Volume 31 Number 4
Wound healing activity of Withania leaf paste and its gel in Wistar albino rats – an experimental evaluation
Shreya D Bhosale, Jagadeesh Mayya A, Hemanth Gowda K, Vijay Kumar, Pramod Yadav, Galib R, Pradeep K Prajapati
Keywords wound, Ashwagandha, Ropana, Withania somnifera, gel
For referencing Bhosale SD et al. Wound healing activity of Withania leaf paste and its gel in Wistar albino rats – an experimental evaluation. Wound Practice and Research 2023; 31(4):182-189.
DOI
10.33235/wpr.31.4.182-189
Submitted 28 June 2023
Accepted 9 August 2023
Abstract
Background During the past few decades, wound healing has gained major attention. The management of wounds depends on various factors; as such, healing poses numerous challenges. The available treatment approaches for wound healing generally include the use of anti-infective agents. The use of traditional medicines for various pathologies is increasing gradually and its use in the management of wounds is not exceptional.
Aim The present study is carried out to investigate the wound healing activity of Ashwagandha (Withania somnifera (L.) Dunal) leaves in the form of paste and gel in excision and incision wound models on Wistar strain albino rats.
Methods The wound healing effect was assessed considering the percentage of wound closure and epithelialisation period in the excision wound model, while the tensile strength of the healed wounds was assessed through the incision wound model. Excision and incision wounds were inflicted on the dorsal surface of Wistar albino rats by following standard protocols.
Result and conclusion Ashwagandha in both the forms shows a significant increase in percentage wound contraction, reduction in the period of epithelialisation, and increase in tensile strength of skin when compared with the normal control group. The results are encouraging and wider studies may be conceptualised to evaluate the efficacy in different wound models.
Introduction
Any damage to the integrity of the skin is called a wound. It has been defined as “disruption of normal anatomic structures and function”1. In everyday pathology, wounds remain a challenging clinical problem, with early and late complications presenting a frequent cause of morbidity and mortality2.
There are 1.51–2.21 chronic wounds per 1000 people worldwide, according to estimates3. The importance of effective wound care cannot be underestimated and wound care should always promote normal healing and prevent complications. In times of scientific development, wound healing is an un-met therapeutic challenge among the medical society since wound assessment and management is a complex procedure including several factors playing a major role in the healing process4.
The improved wound healing process can be performed by shortening the time needed for healing or countering the complications. Antibiotics, antiseptics, de-sloughing agents, extracts, etc. have been used in order to hasten wound healing. However, in every region of the world, antibiotic resistance is increasing to dangerously high levels. Our ability to cure widespread infectious diseases is being threatened by the emergence and global dissemination of new resistance mechanisms. Therefore, it is necessary to discover and develop plants or combinations derived from plants for the management and therapy of wound healing.
There is increasing interest to use medicinal plants in wound healing because of comparatively lower side effects. Many pre-clinical studies have shown that medicinal plants like cinnamon (Cinnamomum verum), neem (Azadirachta indica), bael (Aegle marmelos) etc. improve wound healing in diabetic, infected and chronic open wounds5. The classical texts in Ayurveda also considered the topical applications (Bahir parimarjana) in multiple instances and considered such treatment modalities under three types of treatment strategies6. Acharya Charaka has mentioned 36 types of Upakramas (treatment approaches), while Sushruta referred 60 exclusively for the management of Vrana (wounds)7,8. Among them, Kalka (pastes) is one that is the most effective form that also performs Shodhana (cleansing) and Ropana (healing)9. A huge number of formulations from herbal origin have been described in Ayurveda literature for the management of Vrana.
Ashwagandha (Withania somnifera (L.) Dunal), a commonly used, most popular herb in Ayurvedic medicine, is familiarly known for its anti-microbial, anti-oxidant, anti-inflammatory, anti-tumour, anti-stress, neuro-protective, cardio-protective, hepato-protective and anti-diabetic properties10. More recently, Ashwagandha is being used to treat ulcers and bacterial infections11.
