ill patients166,167, high-risk residents in RACFs168 and people in ICU169,170. This dressing type was shown to be cost-effective171. A trial of two dressing types to prevent PIs found no difference in efficacy or product integrity; however, there was a difference in costs172. One study in neonatal intensive care found there was a high level of consensus on some skin care practices, e.g., changing body position; however, there was a low level on others such as the risk assessment tool used173.
With respect to nutrition management and prevention/treatment of PIs: arginine supplements were associated with quicker healing174–176; individualised nutrition care may have positive outcomes177; and nutrition guidelines for adults with hip fractures were associated with lower PI incidence178. Four studies found that nutrition interventions are cost-effective179–182.
Studies on patient involvement in PI prevention found that patient educational materials were often not available183 and there were barriers in the healthcare environment to patient participation184. However, studies showed there were benefits from education185–187 and patients’ understanding of PI prevention enhanced participation188, and a Patient Participation in PI Prevention scale demonstrated acceptable validity189. Good reliability and validity was found of the Pressure Ulcer Quality of Life-Prevention tool190 which was more sensitive than the Short Form (SF‑12) item survey to differences191.
A number of studies focused on PIs from the perspective of health service delivery, focussing on the practice of health professionals and challenges in delivering PI prevention and management across a range of settings192–209. Cost evaluations were done of: PI management210; non-reimbursed HAPIs211; HAPIs for people with and without dementia212; reduced PI incidence213,214; a PI care bundle215; and use of prophylactic dressings216,217.
Outcomes from evidence implementation studies varied – some studies found uncertain outcomes218,219, others reported significant reduction in PI incidence220,221, documentation222, practice changes and/or decreased PIs214,223–232. One study found registered nurses reported high compliance with the protocol233, another with nurses reported that workload restricted patient education234, while another found skin inspection and interprofessional communication were important strategies235.
Worldwide reviews
Seven EBGs covered the management and prevention of PIs145–148,236–238. Evidence summaries were available on active support surfaces239, seat cushions240, pressure redistribution mattresses241, preventive dressings242, mobilising and repositioning243,244, prevention strategies during surgery245 and heel PIs246. Consensus documents focused on general management of PIs247, MDRPIs154, heel PIs153, nutrition248, preventive dressings249,250, and prevention for critically ill patients251. The SR topics are shown in Table 4.
Venous leg ulcers (VLUs)
The third largest group (17%, n=155) of documents were on VLUs, including 60 Australian studies and 96 worldwide reviews. The study designs and focus topics are shown in Tables 2–4.
Assessment
A total of 28 documents addressed the assessment of VLUs, including 15 Australian studies.
Australian studies
Excellent inter-rater reliability was obtained from a digital planimetry system252, while both the EQ‑5D‑5L Score and SPVU‑5D Score were able to discriminate between healed and unhealed VLUs253. Participants reported that it was useful to include wound care, compression and dressing items to assess QoL254. A study in general practices found vascular assessment is not routinely undertaken, possibly due to a lack of awareness of guidelines, resources or skills255.
Risk factors for delayed healing included larger ulcers, longer duration, higher exudate levels, larger calf circumference256,257, severe pain-depression-fatigue-sleep disturbance symptom cluster group258, dehydration259, living alone, ulcer reduction in 2 weeks, higher ulcer severity score, and not treating with high level compression260. A comparative study found adults receiving home nursing for VLUs were older and had more risk factors for non-healing than those attending wound clinics261. Granulocyte macrophage-colony stimulating factor and matrix metalloprotease‑13 were identified as biomarkers predicting healing262 and uric levels correlated with wound chronicity263. Validation and reliability testing of a risk assessment tool for delayed healing had good results256,260. One study found thermal imaging was not associated with healing264; however, another found textural analysis of thermal imaging could predict healing265.
Worldwide reviews
There were eight EBGs on assessment of VLUs266–273. SR topics are shown in Table 4.
Management
Management of VLUs was the focus of 121 documents, including 37 Australian studies, nine EBGs, 49 SRs, 12 evidence summaries, and four consensus documents.
Australian studies
Compression therapy studies indicated support for four-layer compression bandaging compared to Class three compression hosiery274 and three-layer tubular compression system compared with short stretch compression275. On adherence to compression therapy: funding of compression bandaging did not influence healing or compression use276; and pain, wound size and depth, and age were significant predictors of non-concordance277. Factors influencing adherence to treatment included understanding the management plan, compression-related body image issues, feeling overwhelmed, hot weather and discomfort, cost, ability to wear compression, patience, persistence and remembering self-care instructions278.
Studies on wound dressings or topical interventions found NPWT combined with compression had positive results279, while there were no differences in healing from a trial of wool-derived keratin dressings280. Electrical stimulation therapy was mostly acceptable and easier to use than compression281; however, in people with VLUs who did not use high level compression therapy, it was not associated with improved healing rates281. HBOT was associated with a significantly greater reduction in ulcer area, however no difference in overall healing rates282. One study found no difference in time to healing associated with taking 300mg aspirin daily283, while another reported a reduction in matrix metallopreotease‑1 in wound fluid associated with high-dose oral doxycycline, however no significant change in ulcer area284.
Research on exercise included evaluation of a self-management home-based exercise program which resulted in improved calf muscle pump function and range of ankle motion285, significant relationships between self-efficacy and outcome expectations related to program adherence, and improved healing rates286,287. A qualitative study concluded that while adults with VLUs were interested in exercise, they faced many obstacles288. One study in adults with VLUs found education was positively associated with physical activity289. Self-management studies found that an education program led to successful self-management290 and differing perspectives and priorities of patients and clinicians on management, with patients’ preferences more closely aligned with EBGs291.
Health service management was investigated in a number of studies, finding: suboptimal knowledge and implementation of EBP in VLU management292,293; similar healing rates between home nursing and wound clinic care261; and a lack of awareness of EBGs and a range of enablers and barriers to EBP in VLU care255,294. Health professionals’ perspectives on EBGs in primary care were also noted295. Cost evaluations found EBG management led to lower costs and improved QoL296 and shorter time to healing297, while the amount participants spent on ulcer care varied according to wound severity298.
Worldwide reviews
Nine EBGs covered the management of VLUs266–273,299. The evidence summaries were on adherence to compression therapy300–302, VLU management303, debridement304,305, protease-modulating matrix interventions306, dressings307,308, electromagnetic therapy309, ultrasound310 and surgical interventions311. There were consensus documents on compression312,313, complexities in VLU management314 and holistic management315. SR topics are shown in Table 4.
Prevention
A total of 30 documents covered the prevention of VLUs, including 10 Australian studies, seven EBGs, 10 SRs, one evidence summary and two consensus documents.
Australian studies
Factors identified for increased risk of VLU recurrence included: male sex316; history of deep vein thrombosis (DVT)316,317; multiple previous ulcers and longer ulcer duration317; and decreased mobility, antidepressant medications and haemosiderosis318. Protective factors included: leg elevation316,317, compression (>20mmHg for at least 6 days/week)316, higher social support316,318, higher self-efficacy316,317 and increased walking317. A qualitative study found participants reported traumatic injury, surgery and failure to replace compression as causes of recurrence319. Although compression was perceived as important, some were unaware of different compression levels and required frequency for replacement319. Two studies investigated the validity of a risk assessment tool for recurrence, both finding good discrimination and goodness of fit320.
Good acceptability of compression application devices was reported in one study321; however, non-adherence was higher with higher compression levels and was associated with recurrence322. Adherence to VLU preventive strategies was associated with knowledge, higher self-efficacy and lower depressive symptom scores323. Another study found adherence declined between 6–12 months after healing, with regular follow-up and history of multiple ulcers related to improved adherence, and depressive symptoms and restricted mobility related to decreased adherence324. Research on a multimedia education program found significant improvements in knowledge and self-care behaviours325.
Worldwide reviews
Seven EBGs covered the prevention of VLUs266–270,272,273. The evidence summary301 and consensus documents312,313 focused on compression. SR topics are shown in Table 4.
Mixed chronic wounds
Many studies (n=140) were focused on samples of chronic wounds in general, i.e., samples including persons with a chronic wound of any aetiology, such as chronic leg ulcers, non-healing surgical wounds, PIs and pilonidal sinuses, including 28 Australian studies, 27 evidence summaries, three EBGs, 63 SRs, and 19 consensus documents. Study designs and topics are shown in Tables 2–4.
Assessment
A total of 29 documents addressed the assessment of mixed chronic wounds, including 11 Australian studies.
Australian studies
An IT mobile wound care program facilitated data collection326, while a small study found a high prevalence of wounds in adults with dementia living in RACFs, with skin tears the most common wound identified327. A survey on wound pain assessment found it was undertaken at each visit or dressing change by 61% of respondents, with around two-thirds of practitioners using a validated assessment tool328. High inter-rater reliability was found of a device (SD202) for measuring skin hydration and erythema329, and a telemetric sensor system for temperature, moisture and pressure (under compression) was found reliable and repeatable in pilot testing in the lab and on one human volunteer participant330. The modified TIME-H tool had moderate support to identify the likelihood of healing331.
On infection, a RCT found the Levine wound swab technique had improved outcomes as compared to the Z swabbing technique332, and a study identified varied bacterium types in non-healing wounds333. Delphi surveys achieved consensus on clinical indicators of wound chronicity, infection and biofilm334, and on prevention, identifying and managing chronic wound infections335. A SR of chronic wounds in Australia found costs of healthcare are >A$3.5 billion/year, around 2% of national health expenditure and that EBP improves outcomes4. An education and process change intervention led to a significant decrease in wounds and increased nursing knowledge336.
Worldwide reviews
Guidelines were available on overall assessment of chronic wounds337,338 and on assessment of wound infection339. Evidence summaries focused on sampling techniques for culture340,341 and assessment of cavity wounds342. Consensus documents covered chronic wound assessment315,343,344, ankle brachial pressure index (ABPI)345, biofilms346 and exudate347. SR topics are shown in Table 4.
Management
A large number (n=115) of documents addressed the management of mixed chronic wounds, including 20 Australian studies.
Australian studies
Findings from clinical trials included that: a vitronectin; growth factor complex was safe and re-epithelialisation occurred in most participants348; there was no difference in wound pain from a trial of low intensity laser therapy349; and a standard nutrition supplement led to improved healing compared to a wound-specific supplement350. On patient-reported outcomes, a study found sub-optimal HRQoL and significant costs associated with chronic wounds351, while in another study participants reported the desire to be independent352 and improved HRQoL from self-treating352,353. However, one study found an association between self-treating chronic wounds and reduced HRQoL and financial burden354. Coping strategies for living with chronic wounds included seeking support, solution-focused problem solving, finding new options to stay healthy, distraction and staying positive355.
Health service research reported that: an education and process change intervention led to increased uptake of EBP in RACFs356; link clinicians improved the uptake of TIME principles357; the documentation of wound care occurred in less than one-third of residents with dementia in RACFs with wounds358; the use of an electronic wound management system led to improved communication359; an inconsistent approach was found in product utilisation in RACFs360; there were improved patient outcomes and/or significant cost savings from specialist wound services361–363; there was high satisfaction and viability of a virtual consultant wound specialist service364; and there were positive outcomes from a telephone advisory service365.
Worldwide reviews
Three EBGs covered the management of chronic wounds337,366 or wound infection339. Evidence summaries were found on wound infection367–370, iodophors371, biofilm372, debridement373–378, cavity wounds379, collagen-based dressings380, wet-to-moist dressings381, wet-to-dry saline gauze dressings382, foam with silver dressings383, alginate dressings384, pain management385, biosynthetic skin substitutes386, topical negative pressure (TNP)387, HBOT388 and hydrogen peroxide389. There were consensus documents on biofilms346,390–392, infection393,394, antimicrobial stewardship395, exudate347, chronic wound management337,344,396, NWPT397–399, patient engagement400 and aseptic technique401. SR topics are shown in Table 4.
Prevention
One pre/post study in RACFs found increased uptake of EBP and decreased prevalence of wounds356, and two EBGs covered general prevention of wounds337,338.
Mixed chronic leg and/or foot ulcers
A total of 37 documents were identified on assessment, management and/or prevention of mixed types of leg and foot ulcers, including 20 Australian studies, 14 SRs, two evidence summaries and one consensus document. Study designs and topics are shown in Tables 2–4.
Assessment
A total of 11 documents addressed the assessment of mixed leg ulcers, with ten Australian studies and one SR.
Australian studies
Studies on characteristics of populations with foot ulcers reported that: 7.4% of inpatients in acute hospitals had foot conditions as the primary reason for admission402; characteristics of non-DFU; and 10% of adults on dialysis had foot ulceration which was associated with a history of amputation, PAD and serum albumin403. A later study found additional risk factors of neuropathy and previous ulcers404.
Results from studies on infection included no relationship between the clinical assessment of infection versus bacterial burden from wound swabs, however faster healing in wounds with nil or low bacterial growth at baseline over 2 weeks in a nanocrystalline silver group compared to cadexomer iodine treatments405. Risk factors for infection in chronic leg and foot ulcers included depression, requiring walking aids, a calf-ankle ratio <1.3, larger wound area, and slough406,407. However, not all were validated in a small prospective study408. A small study found a significant relationship between transcutaneous oxygen pressure (TcpO2) assessment and ulcer healing over 4 weeks409, and an evidence implementation study found improved EBP in wound assessment in primary healthcare professionals410.
Worldwide reviews
One SR was found on biomarkers to predict ulceration or recurrence of lower leg ulcers411.
Management
A total of 26 documents covered the management of mixed types of leg and/or foot ulcers, including 11 Australian studies.
Australian studies
Studies on topical treatments reported no differences in healing rates between cadexomer iodine versus nanocrystalline silver405, with both dressings rated favourably by participants412, and evaluation of an acellular synthetic matrix found 36% healing rate at 12 weeks413. Two reports on EMLA® topical analgesic cream found no differences in healing rates, however a significant decrease in pain and improved wellbeing in the EMLA® group412,414. Two symptom clusters were identified in participants with mixed ALUs/VLUs which had a significant impact on HRQoL415. One study found no impact on healing from antibiotics, anticoagulants, steroids or non-steroidal anti-inflammatory drugs416.