Studies suggest that the plant is a promising wound healing agent that warrants further investigation12. To date, no reports are available on the wound healing effects of Ashwagandha leaves when applied topically. Thus, the present study is planned to study the wound healing activity of Ashwagandha leaves in the form of Kalka (paste) and gel on experimentally induced excision and incision wound healing models in Wistar albino rats.
Methods
Plant material and preparation of the formulation
Fresh Ashwagandha leaves were collected from the campus of All India Institute of Ayurveda, New Delhi and the authentication of the drug occurred in the working institute (RRDR/AIIA/phg./80, 13/06/2022). Aerosil 200 and sesame oil were procured from the local market, Khari baoli, New Delhi. All chemicals used in the study were of analytical grade. For preparing Ashwagandha gel, the required ingredients were taken in proportion as listed in Table 1.
Table 1. Formulation composition of Ashwagandha gel
Fresh Ashwagandha leaves were collected, cleaned with potable water, and wiped to remove water. A batch of leaves was ground in a mixer grinder to prepare a fine paste. Another batch of the leaves was ground, and juice was extracted by straining it through a clean cotton cloth. Sesame oil was collected into a stainless steel container, and processed in the presence of the prepared paste and juice by following the classical guidelines13. After getting the chief desired characteristics, the heating of the oil was stopped and it was filtered through the cotton cloth when the contents were at 80˚C temperature, and stored in airtight containers for further process. To convert the medicated oil into a gel, the oil was triturated by the addition of 6% Aerosil 200 in a porcelain mortar until its formation into a gel.
Animals and ethics
The experimental protocols were approved by the Institutional Animal Ethics Committee of the institution (ALNRAO/IAEC/Res No 13/2022-23, 22 October 2022) in accordance with the guideline formulated by CCSEA (Committee for the Control and Supervision on Experiments on Animals), India.
Wistar strain albino rats of either sex weighing 220±20g were used for the experiments. The animals were obtained from the animal house attached to ALN Rao Memorial Ayurvedic Medical College, Koppa, Karnataka. They were housed at standard husbandry conditions of temperature (22±03˚C) with constant humidity (50–70%) and 12 hours of light and dark cycles. Animals were fed with AMRUT brand rat pellet feed supplied by Krishna Valley Aggrotech LLP, India. The drinking water was given ad libitum in polypropylene bottles with stainless steel sipper tubes. All selected animals were kept under acclimatisation for 15 days before experimentation.
Animal grouping and application of medicines
For the evaluation of formulation on excision and incision wounds, Wistar strain albino rats were divided into four groups, each consisting of six rats.
- Group I served as normal control (NC).
- Group II was treated with Ashwagandha paste made in distilled water (AK).
- Group III was treated with Ashwagandha gel (AG).
- Group IV was treated with Betadine® ointment and served as a standard treated group (BT)14.
Equal quantities (0.5g) of ointments were applied once daily after cleaning the wounds with normal saline. The wounds were kept without any dressing during the entire study.
Evaluation of wound healing activity
Wound healing models reported in the literature for excision (Morton & Malone, 1972)15 and incision (Ehrlich & Hunt 1968)16 wounds were employed in the present study.
Excision wound model
The experimental animals were anaesthetised with Isoflurane. The dorsal fur of the animals was shaved with an electric clipper without causing any abrasions and cleaned with 70% v/v alcohol. A full thickness of the excision wound of a circular area of 1cm in diameter and 2mm depth was created on the dorsal thoracic central region 5cm away from the ears. The approximate area thus formed was 80mm2. After achieving full haemostasis, animals were placed in individual cages on a normal diet and were divided in four groups as outlined above.
External application of Ashwagandha paste, Ashwagandha gel and Betadine® was started in different groups from the day of surgery (0th day) (Figures 1–3). On every post-wounding day, wounds were cleaned with normal saline, and a trial drug was applied to the trial group animals once a day for a total duration of 16 days. The wound area was assessed by tracing the wound on 1st, 4th, 8th, 12th and 16th post-wounding days using a transparent OHP sheet and a permanent marker (Figure 4). The point at which the eschar fell off without any residual open wound was considered as period of epithelialisation. The percentage reduction in wound size was calculated using the following equation:
Wound size reduction (%) = [A0 – At] / A0 × 100
Where A0 and At are initial wound area and wound area after time interval (t)17.