Looking at models of care, one study compared health service models found significant differences in EBP and healing outcomes417, and two studies investigated models to increase EBP410,418, with a positive change in EBP in wound assessment and management in primary healthcare professionals410. A survey of specialist providers for chronic leg ulcers found about one-third used HBOT, while the remainder did not believe it had a role or did not have access to HBOT419.
Worldwide reviews
Evidence summaries were available on maggot debridement420 and NPWT for mixed leg ulcers421. A consensus document covered antimicrobial prescribing for leg ulcer infection422. SR topics are shown in Table 4.
Prevention
One quasi-experimental study evaluated an intervention to facilitate uptake of EBP in assessment, management and prevention of wounds. Results were inconclusive due to a small sample size410.
Malignant fungating wounds
Nine articles addressed malignant fungating wounds, one Australian study and eight SRs.
Assessment
A qualitative study investigated the experience of living with malignant wounds with patients, caregivers and nurses, finding malodour was one of the worst aspects423.
Management
There were five SRs on symptom management (to manage odour and/or exudate)424–428, one on topical agents and dressings429, one on management of bleeding from malignant wounds430, and one on microbiome species in malignant wounds431.
Arterial leg ulcers (ALUs)
Two EBGs and two SRs focused on ALUs. There were no Australian studies. The EBGs432,433 both covered the assessment and management of ALUs, with one also providing recommendations for prevention432. The SRs focused on wound dressings434 and autologous bone marrow cell therapy435.
Other chronic wound types
A small number of studies (n=5) were found on wound types not addressed in the main categories, encompassing Buruli ulcers, chronic epidermolysis bullosa wounds, pilonidal sinuses, tophaceous ulcers and ulcers resulting from Hansen’s disease.
Australian studies
Australian studies reported that: Australian Buruli ulcers were mostly located on upper and lower limbs436; wounds healed faster after receiving intralesional allogenic cultured fibroblasts in matched wounds in adults with recessive dystrophic epidermolysis bullosa437; and a registered trial aimed to evaluate metronidazole ointment for pilonidal sinuses438.
Worldwide reviews
Two SRs were found on treatments for ulcers associated with tophaceous gout439 and interventions for ulceration caused by Hansen’s disease440.
Discussion
Over the 12-year period scanned in this review, 365 Australian research studies on chronic wounds were identified. In general, the number of studies steadily increased each year, from 17 in 2010, up to 42 in 2020, followed by a drop in 2021 (34), possibly due to research restrictions during the COVID‑19 pandemic. Over two-thirds of these studies (70%) focused on PIs and DFUs. Information on prevalence of wound types in Australia is scarce; however, a recent Australian study surveyed all (acute and chronic) wound types in hospital, RACFs and community settings, reporting that while 9.9% of the wounds were PIs and 11.9% were foot ulcers, 17.7% were leg ulcers7, the latter being an area which may need increased focus in Australian research. Only one Australian study was found on malignant wounds, a qualitative study with patients, carers and nurses423, representing 0.3% of Australian studies. In comparison, the large Australian survey of wound types above reported malignant wounds represented 2.4% of wounds7.
Most studies in this review focused on wound management or assessment, and a smaller proportion (17%) on prevention, although there is significant potential for prevention of these wound types which are predominantly caused by underlying chronic conditions. There were relatively few Australian studies using RCT designs (10%) compared to observational study designs, such as cohort (31%) and cross-sectional (20%) studies, despite recommendations from previous authors who identified a gap in high quality evidence from well-designed trials441,442. A 2019 scoping review of recommendations, guidelines and standards for chronic wound research identified a lack of RCTs and well-designed, prospective studies441. The lack of these studies subsequently limits the ability to compare and combine data in meta-analyses and SRs441.
The largest proportion (43%) of original Australian studies were on PIs – these were mostly cohort or cross-sectional studies. The increased focus placed on prevention of PIs in health systems, including financial penalties and monitoring of prevalence as a quality indicator, may be a reason for the higher proportion of PI research found in Australia (43%) compared to worldwide reviews (22%).
The second largest proportion (27%) of Australian studies were on DFUs, although lower than the proportion of worldwide reviews (36%), with the largest group of reviews being SRs, most related to efficacy of topical wound applications and dressings (18%). Although the overall research output for DFU research was diverse, the majority concentrated on assessment (29%) and management (64%). Only five Australian studies were on prevention (2%), highlighting the need for greater work in this critical area. Further, Australian DFU studies reported a comparatively low proportion of RCTs (6%) compared to all Australian chronic wound studies (10%), with the vast majority of DFU research utilising observational methodologies. Thus, with a comparatively low focus on DFU studies in Australia compared with worldwide reviews, and an especially low proportion of RCTs, this suggests there may be a comparatively low level of focus and funding available at a national level for DFUs. With DFUs a top 10 leading cause of national and global hospitalisation and disability burden, it is recommended the focus and funding on Australian DFU research needs to improve443–445.
The third largest group of Australian original studies were on VLUs. The most frequent designs were observational studies and randomised trials, conducted primarily in community settings. Nearly two-thirds (62%) of the studies focused on VLU management, in particular health service management, with results reflecting the well-known gaps in access to EBP for this population446, despite studies showing that EBP results in significantly improved outcomes297,447,448.
Studies which combined mixed types of chronic wounds included samples with non-healing wounds of multiple aetiologies. Similar to the other wound groups, most studies focused on assessment or management, with only one study including prevention strategies. A small number of intervention studies were identified; however, most studies were observational in design, with limited high quality evidence.
There is a paucity of good quality research conducted on mixed leg ulcers, with only 20 Australian studies addressing assessment or management, and only one study addressing prevention. Prevalence rates, risk factors for poor outcomes, and evaluations of models of care provide important information; however, some studies indicated non-significant results, concluding that larger sample sizes and more research is needed. Further research is imperative to drive EBP for an increasing number of leg ulcers that are of mixed aetiology.
Overall, topics of Australian research studies were disproportionally focused on either prevalence or risk factors for poor wound outcomes, or implementation of EBP (particularly for PIs), with only half the number of studies on clinical interventions to heal, manage or prevent wounds.
Limitation
These scoping review results have some limitations. Firstly, the level of detail of the findings reported in this scoping review is necessarily brief due to the size of the review. In addition, despite best intentions, the search strategies may have failed to identify all eligible studies.
Conclusions
This review maps the wound research landscape in Australia which demonstrates great variety and diversity of output. Results highlight strengths in areas of risk assessment and implementation research, and a number of gaps – the lack of national evidence being generated on wound prevention, the lack of studies on leg ulcers of mixed or arterial aetiology, and the lack of high quality clinical trials, which is likely related to the lack of national competitive funding in this area of research.
Ethics statement
An ethics statement is not applicable.
Conflict of interest
CP owns shares in a company that manufactures amniotic membrane allografts for wound applications. Other authors have no conflicts of interest to disclose.
Funding
This project was supported by funding from the Australian Government under the Medical Research Future Fund. The funder had no role in the design, conduct or publication of the review.
Author contribution
All authors contributed to study design; UTB, PET, PAL and KF contributed to document identification and review, and UTB and KF to data analysis and synthesis. KF, UTB, CP, PET, PAL and MB-J were responsible for manuscript preparation and all authors for feedback on the manuscript. All authors read and approved the final manuscript.
Author(s)
Ut T Bui1, Peta E Tehan2, Michelle Barakat-Johnson3, Keryln Carville4,5, Emily Haesler5–7,
Peter A Lazzarini8,9, Stephen M Twigg10,11, Christina Parker1, Kathleen Finlayson1
¹School of Nursing, Centre for Healthcare Transformation, Queensland University of Technology, Brisbane, QLD, Australia
2Department of Surgery, School of Clinical Sciences, Faculty of Medicine, Health and Nursing, Monash University, Clayton, VIC, Australia
3Nursing and Midwifery Executive, Sydney Local Health District, Sydney and Faculty of Medicine and Health, University of Sydney, Sydney, NSW, Australia
4Silver Chain Group, Perth, WA, Australia
5Curtin University, Perth, WA, Australia
6La Trobe University, Melbourne, VIC, Australia
7Australian National University, Canberra, ACT, Australia
8School of Public Health and Social Work, Queensland University of Technology, Brisbane, QLD, Australia
9Allied Health Research Collaborative, The Prince Charles Hospital, Brisbane, QLD, Australia
10Department of Endocrinology, Royal Prince Alfred Hospital, Sydney, NSW, Australia
11Central Clinical School, Faculty of Medicine and Health, University of Sydney, Sydney, NSW, Australia
*Corresponding author email thiut.bui@qut.edu.au
References
- Murray RZ, West ZE, McGuiness W, The multifactorial formation of chronic wounds. Perth: Cambridge Publishing; 2018.
- Olsson M, Järbrink K, Divakar U, et al. The humanistic and economic burden of chronic wounds: a systematic review. Wound Repair Regen 2019;27:114–125.
- Han G, Ceilley R. Chronic wound healing: a review of current management and treatments. Adv Therapy 2017;34:599–610.
- McCosker L, Tulleners R, Cheng Q, et al. Chronic wounds in Australia: a systematic review of key epidemiological and clinical parameters. Int Wound J 2019;16:84–95.
- Martinengo L, Olsson M, Bajpai R, et al. Prevalence of chronic wounds in the general population: systematic review and meta-analysis of observational studies. Ann Epidemiol 2019;29:8–15.
- Graves N, Zheng H. Modelling the direct health care costs of chronic wounds in Australia. Wound Pract Res 2014;22:20–33.
- Wilkie J, Carville K, Fu S, et al. Determining the actual cost of wound care in Australia. Wound Pract Res 2023;31:7–18.
- Pacella RE, Tulleners R, McCosker L, et al. Reimbursement for the cost of compression therapy for the management of venous leg ulcers in Australia. Int Wound J 2019;16:1069–1072.
- Tricco AC, Lillie E, Zarin W, et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med 2018;169:467–473.
- Bui UT, Ellen P, Barakat-Johnson M, et al. A scoping review of research in chronic wounds: protocol. Wound Pract Res 2021;29:234–237.
- Frykberg RG, Banks J. Challenges in the treatment of chronic wounds. Adv Wound Care 2015;4:560–582.
- Baba M, Davis WA, Davis TM. A longitudinal study of foot ulceration and its risk factors in community-based patients with type 2 diabetes: the Fremantle Diabetes Study. Diabetes Res Clin Pract 2014;106:42–9.
- Ahmed M, Tannous W, Agho K, Henshaw F, Turner D, Simmons D. Social determinants of diabetes-related foot disease among older adults in New South Wales, Australia: evidence from a population-based study. J Foot Ankle Res 2021;14:65.
- Ahmed MU, Tannous WK, Agho KE, Henshaw F, Turner D, Simmons D. Prevalence and burden of diabetes-related foot disease in New South Wales, Australia: evidence from the 45 and up study survey data linked with health services data. Int J Environ Res Pub Health 2021;18.
- Lazzarini PA, Hurn SE, Kuys SS, et al. The silent overall burden of foot disease in a representative hospitalised population. Int Wound J 2017;14:716–728.
- Lazzarini PA, Hurn SE, Kuys SS, et al. Direct inpatient burden caused by foot-related conditions: a multisite point-prevalence study. BMJ Open 2016;6:10.1136/bmjopen-2015-010811.
- Santamaria N, Ogce F, Gorelik A. Healing rate calculation in the diabetic foot ulcer: comparing different methods. Wound Repair Regen 2012;20:786–789.
- Aliahmad B, Tint AN, Poosapadi Arjunan S, et al. Is thermal imaging a useful predictor of the healing status of diabetes-related foot ulcers? A pilot study. J Diabetes Sci Tech 2019;13:561–567.
- Malone M, Schwarzer S, Walsh A, et al. Monitoring wound progression to healing in diabetic foot ulcers using three-dimensional wound imaging. J Diabetes Complications 2020;34.
- Alahakoon C, Fernando M, Galappaththy C, et al. Repeatability, completion time, and predictive ability of four diabetes-related foot ulcer classification systems. J Diabetes Sci Tech 2021.
- Jelinek HF, Prinz M, Wild T. A digital assessment and documentation tool evaluated for daily podiatric wound practice. Wounds 2013;25:1–6.
- Lasschuit JWJ, Featherston J, Tonks KTT. Reliability of a three-dimensional wound camera and correlation with routine ruler measurement in diabetes-related foot ulceration. J Diab Sci Tech 2020.
- Pena G, Kuang B, Szpak Z, Cowled P, Dawson J, Fitridge R. Evaluation of a novel three-dimensional wound measurement device for assessment of diabetic foot ulcers. Adv Wound Care 2020;9:623–631.
- Fernando M, Crowther RG, Cunningham M, et al. The reproducibility of acquiring three dimensional gait and plantar pressure data using established protocols in participants with and without type 2 diabetes and foot ulcers. J Foot Ankle Res 2016;9:4.
- Rowledge A, Frescos N, Miller C, Perry E, McGuiness W. The diabetic foot ulcer periwound: a comparison of visual assessment and a skin diagnostic device. Wound Pract Res 2016;24:160–168.
- Kuang B, Pena G, Szpak Z, et al. Assessment of a smartphone-based application for diabetic foot ulcer measurement. Wound Repair Regen 2021.
- van Netten JJ, Clark D, Lazzarini PA, Janda M, Reed LF. The validity and reliability of remote diabetic foot ulcer assessment using mobile phone images. Sci Rep 2017;7:9480.
- Travis J, Malone M, Malone M, et al. The microbiome of diabetic foot ulcers: a comparison of swab and tissue biopsy wound sampling techniques using 16S rRNA gene sequencing. BMC Microbiol 2020;20.
- Johani K, Malone M, Jensen S, et al. Microscopy visualisation confirms multi-species biofilms are ubiquitous in diabetic foot ulcers. Int Wound J 2017;14:1160–1169.
- Malone M, Johani K, Jensen SO, et al. Next generation DNA sequencing of tissues from infected diabetic foot ulcers. EBioMedicine 2017;21:142–149.
- Gardiner M, Vicaretti M, Sparks J, et al. A longitudinal study of the diabetic skin and wound microbiome. PeerJ 2017;2017:3543.
- Frescos N, Copnell B. Podiatrists’ views of assessment and management of pain in diabetes-related foot ulcers: a focus group study. J Foot Ankle Res 2020;13:29.
- Dickinson AM, Frescos N, Firth JC, Hamblin PS. The characteristics of wound pain associated with diabetes-related foot ulcers: a pilot study. Wound Pract Res 2016;24:138–148.
- Lipsky BA, Senneville É, Abbas ZG, et al. Guidelines on the diagnosis and treatment of foot infection in persons with diabetes (IWGDF 2019 update). Diabetes Metab Res 2020;36 Suppl 1:e3280.
- Hamilton E, Scheepers J, Ryan H, et al. Australian guideline on wound classification of diabetes-related foot ulcers: part of the 2021 Australian evidence-based guidelines for diabetes-related foot disease. Diabetes Feet Australia; 2021.
- Monteiro-Soares M, Russell D, Boyko EJ, et al. Guidelines on the classification of diabetic foot ulcers (IWGDF 2019). Diabetes Metab Res Rev 2020;36(S1).
- Fong E. Diabetic foot ulcers: assessment and management. JBI EBP Database; 2019.
- Sivapuram MS. Diabetic foot ulcers: assessment and classification. JBI EBP Database; 2021.
- Sun Y, Gao Y, Chen J, et al. Evidence mapping of recommendations on diagnosis and therapeutic strategies for diabetes foot: an international review of 22 guidelines. Metabolism 2019;100.
- Malone M, Johani K, Jensen SO, et al. Effect of cadexomer iodine on the microbial load and diversity of chronic non-healing diabetic foot ulcers complicated by biofilm in vivo. J Antimicrob Chemoth 2017;72:2093–2101.
- Malone M, Schwarzer S, Radzieta M, et al. Effect on total microbial load and community composition with two vs six-week topical Cadexomer Iodine for treating chronic biofilm infections in diabetic foot ulcers. Int Wound J 2019;16:1477–1486.
- Malone M, Radzieta M, Schwarzer S, Jensen SO, Lavery LA. Efficacy of a topical concentrated surfactant gel on microbial communities in non-healing diabetic foot ulcers with chronic biofilm infections: a proof-of-concept study. Int Wound J 2021.
- Henshaw FR, Bolton T, Nube V, et al. Topical application of the bee hive protectant propolis is well tolerated and improves human diabetic foot ulcer healing in a prospective feasibility study. J Diabetes Complications 2014;28:850–857.
- Freeman A, May K, Wraight P. Honey: the bees’ knees for diabetic foot ulcers? Wound Pract Res 2010;18:144–147.
- Manning L, Hamilton EJ, Raby E, et al. Spray on skin for diabetic foot ulcers: an open label randomised controlled trial. J Foot Ankle Res 2019;12:52-.
- Michailidis L, Bergin SM, Haines TP, Williams CM. Healing rates in diabetes-related foot ulcers using low frequency ultrasonic debridement versus non-surgical sharps debridement: a randomised controlled trial. BMC Res Notes 2018;11:732.
- Whitmont K, McKelvey KJ, Fulcher G, et al. Treatment of chronic diabetic lower leg ulcers with activated protein C: a randomised placebo-controlled, double-blind pilot clinical trial. Int Wound J 2015;12:422–427.
- Manning L, Ferreira IB, Gittings P, et al. Wound healing with “spray-on” autologous skin grafting (ReCell) compared with standard care in patients with large diabetes-related foot wounds: an open-label randomised controlled trial. Int Wound J 2022;19:470–481.
- Henshaw FR, Brennan L, MacMillan F. Perceptions of hyperbaric oxygen therapy among podiatrists practicing in high-risk foot clinics. Int Wound J 2018;15:375–382.
- Burns J, Begg L. Optimizing the offloading properties of the total contact cast for plantar foot ulceration. Diabetic Med 2011;28:179–185.
- Berhane T, Jeyaraman K, Hamilton M, Falhammar H. Pressure relieving interventions for the management of diabetes-related foot ulcers: a study from the Northern Territory of Australia. ANZ J Surg 2022;92:723–729.
- Raspovic A, Landorf KB, Gazarek J, Stark M. Reduction of peak plantar pressure in people with diabetes-related peripheral neuropathy: an evaluation of the DH Pressure Relief Shoe™. J Foot Ankle Res 2012;5:25.
- Zhang Y, Cramb S, McPhail S, et al. Factors associated with healing of diabetes-related foot ulcers: observations from a large prospective real-world cohort. Diabetes Care 2021;44:e143-e145.
- Zhang Y, Cramb S, McPhail SM, et al. Multiple factors predict longer and shorter time-to-ulcer-free in people with diabetes-related foot ulcers: survival analyses of a large prospective cohort followed-up for 24-months. Diabetes Res Clin Pract 2022;185.
- Raspovic A, Waller K, Wong WM. The effectiveness of felt padding for offloading diabetes-related foot ulcers, at baseline and after one week of wear. Diabetes Res Clin Pr 2016;121:166–172.
- Begg L, McLaughlin P, Vicaretti M, Fletcher J, Burns J. Total contact cast wall load in patients with a plantar forefoot ulcer and diabetes. J Foot Ankle Res 2016;9:2.
- Malone M, West D, Xuan W, Lau NS, Maley M, Dickson HG. Outcomes and cost minimisation associated with outpatient parenteral antimicrobial therapy (OPAT) for foot infections in people with diabetes. Diabetes Res 2015;31:638–645.
- Hand R, Manning L, Ritter JC, et al. Antimicrobial stewardship opportunities among inpatients with diabetic foot infections: microbiology results from a tertiary hospital multidisciplinary unit. Int Med J 2019;49:533–536.
- Commons RJ, Raby E, Athan E, et al. Managing diabetic foot infections: a survey of Australasian infectious diseases clinicians. J Foot Ankle Res 2018;11:13.
- Gunton JE, Girgis CM, Lau T, Vicaretti M, Begg L, Flood V. Vitamin C improves healing of foot ulcers; a randomised, double-blind, placebo-controlled trial. Br J Nutrition 2020.
- Palaya J, Pearson S, Nash T. Perception of social support in individuals living with a diabetic foot: a qualitative study. Diabetes Res Clin Pr 2018;146:267–277.
- van Netten JJ, Seng L, Lazzarini PA, Warnock J, Ploderer B. Reasons for (non-)adherence to self-care in people with a diabetic foot ulcer. Wound Repair Regen 2019;27:530–539.
- Ploderer B. MyFootCare: a pilot study for a mobile application to engage patients with diabetic foot ulcers in self-care. Australian New Zealand Clinical Trials Registry; 2018.
- McLean A, Gardner M, Perrin B. PodCast: a rural and regional service model for podiatrist-led total contact casting. Aust J Rural Health 2019;27:433–437.
- Sung JA, Gurung S, Lam T, et al. A ‘speed-dating’ model of wound care? Rapid, high-volume assessment of patients with diabetes in a multidisciplinary foot wound clinic. Exp Clin Endocrinol Diabetes 2021;129:837–841.
- West M, Sadler S, Charles J, et al. Yarning about foot care: evaluation of a foot care service for Aboriginal and Torres Strait Islander Peoples. J Foot Ankle Res 2022;15:25.
- Manuel P. A prospective, interventional study of the effectiveness of digital wound imaging, remote consultation and podiatry offloading devices on the healing rates of chronic lower extremity wounds in remote regions of Western Australia. Wound Pract Res 2012;20:103–109.
- Lazzarini PA, Clark D, Mann RD, Perry VL, Thomas CJ, Kuys SS. Does the use of store-and-forward telehealth systems improve outcomes for clinicians managing diabetic foot ulcers? A pilot study. Wound Pract Res 2010;18:164–172.
- Quinton TR, Lazzarini PA, Boyle FM, Russell AW, Armstrong DG. How do Australian podiatrists manage patients with diabetes? The Australian diabetic foot management survey. J Foot Ankle Res 2015;8:16.
- Mullan L, Wynter K, Driscoll A, Rasmussen B. Prioritisation of diabetes-related footcare amongst primary care healthcare professionals. J Clin Nurs 2020;29:4653–4673.
- Cheng Q, Lazzarini PA, Gibb M, et al. A cost-effectiveness analysis of optimal care for diabetic foot ulcers in Australia. Int Wound J 2017;14:616–628.
- Nube VL, Alison JA, Twigg SM. Frequency of sharp wound debridement in the management of diabetes-related foot ulcers: exploring current practice. J Foot Ankle Res 2021;14:52.
- Hinchliffe RJ, Forsythe RO, Apelqvist J, et al. Guidelines on diagnosis, prognosis, and management of peripheral artery disease in patients with foot ulcers and diabetes (IWGDF 2019 update). Diabetes Metab Res Rev 2020;36 Suppl 1:e3276.
- Schaper NC, van Netten JJ, Apelqvist J, Bus SA, Hinchliffe RJ, Lipsky BA. Practical guidelines on the prevention and management of diabetic foot disease (IWGDF 2019 update). Diabetes Metab Res Rev 2020;36 Suppl 1:e3266.
- Schaper NC, van Netten JJ, Apelqvist J, Bus SA, IWGDF guidelines on the prevention and management of diabetic foot disease. www.iwgdfguidelines.org: IWGDF 2019.
- Rayman G, Vas P, Dhatariya K, et al. Guidelines on use of interventions to enhance healing of chronic foot ulcers in diabetes (IWGDF 2019 update). Diabetes Metab Res Rev 2020;36(S1).
- Game FL, Attinger C, Hartemann A, et al. IWGDF guidance on use of interventions to enhance the healing of chronic ulcers of the foot in diabetes. Diabetes Metab Res Rev 2016;32:75–83.
- Bus SA, Armstrong DG, Gooday C, et al. Guidelines on offloading foot ulcers in persons with diabetes (IWGDF 2019 update). Diabetes Metab Res Rev 2020;36 Suppl 1:e3274.
- Fernando ME, Horsley MW, Jones S, et al. Australian guideline on offloading treatment for foot ulcers: part of the 2021 Australian evidence-based guidelines for diabetes-related foot disease. Diabetes Feet Australia; 2021.
- Chen P, Carville K, Swanson T, et al. Australian guideline on wound healing interventions to enhance healing of foot ulcers: part of the 2021 Australian evidence-based guidelines for diabetes-related foot disease. Diabetes Feet Australia; 2021.
- Commons R, Charles J, Cheney J, et al. Australian guideline on management of diabetes-related foot infection: part of the 2021 Australian evidence-based guidelines for diabetes-related foot disease. Diabetes Feet Australia; 2021.
- Chuter V, Quigley F, Tosenovsky P, et al. Australian guideline on diagnosis and management of peripheral artery disease: part of the 2021 Australian evidence-based guidelines for diabetes-related foot disease. Diabetes Feet Australia; 2021.
- Lavery LA, Davis KE, Berriman SJ, et al. WHS guidelines update: diabetic foot ulcer treatment guidelines. Wound Repair Regen 2016;24:112–26.
- Hingorani A, LaMuraglia GM, Henke P, et al. The management of diabetic foot: a clinical practice guideline by the Society for Vascular Surgery in collaboration with the American Podiatric Medical Association and the Society for Vascular Medicine. J Vasc Surg 2016;63:3s–21s.
- Araki E, Goto A, Kondo T, et al. Japanese clinical practice guideline for diabetes 2019. Diabetol Int 2020;11:165–223.
- Botros M, Kuhnke J, Embil JM, et al. Best practice recommendations for the prevention and management of diabetic foot ulcers. Wounds Canada 2017.
- Ewing S. Diabetic foot ulcers: debridement. JBI EBP Database; 2021.
- Lizarondo L. Diabetic foot ulcer: debridement. JBI EBP Database; 2021.
- Marin T. Diabetic foot ulcers: silver based dressings. JBI EBP Database; 2021.
- Wu L, Norman G, Dumville JC, O’Meara S, Bell-Syer SE. Dressings for treating foot ulcers in people with diabetes: an overview of systematic reviews. Cochrane Database Syst Rev 2015.
- Sivapuram MS. Diabetic foot ulcers: pressure relieving interventions. JBI EBP Database; 2021.
- World Union of Wound Healing Societies. WUHS position document: local management in diabetic foot ulcers. Wounds International; 2016.
- Paul C, Mike E, Adam F, et al. Expert panel report: the role of topical oxygen therapy in the management of diabetic foot ulcers. Diabetic Foot J 2019.
- Westphal C, Neame I, Harrison J, Bower V, Gurr J. A diabetic foot ulcer pilot study: does silicone gel sheeting reduce the incidence of reulceration? J Am Podiatr Med Assoc 2011;101:116–23.
- Mullan L, Wynter K, Driscoll A, Rasmussen B. Preventative and early intervention diabetes-related foot care practices in primary care. Aust J Prim Health 2020;26:161–172.
- Kaminski M. Effect of podiatry intervention on foot ulceration and amputations in adults on dialysis. Australian New Zealand Clinical Trials Registry; 2018.
- Ahmed S. Footwear and insole design parameters to prevent occurrence and recurrence of neuropathic plantar forefoot ulcers in patients with diabetes – a series of single participant trials. Australian New Zealand Clinical Trials Registry; 2020.
- Bus SA, Lavery LA, Monteiro-Soares M, et al. Guidelines on the prevention of foot ulcers in persons with diabetes (IWGDF 2019 update). Diabetes Metab Res Rev 2020;36 Suppl 1:e3269.
- van Netten JJ, Lazzarini PA, Armstrong DG, et al. Diabetic Foot Australia guideline on footwear for people with diabetes. J Foot Ankle Res 2018;11:2.
- Kaminski M, Golledge J, Lasschuit J, et al. Australian guideline on prevention of foot ulceration: part of the 2021 Australian evidence-based guidelines for diabetes-related foot disease. Diabetes Feet Australia; 2021.
- Le LK-D. Diabetes: foot care (older people). JBI EBP Database; 2020.
- Pamaiahgari P. Diabetic foot ulcers: Patient education. JBI EBP Database; 2020.