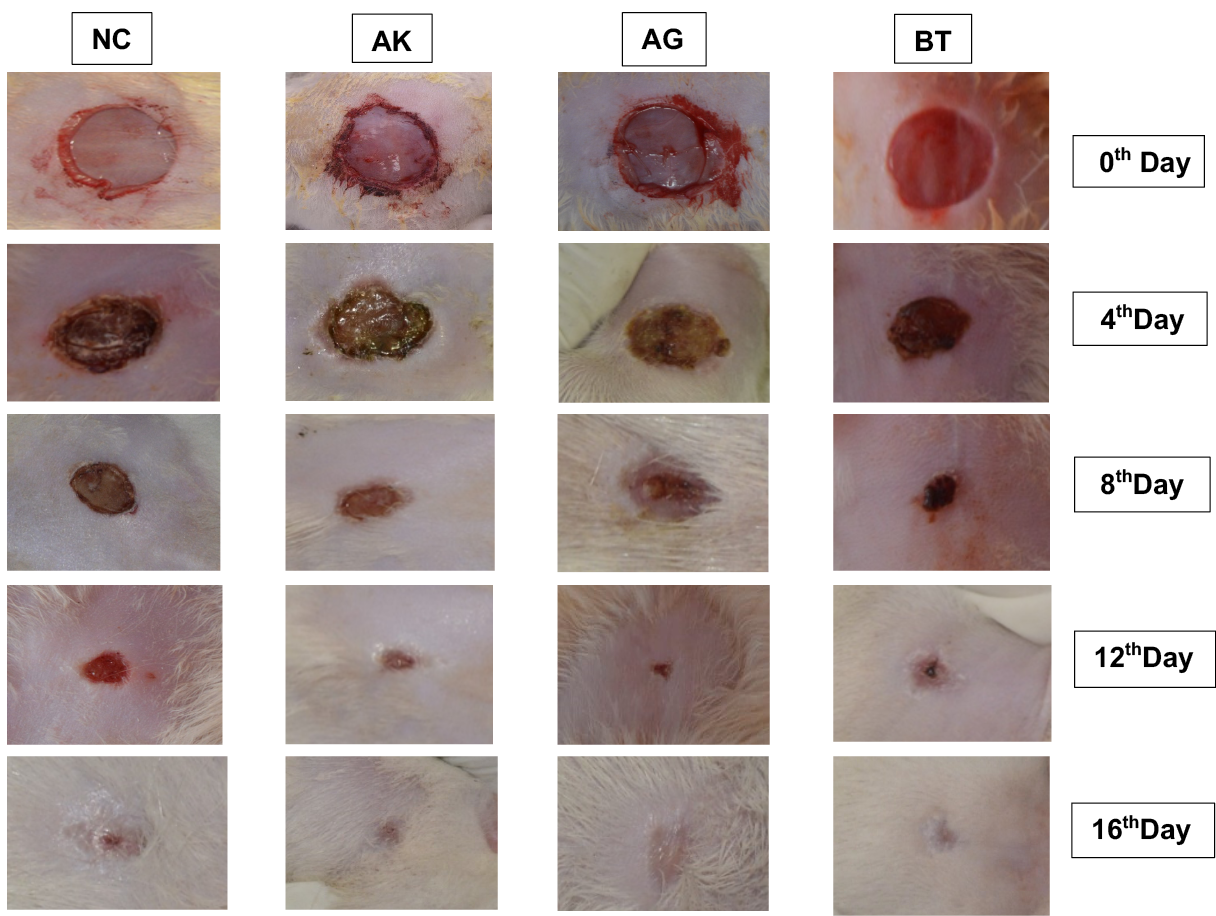
Figure 4: Photographic representation of wound contraction area on different post-excision days of Normal control (NC), Ashwagandha Paste (AK), Ashwagandha Gel (AG) and Standard control (BT) treated rats.