- Barakat-Johnson M, Lai M, Barnett C, et al. Hospital-acquired pressure injuries: are they accurately reported? A prospective descriptive study in a large tertiary hospital in Australia. J Tissue Viability 2018;27:203–210.
- Jorgensen M, Siette J, Georgiou A, Westbrook JI. Longitudinal variation in pressure injury incidence among long-term aged care facilities. Int J Qual Health Care 2018;30:684–691.
- Low ZY, Storer M, Tai R, Yates P. In-hospital morbidity and mortality among patients from residential respite care. Intern Med J 2020;50:85–91.
- Webster J, Coleman K, Mudge A, et al. Pressure ulcers: effectiveness of risk-assessment tools. A randomised controlled trial (the ULCER trial). BMJ Qual Safety 2011;20:297–306.
- Coyer F, Chaboyer W, Lin F, et al. Pressure injury prevalence in Australian intensive care units: a secondary analysis. Aust Crit Care Nurse 2021.
- Yarad E, O’Connor A, Meyer J, et al. Prevalence of pressure injuries and the management of support surfaces (mattresses) in adult intensive care patients: a multicentre point prevalence study in Australia and New Zealand. Aust Crit Care 2021;34:60–66.
- Coyer F, Miles S, Gosley S, et al. Pressure injury prevalence in intensive care versus non-intensive care patients: a state-wide comparison. Aust Crit Care 2017;30:244–250.
- Wang H-RN, Campbell J, Doubrovsky A, Singh V, Collins J, Coyer F. Pressure injury development in critically ill patients with a cervical collar in situ: a retrospective longitudinal study. Int Wound J 2020;17:944–956.
- Labeau SO, Afonso E, Benbenishty J, et al. Prevalence, associated factors and outcomes of pressure injuries in adult intensive care unit patients: the DecubICUs study. Intensive Care Med 2021;47:160–169.
- Xie H, Peel NM, Hirdes JP, Poss JW, Gray LC. Validation of the interRAI pressure ulcer risk scale in acute care hospitals. J Am Geriatr Soc 2016;64:1324–1328.
- Campbell JL, Gosley S, Coleman K, Coyer FM. Combining pressure injury and incontinence-associated dermatitis prevalence surveys: an effective protocol? Wound Pract Res 2016;24:170–177.
- Nowicki JL, Mullany D, Spooner A, et al. Are pressure injuries related to skin failure in critically ill patients? Aust Crit Care 2018;31:257–263.
- Latimer S, Chaboyer W, Thalib L, McInnes E, Bucknall T, Gillespie BM. Pressure injury prevalence and predictors among older adults in the first 36 hours of hospitalisation. J Clin Nurs 2019;28:4119–4127.
- Martinez-Garduno CM, Rodgers J, Phillips R, Gunaratne AW, Drury P, McInnes E. The Surgical Patients’ Pressure Injury Incidence (SPPII) study: a cohort study of surgical patients and processes of care. Wound Pract Res 2019;27:120–128.
- Webster J, Lister C, Corry J, Holland M, Coleman K, Marquart L. Incidence and risk factors for surgically acquired pressure ulcers: a prospective cohort study investigators. J Wound Ostomy Continence Nurs 2015;42:138–144.
- Coyer F, Barakat-Johnson M, Campbell J, et al. Device-related pressure injuries in adult intensive care unit patients: an Australian and New Zealand point prevalence study. Aust Crit Care 2021;34:561–568.
- Coyer F, Cook J-L, Doubrovsky A, Vann A, McNamara G. Exploring medical device-related pressure injuries in a single intensive care setting: a longitudinal point prevalence study. Intensive Crit Care Nurs 2022;68:N.PAG-N.PAG.
- Coyer FM, Stotts NA, Blackman VS. A prospective window into medical device-related pressure ulcers in intensive care. Int Wound J 2014;11:656–664.
- Barakat-Johnson M, Barnett C, Wand T, White K. Medical device-related pressure injuries: an exploratory descriptive study in an acute tertiary hospital in Australia. J Tissue Viability 2017;26:246–253.
- Karki S, Leder K, Cheng AC. Patients under contact precautions have an increased risk of injuries and medication errors: a retrospective cohort study. Infect Control Hosp Epidemiol 2013;34:1118–1120.
- Wilson S, Bremner AP, Hauck Y, Finn J. Evaluation of paediatric nursing-sensitive outcomes in an Australian population using linked administrative hospital data. BMC Health Serv Res 2013;13:396–396.
- Miles SJ, Fulbrook P, Nowicki T, Franks C. Decreasing pressure injury prevalence in an Australian general hospital: a 10-year review. Wound Pract Res 2013;21:148–156.
- Ferguson C, Crouchley K, Mason L. Pressure injury point prevalence: state-wide survey to identify variability in Western Australian hospitals. Australian J Adv Nurs 2019;36:28–36.
- Hunter M, Kelly J, Stanley N, Stilley A, Anderson L. Pressure injury prevention success in a regional hospital. Arch Ital Urol Androl 2014;49:75–82.
- Fulbrook P, Miles S, Coyer F. Prevalence of pressure injury in adults presenting to the emergency department by ambulance. Aust Crit Care 2019;32:509–514.
- Asimus M, Li P. Pressure ulcers in home care settings: is it overlooked? Wound Pract Res 2011;19:88–97.
- Jackson D, Hutchinson M, Neville S, et al. Characteristics of people with pressure ulcers using one year’s routinely collected data in a defined diverse community. J Wound Care 2019;28:576–584.
- Dunk AM, Gardner A, Waddington G. Anatomical location of injury in stage I and stage II heel pressure injuries – a pilot study. Wound Pract Res 2012;20:130–141.
- Floyd E, Hodgkins R, Naylon L, Noon M, Sirotti L, Monaro S. The costs and benefits of pressure injury point prevalence auditing. Wound Pract Res 2020;28:66–74.
- Webster J, Gavin N, Nicholas C, Coleman K, Gardner G. Validity of the Waterlow scale and risk of pressure injury in acute care. Br J Nurs 2010;19:S14-S18.
- Page KN, Barker AL, Kamar J. Development and validation of a pressure ulcer risk assessment tool for acute hospital patients. Wound Repair Regen 2011;19:31–37.
- Leonard P, Hill A, Moon K, Lima S. Pediatric pressure injuries: does modifying a tool alter the risk assessment outcome? Issues Compr Pediatr Nurs 2013;36:279–90.
- Fulbrook P, Anderson A. Pressure injury risk assessment in intensive care: comparison of inter-rater reliability of the COMHON (Conscious level, Mobility, Haemodynamics, Oxygenation, Nutrition) Index with three scales. J Adv Nurs 2016;72:680–692.
- Reaper S, Green C, Gupta S, Tiruvoipati R. Inter-rater reliability of the Reaper Oral Mucosa Pressure Injury Scale (ROMPIS): a novel scale for the assessment of the severity of pressure injuries to the mouth and oral mucosa. Aust Crit Care 2017;30:167–171.
- Fitzgerald S, McTier L, Whitehead C, Masters K, Wynne R. Inter-rater reliability of descriptors for the classification of mucosal pressure injury: a prospective cross-sectional study. Aust Crit Care 2022.
- Scheepers J. Assessment and consideration of foot risk factors is essential for proactive prevention of hospital-acquired foot pressure injuries. Wounds International; 2018;9:29–34.
- Than S, Crabtree A, Moran C. Examination of risk scores to better predict hospital-related harms. Intern Med J 2019;49:1125–1131.
- Curtis K, Qian S, Yu P, et al. Does electronic medical record redesign increase screening of risk for pressure injury, falls and substance use in the Emergency Department? An implementation evaluation. Australas Emerg Care 2021;24:20–27.
- Borzdynski CJ, McGuiness W, Miller C. Comparing visual and objective skin assessment with pressure injury risk. Int Wound J 2016;13:512–518.
- Team V, Tuck M, Reeves J, et al. Pressure injury data in Australian acute care settings: a comparison of three data sets. Int Wound J 2020;17:578–586.
- McInnes E, Chaboyer W, Allen T, Murray E, Webber L. Acute care patient mobility patterns and documented pressure injury prevention – an observational study and survey. Wound Pract Res 2013;21:116–125.
- Ostaszkiewicz J, O’Connell B, Dunning T. Night-time continence care in Australian residential aged care facilities: findings from a grounded theory study. Contemp Nurse 2016;52:152–162.
- EPUAP, NPIAP, PPPIA. Prevention and treatment of pressure ulcers/injuries: clinical practice guideline. EPUAP/NPIAP/PPPIA; 2019.
- Registered Nurses’ Association of Ontario. Assessment and management of pressure injuries for the interprofessional team (3rd ed). Registered Nurses’ Association of Ontario; 2016.
- Wounds Canada. Best practice recommendations for the prevention and management of pressure injuries. Foundations of Best Practice for Skin and Wound Management; 2017.
- Fujiwara H, Isogai Z, Irisawa R, et al. Wound, pressure ulcer and burn guidelines – 2: guidelines for the diagnosis and treatment of pressure ulcers (2nd ed). J Dermatol 2020;47:929–978.
- Abdulsalam A. Pressure injuries (burns): prevalence. JBI EBP Database; 2021.
- Minooee S. Pressure injury prevention (pediatric): risk assessment. JBI EBP Database; 2021.
- Minooee S. Pressure ulcers (older people) risk assessment in community settings. JBI EBP Database; 2021.
- Nguyen DH. Pressure injury prevention strategies (adults): risk assessment. JBI EBP Database; 2021.
- Rivolo M, Dionisi S, Olivari D, et al. Heel pressure injuries: consensus-based recommendations for assessment and management. Adv Wound Care 2020;9:332–347.
- Gefen A, Alves P, Ciprandi G, et al. Device-related pressure ulcers: SECURE prevention. J Wound Care 2020;29:S1–S52.
- Darvall JN, Mesfin L, Gorelik A. Increasing frequency of critically ill patient turns is associated with a reduction in pressure injuries. Critical Care Resus 2018;20:217–222.
- Sharp CA, Schulz Moore JS, McLaws ML. Two-hourly repositioning for prevention of pressure ulcers in the elderly: patient safety or elder abuse? J Bioeth Inq 2019;16:17–34.
- Williams TA, Leslie GD, Bingham R, Brearley L. Optimizing seating in the intensive care unit for patients with impaired mobility. Am J Crit Care 2011;20:e19–e27.
- Kapp S, Gerdtz M, Gefen A, Prematunga R, Santamaria N. An observational study of the maintenance of the 30˚ side-lying lateral tilt position among aged care residents at risk of developing pressure injuries when using the standard care pillow and a purpose-designed positioning device. Int Wound J 2019;16:1080–1086.
- Sousa I, Kapp S, Santamaria N. Positioning immobile critically ill patients who are at risk of pressure injuries using a purpose-designed positioning device and usual care equipment: an observational feasibility study. Int Wound J 2020;17:1028–1038.
- Thom LM, James-McAlpine J. Reducing pressure injuries in children caused by peripheral intravenous cannulae. Nurs Child Young People 2022.
- Barakat-Johnson M, Lai M, Gefen A, Coyer F. Evaluation of a fluidised positioner to reduce occipital pressure injuries in intensive care patients: a pilot study. Int Wound J 2019;16:424–432.
- Dunk AM, Gardner A. The contribution of pressure gradients to advancing understanding of deep tissue injury to sacral regions. Wound Pract Res 2015;23:116–122.
- Malkoun M, Huber J, Huber D. A comparative assessment of interface pressures generated by four surgical theatre heel pressure ulcer prophylactics. Int Wound J 2012;9:259–263.
- Coyer F, Clark M, Slattery P, et al. Exploring pressures, tissue reperfusion and body positioning: a pilot evaluation. J Wound Care 2017;26:583–592.
- Wagstaff MJ, Driver S, Coghlan P, Greenwood JE. A randomized, controlled trial of negative pressure wound therapy of pressure ulcers via a novel polyurethane foam. Wound Repair Regen 2014;22:205–211.
- Cubit K, McNally B, Lopez V. Taking the pressure off in the emergency department: evaluation of the prophylactic application of a low shear, soft silicon sacral dressing on high risk medical patients. Int Wound J 2013;10:579–584.
- Santamaria N, Gerdtz M, Sage S, et al. A randomised controlled trial of the effectiveness of soft silicone multi-layered foam dressings in the prevention of sacral and heel pressure ulcers in trauma and critically ill patients: The Border Trial. Int Wound J 2015;12:302–308.
- Santamaria N, Gerdtz M, Kapp S, Wilson L, Gefen A. A randomised controlled trial of the clinical effectiveness of multi-layer silicone foam dressings for the prevention of pressure injuries in high-risk aged care residents: The Border III Trial. Int Wound J 2018;15:482–490.
- Santamaria N, Gerdtz M, Liu W, et al. Clinical effectiveness of a silicone foam dressing for the prevention of heel pressure ulcers in critically ill patients: Border II trial. J Wound Care 2020;29:S26-S31.
- Santamaria N, Gerdtz M, Liu W, et al. Clinical effectiveness of a silicone foam dressing for the prevention of heel pressure ulcers in critically ill patients: Border II Trial. J Wound Care 2015;24:340–345.
- Santamaria N, Liu W, Gerdtz M, et al. The cost-benefit of using soft silicone multilayered foam dressings to prevent sacral and heel pressure ulcers in trauma and critically ill patients: a within-trial analysis of the Border Trial. Int Wound J 2015;12:344–350.
- Stankiewicz M, Gordon J, Dulhunty JM, Brown W, Pollock H, Barker-Gregory N. A cluster-controlled clinical trial of two prophylactic silicone sacral dressings to prevent sacral pressure injuries in critically ill patients. Wound Pract Res 2019;27:21–26.
- Mishra U, Jani P, Maheshwari R, et al. Skincare practices in extremely premature infants: a survey of tertiary neonatal intensive care units from Australia and New Zealand. J Paediatr Child Health 2021;57:1627–1633.
- Brewer S, Desneves K, Pearce L, et al. Effect of an arginine-containing nutritional supplement on pressure ulcer healing in community spinal patients. J Wound Care 2010;19:311–316.