Incision wound model
The effect of test drugs on incision wound was evaluated by noting the effect on the tensile strength of sutured skin. Incision wounds were made in Isoflurane-anaesthetised Wistar albino rats. The animals were placed in the prone position and the area was cleaned with a 70% alcohol swab. A midline incision of 4cm was made through the skin and cutaneous muscles on the vertebral column in anaesthetised rats and the incision was closed with the help of three interrupted sutures at 1cm apart. Then animals were kept in separate cages. They were maintained on a normal diet and water (ad libitum). The animals were divided into four groups as outlined above.
Drugs were administered by local application respectively until the 9th day, starting from the day of operation. The sutures were opened on the 8th day and wound breaking strength was tested on the 10th post-wounding day18. The anaesthetised animals were secured to the dissection tray in the prone position and a line was drawn on either side of the wound 3mm away from the suture line. The line on either side of the suture was gripped with forceps one at each end opposed to each other. One end of the forceps was supported firmly, whereas the other was connected to a freely suspended lightweight measuring jar. Water was slowly added continuously till the wound began to gap. As soon as the wound gapping appeared, the addition of water was determined and noted as a measure of breaking strength in grams.
Statistical analysis
The data are expressed as Mean ± SEM for six rats per experimental group. One-way analysis of variance (ANOVA) was used to compare the mean values of quantitative variables among the groups by using GraphPad Prism 9.5.1 software to determine significant differences between groups at p<0.05.
Results
On the 16th day, all drug-treated groups including Ashwagandha paste (96.98±0.8173%) (AK), Ashwagandha gel (98.32±0.1682%) (AG) and Betadine® ointment (BT) treated (98.36±0.6129%) groups showed a highly significant increase in the percentage of wound contraction when compared with the normal control (NC) rats (93.08±0.5015%). The percentage of wound contraction of the AG group was higher in comparison to the AK group (Graph 1).
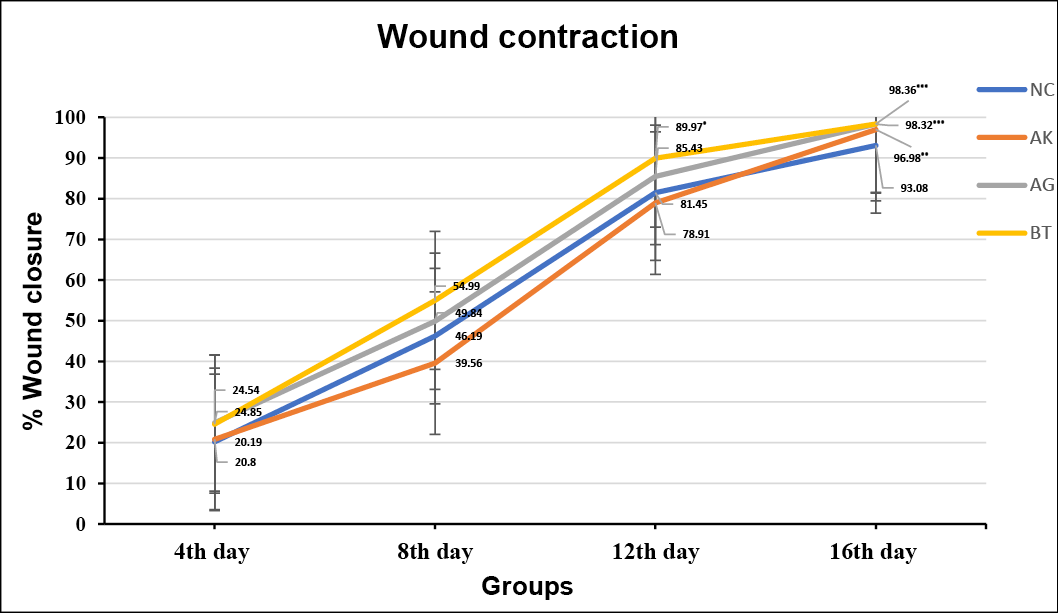
Graph 1. Overall effect of test drugs on percentage contraction of excision wound in rats (n=6)
Normal control (NC), Ashwagandha paste (AK), Ashwagandha gel (AG), Betadine® ointment / standard control (BT)
Data: Mean ± SEM; *p<0.05 **p<0.01 ***p<0.001, compared to normal control group (ANOVA test)
Ashwagandha gel and Betadine® ointment have pronounced activity on wound contraction in Wistar albino rats and showed complete epithelialisation on the 16th day, while the AK-treated group also took approximately 16 days for complete healing of the wound and were significant in comparison to the NC group. Graph 2 shows that, compared to the NC group, there is a 5.7%, 7.6% and 6.7% decrease in the period of complete epithelisation in AK, AG and BT groups respectively.