- Leigh B, Desneves K, Rafferty J, et al. The effect of different doses of an arginine-containing supplement on the healing of pressure ulcers. J Wound Care 2012;21:150–156.
- Chapman BR, Mills KJ, Pearce LM, Crowe TC. Use of an arginine-enriched oral nutrition supplement in the healing of pressure ulcers in patients with spinal cord injuries: an observational study. Nutr Diet 2011;68:208–213.
- Banks MD, Ross LJ, Webster J, et al. Pressure ulcer healing with an intensive nutrition intervention in an acute setting: a pilot randomised controlled trial. J Wound Care 2016;25:384–392.
- Klemm HJ, Bailey JK, Desneves KJ, Crowe TC. Can early dietetic intervention improve outcomes in patients with hip fracture? Nutr Diet 2016;73:336–341.
- Banks MD, Graves N, Bauer JD, Ash S. Cost effectiveness of nutrition support in the prevention of pressure ulcer in hospitals. Eur J Clin Nutr 2013;67:42–46.
- Tuffaha HW, Roberts S, Chaboyer W, Gordon LG, Scuffham PA. Cost-effectiveness analysis of nutritional support for the prevention of pressure ulcers in high-risk hospitalized patients. Adv Skin Wound Care 2016;29:261–267.
- Tuffaha HW, Roberts S, Chaboyer W, Gordon LG, Scuffham PA. Cost-effectiveness and value of information analysis of nutritional support for preventing pressure ulcers in high-risk patients: implement now, research later. Appl Health Econ Health Policy 2015;13:167–179.
- Banks MD, Graves N, Bauer JD, Ash S. The costs arising from pressure ulcers attributable to malnutrition. Clin Nutr 2010;29:180–186.
- Team V, Bouguettaya A, Richards C, et al. Patient education materials on pressure injury prevention in hospitals and health services in Victoria, Australia: availability and content analysis. Int Wound J 2020;17:370–379.
- Latimer S, Chaboyer W, Gillespie B. Patient participation in pressure injury prevention: giving patients a voice. Scand J Caring Sci 2014;28:648–656.
- Gillespie BM, Chaboyer W, Sykes M, O’Brien J, Brandis S. Development and pilot testing of a patient-participatory pressure ulcer prevention care bundle. J Nurs Care Qual 2014;29:74–82.
- McInnes E, Chaboyer W, Murray E, Allen T, Jones P. The role of patients in pressure injury prevention: a survey of acute care patients. BMC Nurs 2014;13:1–15.
- Deakin J, Gillespie BM, Chaboyer W, Nieuwenhoven P, Latimer S. An education intervention care bundle to improve hospitalised patients’ pressure injury prevention knowledge: a before and after study. Wound Pract Res 2020;28:154–162.
- Roberts S, Wallis M, McInnes E, et al. Patients’ perceptions of a pressure ulcer prevention care bundle in hospital: a qualitative descriptive study to guide evidence-based practice. Worldviews Evid Based Nurs 2017;14:385–393.
- Chaboyer W, Harbeck E, Bucknall T, et al. Initial psychometric testing and validation of the patient participation in pressure injury prevention scale. J Adv Nurs 2017;73:2237–2247.
- Rutherford C, Brown JM, Smith I, et al. A patient-reported pressure ulcer health-related quality of life instrument for use in prevention trials (PU-QOL-P): psychometric evaluation. Health Qual Life Outcome 2018;16:227.
- Rutherford C, Campbell R, Brown JM, et al. Comparison of generic and disease-specific measures in their ability to detect differences in pressure ulcer clinical groups. Wound Repair Regen 2019;27:396–405.
- Rose A, Mackenzie L. ‘Beyond the cushion’: a study of occupational therapists’ perceptions of their role and clinical decisions in pressure care. Disabil Rehabil 2010;32:1099–1108.
- Macens K, Rose A, Mackenzie L. Pressure care practice and occupational therapy: findings of an exploratory study. Aust Occup Ther J 2011;58:346–354.
- Chaboyer W, Gillespie BM. Understanding nurses’ views on a pressure ulcer prevention care bundle: a first step towards successful implementation. J Clin Nurs 2014;23:3415–3423.
- Barakat-Johnson M, Lai M, Wand T, White K. A qualitative study of the thoughts and experiences of hospital nurses providing pressure injury prevention and management. Collegian 2019;26:95–102.
- Coyer F, Cook J-L, Doubrovsky A, Campbell J, Vann A, McNamara G. Understanding contextual barriers and enablers to pressure injury prevention practice in an Australian intensive care unit: an exploratory study. Aust Crit Care 2019;32:122–130.
- Fulbrook P, Lawrence P, Miles S. Australian nurses’ knowledge of pressure injury prevention and management: a cross-sectional survey. J Wound Ostomy Continence Nurs 2019;46:106–112.
- Bundz J, Schuurs S, Kendall M, Amsters D. What prompts nurses to seek help from wound care consultants in spinal cord injury management? Aust J Adv Nurs 2016;34:6–15.
- Jackson D, Hutchinson M, Barnason S, et al. Towards international consensus on patient harm: perspectives on pressure injury policy. J Nurs Manag 2016;24:902–914.
- Lovegrove J, Fulbrook P, Miles S. Prescription of pressure injury preventative interventions following risk assessment: an exploratory, descriptive study. Int Wound J 2018;15:985–992.
- Lovegrove J, Fulbrook P, Miles S. Relationship between prescription and documentation of pressure injury prevention interventions and their implementation: an exploratory, descriptive study. Worldviews Evid Based Nurs 2020;17:465–475.
- Coyer F, Cook JL, Brown W, Vann A, Doubrovsky A. Securement to prevent device-related pressure injuries in the intensive care unit: a randomised controlled feasibility study. Int Wound J 2020;17:1566–1577.
- Latimer S, Chaboyer W, Gillespie B. Pressure injury prevention strategies in acute medical inpatients: an observational study. Contemp Nurse 2016;52:326–340.
- Chaboyer W, Bucknall T, Gillespie B, et al. Adherence to evidence-based pressure injury prevention guidelines in routine clinical practice: a longitudinal study. Int Wound J 2017.
- Latimer S, Gillespie BM, Chaboyer W. Predictors of pressure injury prevention strategies in at-risk medical patients: an Australian multi-centre study. Collegian 2017;24:155–163.
- Hewson-Conroy KM, Burrell AR, Elliott D, et al. Compliance with processes of care in intensive care units in Australia and New Zealand – a point prevalence study. Anaesth Intensive Care 2011;39:926–935.
- Levido A, Fulbrook P, Barakat-Johnson M, et al. Pressure injury prevention practice in Australian intensive care units: a national cross-sectional survey. Aust Crit Care 2021.
- Kapp S, Annells M. Pressure ulcers: home-based nursing. Br J Community Nurs 2010;15:S6-S13.
- Asimus M, Maclellan L, Li PI. Pressure ulcer prevention in Australia: the role of the nurse practitioner in changing practice and saving lives. Int Wound J 2011;8:508–513.
- Wilson L, Kapp S, Santamaria N. The direct cost of pressure injuries in an Australian residential aged care setting. Int Wound J 2019;16:64–70.
- McNair PD, Jackson TJ, Borovnicar DJ, McNair PD, Jackson TJ, Borovnicar DJ. The US Medicare policy of not reimbursing hospital-acquired conditions: what impact would such a policy have in Victorian hospitals? Med J Aust 2010;193:22–25.
- Bail K, Goss J, Draper B, Berry H, Karmel R, Gibson D. The cost of hospital-acquired complications for older people with and without dementia; a retrospective cohort study. BMC Health Serv Res 2015;15:91.
- Barakat-Johnson M, Lai M, Wand T, White K, De Abreu Lourenco R. Costs and consequences of an intervention-based program to reduce hospital-acquired pressure injuries in one health district in Australia. Aust Health Rev 2019;43:516–525.
- Antonio T, Conrad K. Clinical and economic improvements in pressure injury care at Ballarat Health Services. Wound Pract Res 2013;21:4–10.
- Whitty JA, McInnes E, Bucknall T, et al. The cost-effectiveness of a patient centred pressure ulcer prevention care bundle: findings from the INTACT cluster randomised trial. Int J Nurs Stud 2017;75:35–42.
- Santamaria N, Santamaria H. An estimate of the potential budget impact of using prophylactic dressings to prevent hospital-acquired PUs in Australia. J Wound Care 2014;23:583.
- Padula WV, Chen YH, Santamaria N. Five-layer border dressings as part of a quality improvement bundle to prevent pressure injuries in US skilled nursing facilities and Australian nursing homes: a cost-effectiveness analysis. Int Wound J 2019;16:1263–1272.
- Burston S, Chaboyer W, Gillespie B, Carroll R. The effect of a transforming care initiative on patient outcomes in acute surgical units: a time series study. J Adv Nurs 2015;71:417–429.
- Chaboyer W, Bucknall T, Webster J, et al. The effect of a patient centred care bundle intervention on pressure ulcer incidence (INTACT): a cluster randomised trial. Int J Nurs Stud 2016;64:63–71.
- Tinker M, Roach V, Elliott R. Save our skin: a pressure injury reduction project targeting pressure injuries acquired in the intensive care unit. Wound Pract Res 2020;28:106–114.
- Coyer F, Gardner A, Doubrovsky A, et al. Reducing pressure injuries in critically ill patients by using a patient skin integrity care bundle (InSPiRE). American J Crit Care 2015;24:199–209.
- Considine J, McGillivray B. An evidence-based practice approach to improving nursing care of acute stroke in an Australian Emergency Department. J Clin Nurs 2010;19:138–144.
- Barakat-Johnson M, Lai M, Wand T, Coyer F, White K. Systemwide practice change program to combat hospital-acquired pressure injuries: translating knowledge into practice. J Nurs Care Qual 2020;35:51–57.
- Coyer F, Cook J-L, Doubrovsky A, et al. Implementation and evaluation of multilayered pressure injury prevention strategies in an Australian intensive care unit setting. Aust Crit Care 2022;35:143–152.
- Waird A, Monaro S. Reducing the incidence and severity of pressure injuries in a high level care residential aged facility: a quality improvement project. Wound Pract Res 2021;29:77–85.
- Hada A, Coyer F. Shift-to-shift nursing handover interventions associated with improved inpatient outcomes – falls, pressure injuries and medication administration errors: an integrative review. Nurs Health Sci 2021;23:337–351.
- Holbrook S, O’Brien-Malone C, Barton A, Harper K. A quality improvement initiative to reduce hospital-acquired pressure injuries (HAPI) in an acute inpatient setting by improving patient education and seating. Wound Pract Res 2021;29:198–205.
- Tayyib N, Coyer F. Translating pressure ulcer prevention into intensive care nursing practice: overlaying a care bundle approach with a model for research implementation. J Nurs Care Qual 2017;32:6–14.
- Kapp S. Successful implementation of clinical practice guidelines for pressure risk management in a home nursing setting. J Eval Clin Pract 2013;19:895–901.
- Barker AL, Kamar J, Tyndall TJ, et al. Implementation of pressure ulcer prevention best practice recommendations in acute care: an observational study. Int Wound J 2013;10:313–320.
- Edwards HE, Chang AM, Gibb M, et al. Reduced prevalence and severity of wounds following implementation of the Champions for Skin Integrity model to facilitate uptake of evidence-based practice in aged care. J Clin Nurs 2017;26:4276–4285.
- Smith SK, Ashby SE, Thomas L, Williams F. Evaluation of a multifactorial approach to reduce the prevalence of pressure injuries in regional Australian acute inpatient care settings. Int Wound J 2018;15:95–105.
- Tayyib N, Coyer F, Lewis PA. Implementing a pressure ulcer prevention bundle in an adult intensive care. Intensive Crit Care Nurs 2016;37:27–36.
- Latimer SL, Deakin JL, Chaboyer WP, Gillespie BM. Feasibility and acceptability of implementing a patient education pressure injury prevention care bundle in acute care: an interview study. Wound Pract Res 2021;29:163–170.
- Wang I, Walker R, Gillespie BM. How well do perioperative practitioners implement pressure injury prevention guidelines? An observational study. Wound Pract Res 2018;28:24–33.
- Vélez-Díaz-Pallarés M, Lozano-Montoya I, Correa-Pérez A, et al. Non-pharmacological interventions to prevent or treat pressure ulcers in older patients: Clinical practice recommendations. The SENATOR-ONTOP series. Eur Geriatr Med 2016;7:142–148.
- Gould L, Stuntz M, Giovannelli M, et al. Wound Healing Society 2015 update on guidelines for pressure ulcers. Wound Repair Regen 2016;24:145–62.
- Australian Wounds Management Association. Pan Pacific clinical practice guideline for the prevention and management of pressure injury. Perth, WA: Cambridge Media; 2012.
- Haesler E, Le LK-D. Pressure injury prevention strategies: active support surfaces. JBI EBP Database; 2020.
- Lizarondo L. Pressure injury prevention strategies: seat cushion. JBI EBP Database; 2021.
- Abdulsalam A. Pressure injuries (burns): pressure relieving mattress. JBI EBP Database; 2021.
- Kunde L. Pressure injury prevention strategies: dressings. JBI EBP Database; 2021.
- Manuel B. Pressure injury prevention strategies: mobilizing and repositioning. JBI EBP Database; 2020.
- Xue J, Gu X, Jiang L, Cheng N, Huang Q, Wang M. Summary of the best evidence for postural change in the prevention of pressure injury in critically ill adult patients. Ann Palliat Med 2021;10:11445–11453.
- Wise V. Pressure injury prevention strategies: surgical procedures. JBI EBP Database; 2019.
- Haesler E. Pressure injuries: preventing heel pressure injuries with positioning. JBI EBP Database; 2017.
- New Zealand Wound Care Society. Guiding principles for pressure injury prevention and management in New Zealand. NZWCS; 2017.
- Posthauer ME, Banks M, Dorner B, Schols JMGA. The role of nutrition for pressure ulcer management: National Pressure Ulcer Advisory Panel, European Pressure Ulcer Advisory Panel, and Pan Pacific Pressure Injury Alliance white paper. Adv Skin Wound Care 2015;28:175–188.
- Black J, Clark M, Dealey C, et al. Dressings as an adjunct to pressure ulcer prevention: consensus panel recommendations. Int Wound J 2015;12:484–488.
- World Union of Wound Healing Societies. Role of dressings in pressure ulcer prevention. Wounds International; 2016.
- Lovegrove J, Fulbrook P, Miles S. International consensus on pressure injury preventative interventions by risk level for critically ill patients: a modified Delphi study. Int Wound J 2020;17:1112–1127.
- Stacey MC, Phillips SA, Farrokhyar F, Swaine JM. Reliability and measurement error of digital planimetry for the measurement of chronic venous leg ulcers: reliability of digital planimetry. Wound Repair Regen 2017;25:901–905.
- Cheng Q, Kularatna S, Lee XJ, Graves N, Pacella RE. Comparison of EQ-5D-5L and SPVU-5D for measuring quality of life in patients with venous leg ulcers in an Australian setting. Qual Life Res 2019;28:1903–1911.
- Ruseckaite R, Richards C, Rutherford C, et al. A conceptual framework of patient-reported outcomes in people with venous leg ulcers. Wound Repair Regen 2020;28:355–363.
- Weller C, Richards C, Turnour L, Green S, Team V. Vascular assessment in venous leg ulcer diagnostics and management in Australian primary care: clinician experiences. J Tissue Viability 2020;29:184–189.
- Edwards HE, Parker CN, Miller C, et al. Predicting delayed healing: the diagnostic accuracy of a venous leg ulcer risk assessment tool. Int Wound J 2018;15:258–265.
- Weller CD, Bouguettaya A, Team V, Flegg J, Kasza J, Jayathilake C. Associations between patient, treatment, or wound-level factors and venous leg ulcer healing: wound characteristics are the key factors in determining healing outcomes. Wound Repair Regen 2020;28:211–218.
- Finlayson K, Miaskowski C, Alexander K, et al. Distinct wound healing and quality of life outcomes in subgroups of patients with venous leg ulcers with different symptom cluster experiences. J Pain Symptom Manage 2017;53:871–879.
- Koh JH, Miller C, McKenzie G, McGuiness W. The relationship between periwound skin condition and venous leg ulcer chronicity. Wound Pract Res 2015;23:82–89.
- Parker C, Finlayson K, Edwards H. Predicting the likelihood of delayed healing and recurrence: development and reliability of venous leg ulcer risk assessment tools. Ostomy Wound Manag 2017;63.
- Ogrin R, Parker CN, Finlayson KJ, Anderson J, Edwards HE. Characteristics of people receiving wound care at home versus in a clinic. Collegian 2021;28:385–392.
- Stacey MC, Phillips SA, Farrokhyar F, Swaine JM. Evaluation of wound fluid biomarkers to determine healing in adults with venous leg ulcers: a prospective study. Wound Repair Regen 2019;27:509–518.
- Fernandez ML, Upton Z, Edwards H, Finlayson K, Shooter GK. Elevated uric acid correlates with wound severity. Int Wound J 2012;9:139–149.
- Ogrin R, Motin MA, Aliahmad B, Elder K, Anderson J, Kumar D. Can thermal imaging technique be used to predict the healing status of a venous leg ulcer? Int J Low Extrem Wounds 2023;22:85–92.
- Monshipouri M, Aliahmad B, Ogrin R, et al. Thermal imaging potential and limitations to predict healing of venous leg ulcers. Sci Rep 2021;11:13239.
- Kelechi TJ, Brunette G, Bonham PA, et al. 2019 guideline for management of wounds in patients with lower-extremity venous disease (LEVD): an executive summary. J Wound Ostomy Continence Nurs 2020;47:97–110.
- Wounds Canada. Best practice recommendations for the prevention and management of venous leg ulcers. Foundations of Best Practice for Skin and Wound Management; 2019.
- Couch KS, Corbett L, Gould L, Girolami S, Bolton L. The international consolidated venous ulcer guideline update 2015: process improvement, evidence analysis, and future goals. Ostomy Wound Manag 2017;63:42–46.
- Marston W, Tang J, Kirsner RS, Ennis W. Wound Healing Society 2015 update on guidelines for venous ulcers. Wound Repair Regen 2016;24:136–44.
- Neumann HAM, Cornu-Thenard A, Jünger M, et al. Evidence-based (S3) guidelines for diagnostics and treatment of venous leg ulcers. J Eur Acad Dermatol Venereol 2016;30:1843–1875.
- National Institute for Health and Care Excellence, editor. Guideline CG168: varicose veins in the legs: diagnosis and management. UK: NICE; 2013.
- The Australian Wound Management Association Inc & the New Zealand Wound Care Society Inc. Australian and New Zealand clinical practice guideline for prevention and management of venous leg ulcers. 2011.
- O’Donnell TF, Passman MA, Marston WA, et al. Management of venous leg ulcers: clinical practice guidelines of the Society for Vascular Surgery® and the American Venous Forum. J Vasc Surg 2014;60:3S–59S.
- Finlayson KJ, Courtney MD, Gibb MA, O’Brien JA, Parker CN, Edwards HE. The effectiveness of a four-layer compression bandage system in comparison to Class 3 compression hosiery on healing and quality of life for patients with venous leg ulcers: a randomised controlled trial. Int Wound J 2012;11:21–27.
- Weller CD, Evans SM, Staples MP, Aldons P, McNeil JJ. Randomized clinical trial of three-layer tubular bandaging system for venous leg ulcers. Wound Repair Regen 2012;20:822–829.
- Kapp S, Miller C, Elder K. The impact of providing product funding for compression bandaging and medical footwear on compression use, wound healing and quality of life. Int Wound J 2012;9:494–504.
- Miller C, Kapp, Newall N, et al. Predicting concordance with multilayer compression bandaging. J Wound Care 2011;20:101–112.
- Carolina DW, Catelyn R, Louise T, Victoria T. Patient explanation of adherence and non-adherence to venous leg ulcer treatment: a qualitative study. Front Pharmacol 2021;12.
- Wang E, Tang R, Walsh N, et al. Topical negative pressure therapy and compression in the management of venous leg ulcers: a pilot study. Wound Pract Res 2017;25:36–40.
- Jull A, Wadham A, Bullen C, Parag V, Waters J. Wool-derived keratin dressings versus usual care dressings for treatment of slow-healing venous leg ulceration: study protocol for a randomised controlled trial (Keratin4VLU). BMJ Open 2018;8:e020319.
- Miller C, McGuiness W, Wilson S, et al. Venous leg ulcer healing with electric stimulation therapy: a pilot randomised controlled trial. J Wound Care 2017;26:88–98.
- Thistlethwaite KR, Finlayson KJ, Cooper PD, et al. The effectiveness of hyperbaric oxygen therapy for healing chronic venous leg ulcers: a randomized, double-blind, placebo-controlled trial. Wound Repair Regen 2018;26:324–331.
- Weller CD, Martin C, Bouguettaya A, Underwood M, Barker AL, Haines T, Pouniotis D, Wolfe R. Once daily 300mg aspirin with compression versus compression alone in patients with chronic venous leg ulcers (ASPiVLU): a randomised, double-blinded, multicentre, placebo-controlled, clinical trial. J Tissue Viability 2021;30:509–516.
- Sadler GM, Wallace HJ, Stacey MC. Erratum to: oral doxycycline for the treatment of chronic leg ulceration. Arch Dermatol Res 2012;304:495–495.
- O’Brien J, Edwards H, Stewart I, Gibbs H. A home-based progressive resistance exercise programme for patients with venous leg ulcers: a feasibility study. Int Wound J 2013;10:389–396.
- O’Brien J, Finlayson K, Kerr G, Edwards H. Evaluating the effectiveness of a self-management exercise intervention on wound healing, functional ability and health-related quality of life outcomes in adults with venous leg ulcers: a randomised controlled trial. Int Wound J 2017;14:130–137.
- O’Brien J, Finlayson K, Kerr G, Shortridge-Baggett L, Edwards H. Using a theoretical approach to identify factors influencing adherence to an exercise programme for adults with venous leg ulcers. J Health Psychol 2018;23:691–700.
- O’Brien J, Finlayson K, Kerr G, Edwards H. The perspectives of adults with venous leg ulcers on exercise: an exploratory study. J Wound Care 2014;23:496.
- Smith D, Team V, Barber G, et al. Factors associated with physical activity levels in people with venous leg ulcers: a multicentre, prospective, cohort study. Int Wound J 2018;15:291–296.
- Kapp S, Miller C. The experience of self-management following venous leg ulcer healing. J Clin Nurs 2015;24:1300–1309.
- Weller CD, Richards C, Turnour L, Team V. Venous leg ulcer management in Australian primary care: patient and clinician perspectives. Int J Nurs Stud 2021;113:103774–103774.
- Weller C, Evans S. Venous leg ulcer management in general practice: Practice nurses and evidence based guidelines. Aust Fam Physician 2012;41:331.
- Weller CD, Bouguettaya A, Britt H, Harrison C. Management of people with venous leg ulcers by Australian general practitioners: an analysis of the national patient-encounter data. Wound Repair Regen 2020;28:553–560.
- Weller CD, Richards C, Turnour L, Patey AM, Russell G, Team V. Barriers and enablers to the use of venous leg ulcer clinical practice guidelines in Australian primary care: a qualitative study using the theoretical domains framework. Int J Nurs Stud 2020;103:103503–12.
- Weller CD, Richards C, Turnour L, Team V. Understanding factors influencing venous leg ulcer guideline implementation in Australian primary care. Int Wound J 2020;17:804–818.
- Cheng Q, Gibb M, Graves N, Finlayson K, Pacella RE. Cost-effectiveness analysis of guideline-based optimal care for venous leg ulcers in Australia. BMC Health Serv Res 2018;18:421–421.
- Barnsbee L, Cheng Q, Tulleners R, Lee X, Brain D, Pacella R. Measuring costs and quality of life for venous leg ulcers. Int Wound J 2019;16:112–121.
- Smith E, McGuiness W. Managing venous leg ulcers in the community: personal financial cost to sufferers. Wound Pract Res 2010;18:134–139.
- Lurie F, Lal BK, Antignani PL, et al. Compression therapy after invasive treatment of superficial veins of the lower extremities: Clinical practice guidelines of the American Venous Forum, Society for Vascular Surgery, American College of Phlebology, Society for Vascular Medicine, and International Union of Phlebology. J Vasc Surg 2019;7:17–28.
- Lizarondo L. Venous leg ulcer: compression therapy. JBI EBP Database; 2021.
- Fong E. Venous leg ulcers (compression therapy): patient adherence. JBI EBP Database; 2021.
- Swe KK. Leg ulcers (venous): intermittent pneumatic compression. JBI EBP Database; 2020.
- Nelson EA, Adderley U. Venous leg ulcers. BMJ Clin Evid 2016;2016.
- Fong E. Wound management: enzymatic debridement of venous leg ulcers. JBI EBP Database; 2017.
- Lizarondo L. Venous leg ulcer: debridement. JBI EBP Database; 2021.
- Ishaque S. Venous leg ulcers: protease-modulating matrix interventions. JBI EBP Database; 2021.
- Mattis P. Venous leg ulcers: choosing an appropriate dressing. JBI EBP Database; 2021.
- Nguyen DH. Venous leg ulcers: effectiveness of wound dressing treatment. JBI EBP Database; 2021.
- Soumya. Venous leg ulcers: electromagnetic therapy. JBI EBP Database; 2021.
- Soumya. Venous leg ulcers: therapeutic ultrasound. JBI EBP Database; 2021.
- Soumya. Venous leg ulcers: surgical interventions. JBI EBP Database; 2021.
- Wounds UK. Best practice statement. Compression hosiery (2nd ed). Wounds UK; 2015.
- Wounds International. Principles of compression in venous disease: a practitioner’s guide to treatment and prevention of venous leg ulcers. Wounds International; 2013.
- Wounds UK. Best practice statement: addressing complexities in the management of venous leg ulcers. Wounds UK; 2019.
- Wounds UK. Best practice statement: Improving holistic assessment of chronic wounds. Wounds UK; 2018.
- Finlayson K, Edwards H, Courtney M. Relationships between preventive activities, psychosocial factors and recurrence of venous leg ulcers: a prospective study. J Adv Nurs 2011;67:2180–2190.
- Finlayson K, Wu M-L, Edwards HE. Identifying risk factors and protective factors for venous leg ulcer recurrence using a theoretical approach: a longitudinal study. Int J Nurs Stud 2015;52:1042.
- Finlayson K, Parker CN, Miller C, Edwards H, Campbell J. Decreased mobility, lack of social support, haemosiderosis and use of antidepressant medications may predict recurrent venous leg ulcers within 12 months of healing: a prospective longitudinal study. Phlebology 2022;37:206–215.
- Stewart A, Edwards H, Finlayson K. Reflection on the cause and avoidance of recurrent venous leg ulcers: an interpretive descriptive approach. J Clin Nurs 2018;27:e931-e939.
- Finlayson KJ, Parker CN, Miller C, et al. Predicting the likelihood of venous leg ulcer recurrence: the diagnostic accuracy of a newly developed risk assessment tool. Int Wound J 2018;15:686–694.
- Kapp S, Miller C, Donohue L. The use and acceptability of devices for compression stocking application and removal. Wound Pract Res 2014;22:34–43.
- Kapp S, Miller C, Donohue L. The clinical effectiveness of two compression stocking treatments on venous leg ulcer recurrence: a randomized controlled trial. Int J Low Extrem Wounds 2013;12:189–198.
- Finlayson K, Edwards H, Courtney M. The impact of psychosocial factors on adherence to compression therapy to prevent recurrence of venous leg ulcers. J Clin Nurs 2010;19:1289–1297.
- Finlayson KJ, Edwards HE, Courtney MD. Venous leg ulcer recurrence: deciphering long-term patient adherence to preventive treatments and activities. Wound Pract Res 2014;22:91–97.
- Kapp S, Miller C, Donohue L. The leg ulcer prevention program: effectiveness of a multimedia client education package for people with venous leg ulcers. Wound Pract Res 2010;80:84–90.