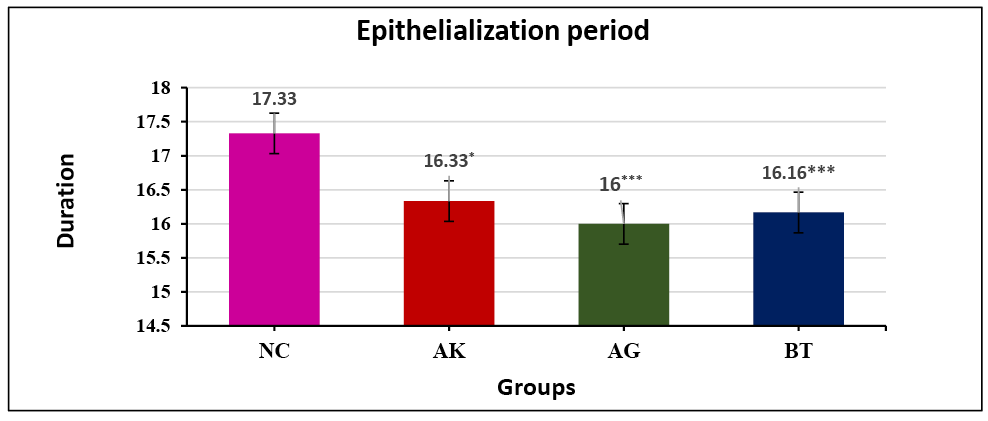
Graph 2. Effect of test drugs on complete epithelialisation period in excision wound healing activity (n=6)
Normal control (NC), Ashwagandha paste (AK), Ashwagandha gel (AG), Betadine® ointment / standard control (BT)
Data: Mean ± SEM; *p<0.05 **p<0.01 ***p<0.001, compared to NC group (ANOVA test)
In the incision wound healing model, the AK-treated group showed a significant increase in the tensile strength of healed wounded skin when compared with the NC group, while a highly significant increase in the tensile strength was observed in the AG-treated group and was highest among all the drug-treated groups when compared with the NC group. Graph 3 shows that, compared to the NC group, there is a 19.47%, 44.14% and 38.95% increase in tensile strength of healed skin in the AK, AG and BT groups respectively.