- Walker J, Cullen M, Chambers H, Mitchell E, Steers N, Khalil H. Identifying wound prevalence using the mobile wound care program: identifying wound prevalence using the MWC program. Int Wound J 2014;11:319–325.
- Parker CN, Finlayson KJ, Edwards HE, MacAndrew M. Exploring the prevalence and management of wounds for people with dementia in long-term care. Int Wound J 2020.
- Frescos N. Assessment of pain in chronic wounds: a survey of Australian health care practitioners. Int Wound J 2018;15:943–949.
- Huimin K, Rowledge AM, Borzdynski CJ, et al. Reliability of a skin diagnostic device in assessing hydration and erythema. Adv Skin Wound Care 2017;30:452–459.
- Mehmood N, Hariz A, Templeton S, Voelcker NH. A flexible and low power telemetric sensing and monitoring system for chronic wound diagnostics. Biomed Eng Online 2015;14:1–17.
- Lim K, Free B, Sinha S. Modified TIME-H: a simplified scoring system for chronic wound management. J Wound Care 2015;24:415–419.
- Angel DE, Lloyd P, Carville K, Santamaria N. The clinical efficacy of two semi-quantitative wound-swabbing techniques in identifying the causative organism(s) in infected cutaneous wounds. Int Wound J 2011;8:176–185.
- Hussain MA, Rathnayake IU, Huygens F. The importance of anaerobic bacteria in non-healing wounds. Wound Pract Res 2016;24:218–223.
- Haesler E, Swanson T, Ousey K, Carville K. Clinical indicators of wound infection and biofilm: reaching international consensus. J Wound Care 2019;28:s4–s12.
- Swanson T, et al. Preventing and treating infection in wounds: translating evidence and recommendations into practice. Wounds International; 2020;11:82–87.
- Price K, Kennedy KJ, Rando TL, Dyer AR, Boylan J. Education and process change to improve skin health in a residential aged care facility. Int Wound J 2017;14:1140–1147.
- Wounds Canada. Best practice recommendations for the prevention and management of wounds. Foundations of Best Practice for Skin and Wound Management; 2017.
- Wounds Australia. Standards for wound prevention and management (3rd ed). Perth, WA: Cambridge Media; 2016.
- Association for the Advancement of Wound Care. Major recommendations for the International Consolidated Wound Infection Guideline (ICWIG); 2018. Available from: https://aawconline.memberclicks.net/resources
- Stallard Y. When and how to perform cultures on chronic wounds? J Wound Ostomy Continence Nurs 2018;45:179–186.
- Sivapuram MS. Wound specimens: techniques for identification of infections. JBI EBP Database; 2021.
- Podder V. Cavity wounds: assessment. JBI EBP Database; 2021.
- World Union of Wound Healing Societies. Position document. Advances in wound care: the triangle of wound assessment. Wounds International; 2016.
- Gupta S, Andersen C, Black J, et al. Management of chronic wounds: diagnosis, preparation, treatment, and follow-up. Wounds 2017;29:S19–S36.
- Wounds UK. Best practice statement: ankle brachial pressure index (ABPI) in practice. Wounds UK; 2019.
- Schultz G, Bjarnsholt T, James GA, et al. Consensus guidelines for the identification and treatment of biofilms in chronic nonhealing wounds. Wound Repair Regen 2017;25:744–757.
- World Union of Wound Healing Societies. Consensus document. Wound exudate: effective assessment and management. Wounds International; 2019.
- Upton Z, Wallace HJ, Shooter GK, et al. Human pilot studies reveal the potential of a vitronectin: growth factor complex as a treatment for chronic wounds. Int Wound J 2011;8:522–532.
- Karimi L, Miller CN, Donohue LA, et al. Resolving chronic wound pain using low intensity laser therapy (LILT): a proof of concept study. Wound Pract Res 2012;20:159–168.
- Bauer JD, Isenring E, Waterhouse M. The effectiveness of a specialised oral nutrition supplement on outcomes in patients with chronic wounds: a pragmatic randomised study. J Human Nutrition Dietetics 2013;26:452–458.
- Kapp S, Santamaria N. The financial and quality-of-life cost to patients living with a chronic wound in the community. Int Wound J 2017;14:1108–1119.
- Kapp S, Santamaria N. How and why patients self-treat chronic wounds. Int Wound J 2017;14:1269–1275.
- Kapp S, Santamaria N. The effect of self-treatment of wounds on quality of life: a qualitative study. J Wound Care 2020;29:260–268.
- Kapp S, Miller C, Santamaria N. The quality of life of people who have chronic wounds and who self-treat. J Clin Nurs 2018;27:182–192.
- Upton P, Cartwright M, Upton D. Living with chronic wounds: an exploration of adaptive and maladaptive coping strategies and their association with wellbeing. Wounds International; 2021;12:12–17.
- Price K, Kennedy KJ, Rando TL, Dyer AR, Boylan J. Education and process change to improve skin health in a residential aged care facility. Int Wound J 2017;14:1140–1147.
- Munro G. Barriers and enablers for effective implementation of the TIME framework for chronic wounds in a district nursing service. Wounds International; 2021;12:26–32.
- Parker CN, Finlayson KJ, Edwards HE, MacAndrew M. Exploring the prevalence and management of wounds for people with dementia in long-term care. Int Wound J 2020;17:650–659.
- Khalil H, Cullen M, Chambers H, Steers N, Walker J. Implementation of a successful electronic wound documentation system in rural Victoria, Australia: a subject of collaboration and community engagement. Int Wound J 2014;11:314–318.
- Baines C, McGuiness B. Improving wound management outcomes in residential aged care. Wound Pract Res 2014;22:124–130.
- Brain D, Tulleners R, Lee X, Cheng Q, Graves N, Pacella R. Cost-effectiveness analysis of an innovative model of care for chronic wounds patients. PloS One 2019;14:e0212366.
- Tulleners R, Brain D, Lee X, Cheng Q, Graves N, Pacella RE. Health benefits of an innovative model of care for chronic wounds patients in Queensland. Int Wound J 2019;16:334–342.
- Graves N, Finlayson K, Gibb M, O’Reilly M, Edwards H. Modelling the economic benefits of gold standard care for chronic wounds in a community setting. Wound Pract Res 2014;22:163–168.
- Barakat-Johnson M, Kita B, Jones A, Burger M, Airey D, Stephenson J, Leong T, Pinkova J, Frank G, Ko N, Kirk A, Frotjold A, White K, Coyer F. The viability and acceptability of a virtual wound care command centre in Australia. Int Wound J 2022.
- Edmondson M, Prentice J, Fielder K, Mulligan S. WoundsWest advisory service pilot: an innovative delivery of wound management. Wound Pract Res 2010;18:180–188.
- Hasegawa M, Inoue Y, Kaneko S, et al. Wound, pressure ulcer and burn guidelines – 1: guidelines for wounds in general (2nd ed). J Dermatol 2020;47:807–833.
- Deakin L. Chronically infected wounds: topical silver-sulphadiazine. JBI EBP Database; 2021.
- Deakin L. Nanocrystalline silver dressings for wounds (other than burns and donor sites). JBI EBP Database; 2021.
- Fong E. Chronically infected wounds: silver-releasing alginate dressings. JBI EBP Database; 2021.
- Oerlemans S. Wound infection: silver products and biofilms. JBI EBP Database; 2021.
- Sivapuram MS. Wound infection: iodophors and biofilms. JBI EBP Database; 2021.
- Whitehorn A. Wound infection biofilms: management. JBI EBP Database; 2021.
- Gyi AA. Autolytic debridement: efficacy and safety. JBI EBP Database; 2021.
- Podder V. Wound management: overview of mechanical debridement. JBI EBP Database; 2019.
- Podder V. Wound management: debridement – sharp and conservative sharp. JBI EBP Database; 2021.
- The Wound Healing and Management Node Group. Surgical and conservative sharp wound debridement for chronic wounds. Wound Pract Res 2011;19:29–31.
- Wound Healing and Management Node Group. Larval therapy for debridement of chronic wounds. Wound Pract Res 2012;20:197–199.
- Mathew S. High-powered hydrosurgical debridement of chronic wounds. JBI EBP Database; 2020.
- Fong E. Wound packing (older people): cavity wounds. JBI EBP Database; 2021.
- Haesler E, Deakin L. Biological dressings: collagen-based dressings. JBI EBP Database; 2021.
- Le LK-D. Wound care: wet-to-moist dressings. JBI EBP Database; 2021.
- Le LK-D. Lower limb wounds: wet-to-dry saline moistened gauze dressing. JBI EBP Database; 2021.
- Le LK-D. Wound dressings: foam with silver. JBI EBP Database; 2021.
- Sivapuram MS. Wound management: dressing – alginate. JBI EBP Database; 2021.
- Moola S. Pain management during wound care: wound care technique. JBI EBP Database; 2021.
- Moola S, Nnaji C. Biosynthetic skin substitues: burns/wounds care. JBI EBP Database; 2019.
- Romano M. Chronic wounds: topical negative pressure. JBI EBP Database; 2021.
- Sivapuram MS. Chronic wounds: hyperbaric oxygen therapy. JBI EBP Database; 2021.
- Oerlemans S. Wound management: hydrogen peroxide. JBI EBP Database; 2021.
- World Union of Wound Healing Societies. Position document. Management of biofilm. Wounds International; 2016.
- Wounds UK. Best practice statement: making day-to-day management of biofilm simple. Wounds UK; 2017.
- Murphy C, Atkin L, Swanson T, et al. Defying hard-to-heal wounds with an early antibiofilm intervention strategy: wound hygiene. J Wound Care 2020;29:S5–S10.
- International Wound Infection Institute. International consensus updated: wound infection in clinical practice – principles of best practice. Wounds International; 2016.
- World Union of Wound Healing Societies. The role of non-medicated dressings for the management of wound infection. Wounds International; 2020.
- Wounds UK. Best practice statement: antimicrobial stewardship strategies for wound management. Wounds UK; 2020.
- Keast DH, Bain K, Hoffmann C, et al. Managing the gap to promote healing in chronic wounds – an international consensus. Wounds International; 2020;11:58–63.
- Vig S, Dowsett C, Berg L, et al. Evidence-based recommendations for the use of negative pressure wound therapy in chronic wounds: steps towards an international consensus. J Tissue Viability 2011;20:S1–S18.
- Apelqvist J, Willy C, Fagerdahl AM, et al. EWMA document: negative pressure wound therapy. J Wound Care 2017;26:S1–S154.
- Kim PJ, Attinger CE, Constantine T, et al. Negative pressure wound therapy with instillation: international consensus guidelines update. Int Wound J 2020;17:174–186.
- World Union of Wound Healing Societies. Optimising wound care through patient engagement. Wounds International; 2020.
- Wounds Australia. Application of aseptic technique in wound dressing procedure: a consensus document (3rd ed); 2020.
- Lazzarini PA, Hurn SE, Kuys SS, et al. Direct inpatient burden caused by foot-related conditions: a multisite point-prevalence study. BMJ Open 2016;6:e010811-e010811.
- Kaminski MR, Raspovic A, McMahon LP, et al. Factors associated with foot ulceration and amputation in adults on dialysis: a cross-sectional observational study. BMC Nephrol 2017;18:293–293.
- Kaminski MR, Lambert KA, Raspovic A, et al. Risk factors for foot ulceration in adults with end-stage renal disease on dialysis: a prospective observational cohort study. BMC Nephrol 2019;20:423–423.
- Miller CN, Carville K, Newall N, et al. Assessing bacterial burden in wounds: comparing clinical observation and wound swabs. Int Wound J 2011;8:45–55.
- Bui UT, Finlayson K, Edwards H. Risk factors for infection in patients with chronic leg ulcers: a survival analysis. Int J Clin Pract 2018;72:e13263-n/a.
- Bui UT, Edwards H, Finlayson K. Identifying risk factors associated with infection in patients with chronic leg ulcers. Int Wound J 2018;15:283–290.
- Bui UT, Finlayson K, Edwards H. Validation of predictive factors for infection in adults with chronic leg ulcers: a prospective longitudinal study. J Clin Nurs 2020;29:1074–1084.
- Ogrin R, Woodward M, Sussman G, Khalil Z. Oxygen tension assessment: an overlooked tool for prediction of delayed healing in a clinical setting. Int Wound J 2011;8:437–445.
- Parker CN, Shuter P, Maresco-Pennisi D, et al. Implementation of the champions for skin integrity model to improve leg and foot ulcer care in the primary health care setting. J Clin Nurs 2019.
- Sen CK, Gordillo GM, Roy S, et al. Human skin wounds: a major and snowballing threat to public health and the economy. Wound Repair Regen 2009;17:763–771.
- Karimi L, Miller C, Kapp S, et al. Client perceptions of two types of antimicrobial dressings and compression bandaging. Wound Pract Res 2010;18:124–132.
- Harding K, Aldons P, Edwards H, et al. Effectiveness of an acellular synthetic matrix in the treatment of hard-to-heal leg ulcers. Int Wound J 2014;11:129–137.
- Purcell A, Buckley T, Fethney J, King J, Moyle W, Marshall AP. The effectiveness of EMLA as a primary dressing on painful chronic leg ulcers: effects on wound healing and health-related quality of life. Int J Low Extrem Wounds 2017;16:163–172.
- Do HTT, Edwards H, Finlayson K. Identifying relationships between symptom clusters and quality of life in adults with chronic mixed venous and arterial leg ulcers. Int Wound J 2016;13:904–911.
- Khalil H, Cullen M, Chambers H, McGrail M. Medications affecting healing: an evidence-based analysis. Int Wound J 2017;14:1340–1345.
- Edwards H, Finlayson K, Courtney M, Graves N, Gibb M, Parker C. Health service pathways for patients with chronic leg ulcers: identifying effective pathways for facilitation of evidence based wound care. BMC Health Serv Res 2013;13:86.
- Edwards H, Finlayson K, Maresco-Pennisi D, Gibb M, Parker C, Graves N. The long and winding road: health services for clients with chronic leg ulcers in the community. Wound Pract Res 2014;22:226–233.