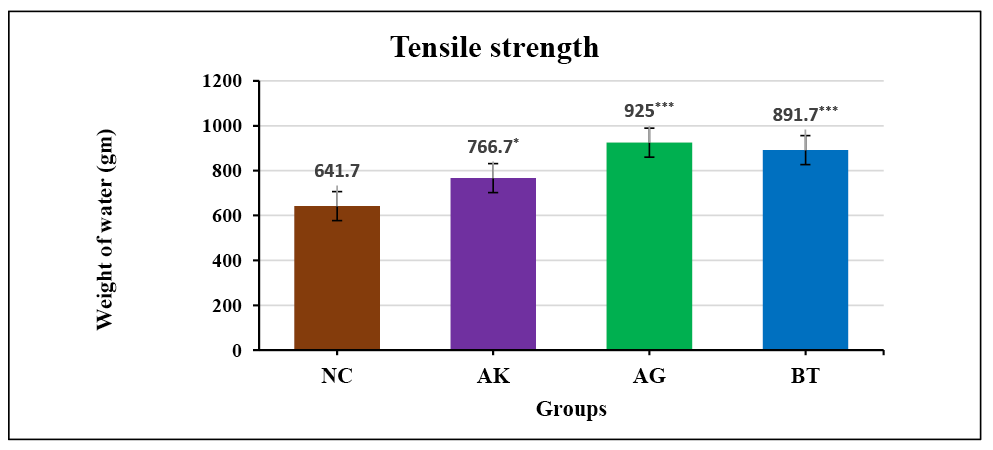
Graph 3. Effect of test drugs on tensile strength of healed skin in incision wound healing (n=6)
Normal control (NC), Ashwagandha paste (AK), Ashwagandha gel (AG), Betadine® ointment / standard control (BT)
Data: Mean ± SEM; *p<0.05 **p<0.01 ***p<0.001, compared to NC group (ANOVA test)
Discussion
Wound healing is the physiological response to the tissue injury that results in the replacement of destroyed tissue by living tissue and thus restoration of tissue integrity. It involves a highly coordinated cascade of cellular responses encompassing the interaction of many cell types over long periods of time. Wound contracture occurs throughout the healing process and it mainly depends on the extent of tissue damage, its repairing ability, and the general state of the health of the tissue. The wound healing process can be broadly categorised into three stages: the inflammatory phase comprising the establishment of haemostasis and inflammation; the proliferative phase consisting of granulation, contraction and epithelialisation; and finally the remodelling phase which ultimately determines the strength and the appearance of the healed tissue19.
The goal of the inflammatory response is to prevent microbial wound infection and promote wound closure. Inflammation’s early stage seeks to restore tissue integrity. Further, fibroblasts are infiltrated at the wound site initiating the proliferative phase. Within the first 3 days following tissue damage, collagen and fibronectin synthesis are increased. Collagen is deposited by fibroblasts in the dermal wound area, modulating the healing process. Cytokines are also secreted by fibroblast which release keratinocyte cells to the site of injury. Keratinocytes help the wound to re-epithelialise. Keloid and hypertrophic scars develop when there is an imbalance between excessive collagen production and reduced collagen catabolism. Newly formed blood vessels continue to mature in this stage, forming a functional vascular network20. As such, wound contraction, collagenation and epithelialisation become the crucial phases of wound healing21.
In the present investigation, from the percentage of wound contraction, topically applied Withania paste and its gel significantly accelerated wound healing and improved the quality of vascularity of granulation tissue, possibly by increasing fibroblast proliferation, maturation of collagen content on one hand and decreasing collagenase activity on the other22. This could be possibly related to the anti-inflammatory23, anti-oxidant24 and antimicrobial properties25 of the drug.
The time required for complete epithelialisation of the excision wound is an important parameter to assess the wound healing process. On the 16th day, a significant reduction in wound size was observed in both the AK and AG-treated groups. Further, a rapid wound closure rate was observed in the AG group when compared to the NC group. The enhanced rate of wound contraction and a significant reduction in healing time in this group might be due to enhanced epithelialisation.
The leaves of Ashwagandha have Shodhana (purifying) and Ropana (~healing) properties26. Ashwagandha has known for its anti-microbial and anti-bacterial properties27. Ashwagandha leaves have remarkable antioxidant properties and have the highest ascorbic acid and anthocyanin content. Leaves possess significant anti-bacterial properties against Gram-negative organisms, particularly against Salmonella typhi24, Escherichia coli, Pseudomonas fluorescens, Bacillus subtilis, Staphylococcus aureus28 and Pseudomonas aeruginosa29. These properties may assist in cleaning the wound and help to inhibit the growth of microorganisms; therefore Ashwagandha gel can be effective against pathogenic bacteria to clear the infection and improve healing. Paste of leaves and roots is considered to have wound healing ability30.
It has been demonstrated that non-steroidal anti-inflammatory medicines (NSAIDs) inhibit wound healing while concurrently reducing the granulocytic inflammatory response23. NSAIDs can lessen pain by preventing the formation of PGE2, a prostaglandin that mediates inflammation24. NSAIDs may promote the production of scars because they inhibit PGE2, which also occurs with severe wound scarring, particularly if they are used during the proliferative stage of healing. Ashwagandha has anti-inflammatory24, analgesic31 and healing properties along with antioxidant properties. These properties may also be responsible for the wound healing activity. Flavonoids are polyphenolic compounds present in the Ashwagandha that have free radical scavenging, inhibition of hydrolytic and oxidative enzymes, and anti-inflammatory action32.