- Drovandi A, Fernando ME, Singh TP, Woolley T, Golledge J. Opinions of vascular surgeons and podiatrists in Australia and New Zealand on the use of hyperbaric oxygen therapy for lower limb ulcers. BMJ Open Diabetes Res Care 2020;8.
- Owaya A. Maggot debridement therapy: chronic lower extremities wounds. JBI EBP Database; 2021.
- Watts R, Rayner R, Solomon T. Leg ulcers: managing leg ulcers – venous, arterial, mixed – with negative pressure wound therapy. JBI EBP Database; 2021.
- National Institute for Health and Care Excellence. Leg ulcer infection: antimicrobial prescribing. UK: NICE; 2020.
- Alexander SJ. An intense and unforgettable experience: the lived experience of malignant wounds from the perspectives of patients, caregivers and nurses. Int Wound J 2010;7:456–465.
- Tsichlakidou A, Govina O, Vasilopoulos G, Kavga A, Vastardi M, Kalemikerakis I. Intervention for symptom management in patients with malignant fungating wounds – a systematic review. J BUON 2019;24:1301–1308.
- Finlayson K, Teleni L, McCarthy AL. Topical opioids and antimicrobials for the management of pain, infection, and infection-related odors in malignant wounds: a systematic review. Oncol Nurs Forum 2017;44:626–632.
- de Castro DLV, Santos VLCG. Odor management in fungating wounds with metronidazole. J Hosp Palliat Nurs 2015;17:73–79.
- da Costa Santos CM, de Mattos Pimenta CA, Nobre MRC. A systematic review of topical treatments to control the odor of malignant fungating wounds. J Pain Symptom Manage 2010;39:1065–1076.
- Ramasubbu DA, Smith V, Hayden F, Cronin P. Systemic antibiotics for treating malignant wounds. Cochrane Database Syst Rev 2017;8:CD011609.
- Adderley UJ, Holt IG. Topical agents and dressings for fungating wounds. Cochrane Database Syst Rev 2014.
- Firmino F, Villela-Castro DL, Santos Jd, Conceição de Gouveia Santos VL. Topical management of bleeding from malignant wounds caused by breast cancer: a systematic review. J Pain Symptom Manage 2021;61:1278–1286.
- Tilley CP, Fu MR, Qiu JM, et al. The microbiome and metabolome of malignant fungating wounds: a systematic review of the literature from 1995 to 2020. J Wound Ostomy Continence Nurs 2021;48:124–135.
- Wounds Canada. Best practice recommendations for the prevention and management of peripheral arterial ulcers. Foundations of Best Practice for Skin and Wound Management; 2020.
- Federman DG, Ladiiznski B, Dardik A, et al. Wound Healing Society 2014 update on guidelines for arterial ulcers. Wound Repair Regen 2016;24:127–35.
- Broderick C, Pagnamenta F, Forster R. Dressings and topical agents for arterial leg ulcers. Cochrane Database Syst Rev 2020;2020.
- Wen Y, Meng L, Gao Q. Autologous bone marrow cell therapy for patients with peripheral arterial disease: a meta-analysis of randomized controlled trials. Expert Opin Biol Ther 2011;11:1581–1589.
- Yerramilli A, Tay EL, Stewardson AJ, et al. The location of Australian Buruli ulcer lesions – implications for unravelling disease transmission. PLoS Negl Trop Dis 2017;11:e0005800.
- Venugopal SS, Yan W, Frew JW, et al. A phase II randomized vehicle-controlled trial of intradermal allogeneic fibroblasts for recessive dystrophic epidermolysis bullosa. J Am Acad Dermatol 2013;69:898-908.e7.
- Lubowski D. Randomised, double-blind, placebo-controlled study of the, efficacy and safety metronidazole ointment in facilitating resolution of non-healing pilonidal sinus. Australian New Zealand Clinical Trials Registry; 2018.
- Lam G, Ross FL, Chiu ES. Nonhealing ulcers in patients with tophaceous gout: a systematic review. Adv Skin Wound Care 2017;30:230–237.
- Reinar LM, Forsetlund L, Lehman LF, Brurberg KG. Interventions for ulceration and other skin changes caused by nerve damage in leprosy. Cochrane Database Syst Rev 2019.
- Parker CN, Francis A, Finlayson K. Methods for chronic wound research – a scoping systematic review of the recommendations, guidelines and standards. Wound Pract Res 2019;27:62–107.
- Sonnad S, Goldsack J, Mohr P, Tunis S. Methodological recommendations for comparative research on the treatment of chronic wounds. J Wound Care 2013;22:470–80.
- Lazzarini PA, Pacella R, Armstrong D, Van Netten J. Diabetes-related lower-extremity complications are a leading cause of the global burden of disability. Diabet Med 2018;35:1297–9.
- Zhang Y, van Netten JJ, Baba M, et al. Diabetes-related foot disease in Australia: a systematic review of the prevalence and incidence of risk factors, disease and amputation in Australian populations. J Foot Ankle Res 2021;14:8.
- Lazzarini PA, van Netten JJ, Fitridge RA, et al. Pathway to ending avoidable diabetes-related amputations in Australia. Med J Aust 2018;209:288–90.
- Weller CD, Bouguettaya A, Britt H, Harrison C. Management of people with venous leg ulcers by Australian general practitioners: an analysis of the national patient-encounter data. Wound Repair Regen 2020;28:553–560.
- Edwards H, Finlayson K, Courtney M, Graves N, Gibb M, Parker C. Health service pathways for patients with chronic leg ulcers: identifying effective pathways for facilitation of evidence based wound care. BMC Health Serv Res 2013;13:86.
- Cheng Q, Gibb M, Graves N, Finlayson K, Pacella RE. Cost-effectiveness analysis of guideline-based optimal care for venous leg ulcers in Australia. BMC Health Serv Res 2018;18:421.
- Lee M, van Netten JJ, Sheahan H, Lazzarini PA. Moderate-to-vigorous-intensity physical activity observed in people with diabetes-related foot ulcers over a one-week period. J Diabetes Sci Technol 2019;13:827–835.
- Ahmed MU, Tannous WK, Agho KE, Henshaw F, Turner D, Simmons D. Social determinants of diabetes-related foot disease among older adults in New South Wales, Australia: evidence from a population-based study. J Foot Ankle Res 2021;14:65.
- Fernando ME, Crowther RG, Lazzarini PA, et al. Plantar pressures are elevated in people with longstanding diabetes-related foot ulcers during follow-up. PloS one 2017;12:e0181916.
- Fernando ME, Crowther RG, Lazzarini PA, et al. Plantar pressures are higher in cases with diabetic foot ulcers compared to controls despite a longer stance phase duration. BMC Endocr Disord 2016;16:1–10.
- Lazzarini PA, Hurn SE, Kuys SS, et al. The silent overall burden of foot disease in a representative hospitalised population. Int Wound J 2017;14:716–728.
- Lazzarini PA, Hurn SE, Kuys SS, et al. Direct inpatient burden caused by foot-related conditions: a multisite point-prevalence study. BMJ Open 2016;6.
- Baba M, Davis WA, Norman PE, Davis TME. Temporal changes in the prevalence and associates of foot ulceration in type 2 diabetes: the Fremantle diabetes study. J Diabetes Complicat 2015;29:356–361.
- Kaminski M, Frescos N, Tucker S. Prevalence of risk factors for foot ulceration in patients with end-stage renal disease on haemodialysis. Intern Med J 2012;42:e120-e128.
- Perrin BM, Gardner MJ, Kennett SR. The foot-health of people with diabetes in a regional Australian population: a prospective clinical audit. J Foot Ankle Res 2012;5:6–6.
- Fernando ME, Crowther RG, Lazzarini PA, et al. Gait in people with nonhealing diabetes-related plantar ulcers. Physical Therapy 2019;99:1602–1615.
- Zaine N, Burns J, Vicaretti M, Fletcher J, Begg L, Hitos K. Characteristics of diabetic foot ulcers in Western Sydney, Australia. J Foot Ankle Res 2014;7:39.
- Corbett C, Jolley J, Barson E, Wraight P, Perrin B, Fisher C. Cognition and understanding of neuropathy of inpatients admitted to a specialized tertiary diabetic foot unit with diabetes-related foot ulcers. Int J Low Extrem Wounds 2019;18:294–300.
- Whitmont K, Fulcher G, Reid I, et al. Low circulating protein C levels are associated with lower leg ulcers in patients with diabetes. BioMed Research International 2013;2013.
- Featherston J, Wijlens AM, van Netten JJ. Is a left-to-right >2.2˚C difference a valid measurement to predict diabetic foot ulceration in people with diabetes and a history of diabetic foot ulceration? Int J Low Extrem Wounds 2021.
- Beulens JWJ, Yauw JS, Elders PJM, et al. Prognostic models for predicting the risk of foot ulcer or amputation in people with type 2 diabetes: a systematic review and external validation study. Diabetologia 2021;64:1550–1562.
- Pena G, Kuang B, Cowled P, et al. Micronutrient status in diabetic patients with foot ulcers. Adv Wound Care 2020;9:9–15.
- Jia L, Parker CN, Parker TJ, et al. Incidence and risk factors for developing infection in patients presenting with uninfected diabetic foot ulcers. PloS One 2017;12.
- Hamilton EJ, Davis WA, Siru R, Baba M, Norman PE, Davis TME. Temporal trends in incident hospitalization for diabetes-related foot ulcer in type 2 diabetes: the Fremantle diabetes study. Diabetes Care 2021;44:722–730.
- Rhou YJJ, Henshaw FR, McGill MJ, Twigg SM. Congestive heart failure presence predicts delayed healing of foot ulcers in diabetes: an audit from a multidisciplinary high-risk foot clinic. J Diabetes Complicat 2015;29:556–562.
- Zhang Y, Cramb S, McPhail SM, et al. Multiple factors predict longer and shorter time-to-ulcer-free in people with diabetes-related foot ulcers: survival analyses of a large prospective cohort followed-up for 24-months. Diabetes Res Clin Pract 2022;185:109239.
- Pena G, Kuang B, Edwards S, Cowled P, Dawson J, Fitridge R. Factors associated with key outcomes in diabetes related foot disease: a prospective observational study. Eur J Vasc Endovasc Surg 2021;62:233–240.
- Brookes JDL, Jaya JS, Tran H, et al. Broad-ranging nutritional deficiencies predict amputation in diabetic foot ulcers. Int J Low Extrem Wounds 2020;19:27–33.
- Jeyaraman K, Berhane T, Hamilton M, Chandra AP, Falhammar H. Amputations in patients with diabetic foot ulcer: a retrospective study from a single centre in the Northern Territory of Australia. ANZ J Surg 2019;89:874–879.
- Rodrigues BT, Vangaveti VN, Malabu UH. Prevalence and risk factors for diabetic lower limb amputation: a clinic-based case control study. J Diabetes Res 2016;2016.
- Tehan PE, Hawes MB, Hurst J, Sebastian M, Peterson BJ, Chuter VH. Factors influencing lower extremity amputation outcomes in people with active foot ulceration in regional Australia: a retrospective cohort study. Wound Repair Regen 2022;30:24–33.
- Jeyaraman K, Berhane T, Hamilton M, Chandra AP, Falhammar H. Mortality in patients with diabetic foot ulcer: a retrospective study of 513 cases from a single centre in the Northern Territory of Australia. BMC Endocr Disord 2019;19:1.
- Rosi LM, Jones AS, Topliss DJ, Bach LA. Demographics and outcomes of inpatients with diabetic foot ulcers treated conservatively and surgically in a metropolitan hospital network. Diabetes Res Clin Pract 2021;175:108821.
- Banks M, Bauer J, Graves N, Ash S. Malnutrition and pressure ulcer risk in adults in Australian health care facilities. Nutrition 2010;26:896–901.
- Ness SJ, Hickling DF, Bell JJ, Collins PF. The pressures of obesity: the relationship between obesity, malnutrition and pressure injuries in hospital inpatients. Clin Nutr 2018;37:1569–1574.
- Woodward T, Josephson C, Ross L, et al. Hospital acquired malnutrition (HAM): incidence and contributors across five Australian public hospitals over 3 years. Sth Afr J Clin Nutr 2021;34:42.
- Dunk AM, Gardner A. Body shape: a predictor for pressure injury risk. Wound Pract Res 2016;24:92–98.
- Chaboyer W, Mills PM, Roberts S, Latimer S. Physical activity levels and torso orientations of hospitalized patients at risk of developing a pressure injury: an observational study. Int J Nurs Pract 2015;21:11–17.
- McCarthy A, Robertson V, Roberts K, Lannin NA. Audit of sitting time in older inpatients and implications for pressure-injury management. Phys Occup Ther Geriatr 2019;37:183–195.
- Loorham-Battersby CM, McGuiness W. Heel damage and epidural analgesia: is there a connection? J Wound Care 2011;20:28.
- McEvoy L, Richter M, Chen T, et al. Frailty among older surgical patients and risk of hospital acquired adverse events: the south-western Sydney frailty and nurse sensitive indicators study. J Clin Nurs 2022.
- Barakat-Johnson M, Barnett C, Lai M, Wand T, White K. Incontinence, incontinence-associated dermatitis, and pressure injuries in a health district in Australia: a mixed-methods study. J Wound Ostomy Continence Nurs 2018;45:349–355.
- Hampson J, Green C, Stewart J, et al. Impact of the introduction of an endotracheal tube attachment device on the incidence and severity of oral pressure injuries in the intensive care unit: a retrospective observational study. BMC Nurs 2018;17:1–1.
- Wright KM, Van Netten Y, Dorrington CA, Hoffman GR. Pressure injury can occur in patients undergoing prolonged head and neck surgery. J Oral Maxillofac Surg 2014;72:2060–2065.
- Kumta N, Coyer F, David M. Perioperative factors and pressure ulcer development in postoperative ICU patients: a retrospective review. J Wound Care 2018;27:475–485.
- Brimelow RE, Wollin JA. The impact of care practices and health demographics on the prevalence of skin tears and pressure injuries in aged care. J Clin Nurs 2018;27:1519–1528.