The collagen molecules synthesised are laid down at the wound site and become cross-linked to form fibres. Wound strength is acquired from both the remodelling of collagen and the formation of stable intra- and inter-molecular cross-links. Incision wounds treated with the Ashwagandha gel showed a significant increase in the tensile strength of wounded skin therefore, future studies should be conducted to evaluate the collagen synthesis per cell as well as cross-linking of the protein.
The drug treatment may have beneficial effects on various phases of wound healing like collagen and elastin synthesis and contraction and epithelialisation period, resulting in faster healing of wounds in albino rats. The results in the healing and sealing of wounds make Ashwagandha an important natural product for assistance in the process of wound healing which may be due to the synergistic actions of biologically active ingredients present in this plant.
Conclusions
The present study demonstrated that the Ashwagandha leaves, when applied in the form of paste and gel, fulfil most of the possible levels of the ideal wound healing agent. But it is not always possible to get fresh leaves across the seasons and in the same condition. Preparing leaf paste is also not feasible commercially. On the other hand, converting the formulation into a gel will facilitate its application. In the current study, better wound healing potency was observed with Ashwagandha gel. The rate of wound contraction and higher tensile strength encourage future research into the efficacy of Ashwagandha gel as a potential topical wound management agent.
Limitations and future scope
Even though a significant wound healing property of leaf paste and its gel was observed in the present study, histopathology of the healed skin and the estimation of marker enzymes like hyaluronidase, collagenase and elastase in the granulation tissue formed beneath the healed wound could not be evaluated.
Acknowledgments
The authors are truly thankful to Mr Ramesh Rao, Dr Sanjay KS, Dr Pradeep HR, Dr Vishnu V Nath, Dr Karthik, Dr Anusha MS, and Mr Ramesh from ALN Rao Memorial Ayurvedic Medical College, Koppa, Karnataka, for their support in conducting the animal experimentation. The authors are also thankful to Prof Dr Tanuja Manoj Nesari, Director, All India Institute of Ayurveda, Sarita Vihar, New Delhi, for supporting and facilitating this study.
Conflict of interest
The authors declare no conflicts of interest.
Ethics statement
An ethics statement is not applicable.
Funding
The authors received no funding for this study.
Author contribution
Dr Galib R and Dr Jagadeesha Mayya have participated sufficiently in the work and made substantive intellectual contributions to the submitted work in the form of conception and design. Dr Hemanth Gowda and Dr Vijay Kumar helped in acquisition of data along with analysis and interpretation of the data. Dr Galib R, Dr Vijay Kumar, Dr Pramod Yadav and Prof Pradeep Kumar Prajapati have helped in drafting the article as well as revised it critically. Hence, all of them meet the criteria for authorship of the manuscript.
Author(s)
Shreya D Bhosale1*, Jagadeesh Mayya A2, Hemanth Gowda K3, Vijay Kumar4, Pramod Yadav5, Galib R6, Pradeep K Prajapati7,8
¹PG Scholar, Department of Rasashastra and Bhaishajya Kalpana, All India Institute of Ayurveda, New Delhi-110076, India
2Associate Professor, Department of Rasashastra and Bhaishajya Kalpana, ALN Rao Memorial Ayurvedic Medical College, Koppa, Karnataka-577126, India
3Assistant Professor, Department of Veterinary Physiology and Biochemistry, KVAFSU, Hassan, Karnataka-573201, India
4Assistant Professor, Pharmacology, All India Institute of Ayurveda, New Delhi-110076, India
5Assistant Professor, Department of Rasashastra and Bhaishajya Kalpana, All India Institute of Ayurveda, New Delhi-110076, India
6Associate Professor, Department of Rasashastra and Bhaishajya Kalpana, All India Institute of Ayurveda, New Delhi-110076, India
7Vice Chancellor, DSRR Ayurved University, Jodhpur, Rajasthan, India
8Professor and Head of the Department of Rasashastra and Bhaishajya Kalpana, All India Institute of Ayurveda, New Delhi- 110076, India
*Corresponding author email shreyab0496@gmail.com
References
- Robson MC, Steed DL, Franz MG. Wound healing: biologic features and approaches to maximize healing trajectories. Curr Probl Surg 2001;38:72–140. doi:10.1016/S0011-3840(01)70035-4.
- Natarajan S, Williamson D, Stiltz AJ, Harding K. Advances in wound care and healing technology. Am J Clin Dermatol 2000;1(5):269–75.
- Martinengo L, Olsson M, Bajpai R, Soljak M, Upton Z, Schmidtchen A, Car J, Järbrink K. Prevalence of chronic wounds in the general population: systematic review and meta-analysis of observational studies. Annal Epidemiol 2019;29:8–15.
- Mehmet EO, Karantas ID, Zeynep S, Neslihan UO, Panoraia IS. Recent trends on wound management: new therapeutic choices based on polymeric carriers. Asian J Pharm Sci 2020;15(6):661–684.
- Farahpour MR. Medicinal plants in wound healing; 2019. Available from: https://www.intechopen.com/books/wound-healing-current-perspectives/medicinalplants-in-wound-healing
- Shukla AV, Tripathi R, editors. Charak Samhita of Agnivesh, Sutrasthana. Chapter 11/55. Chaukhambha Sanskrit Pratishthan, Delhi, p.179.
- Tripathi B, editor. Charak Samhita of Agnivesh, Chikitsasthan. Chapter 25/39–43. Chaukaumbha Surbharati Prakashan, Varanasi, p.843.
- Shastri A, editor. Sushrutsamhita of Sushruta, Chikitsasthan. Chapter 1/8. Chaukhamba Sanskrit Sansthan, Varanasi, p.5.
- Trikamji VY, editor. Sushrutsamhita with Nimbandh Sangrah commentary of Dalhanacharya Sutrasthan 37/23. Chaukaumbha Surbharati Prakashan, Varanasi, p.162.
- Dar NJ, Hamid A, Ahmad M. Pharmacologic overview of Withania somnifera, the Indian Ginseng. Cell Mol Life Sci 2015;72(23):4445–60. doi:10.1007/s00018015-2012-1.
- Gupta GL, Rana AC. Plant review Withania somnifera (Ashwagandha): a review. Department of Pharmacology, BN College of Pharmacy, Udaipur, 2007.
- Sikandan A, Shinomiya T, Nagahara Y. Ashwagandha root extract exerts anti-inflammatory effects in HaCaT cells by inhibiting the MAPK/NF-κB pathways and by regulating cytokines. Int J Molec Med 2018;42(1):425–434.
- Vidyasagar PS, editor. Sharangdhar Samhita of Sharangadharacharya, Madhyama Khanda 9/1, Chaukaumbha Surbharati Prakashan, Varanasi, p.212.
- Sembian S, Kalidass S, Femina W, Karthikeyan G. Evaluation of wound healing activity of Acacia leucophloea bark in rats. Rev Bras Farmacogn 2012;22(6):1338–1343.
- Morton JJP, Malone MH. Evaluation of vulnerary activity by an open wound procedure in rats. Architect Int J Pharmacol Ther 1972;196–117.
- Ehrlich HP, Hunt TK. The effect of cortisone and anabolic steroids on the tensile strength of healing wounds. Ann of Surg 1968;57:117–121.
- Balakrishnan B, Mohanty M, Fernandez AC, Jayakrishnan A. Evaluation of the effect of incorporation of dibutyryl cyclic adenosine monophosphate in an in situ forming hydrogel wound dressing based on oxidized alginate and gelatin. Biomaterial 2006;27(8):1355–1361.
- Lee KH. Studies on the mechanism of action of salicylate II. Retardation of wound healing by aspirin. J Pharma Sci 1968;57(6):1042–1043. doi:10.1002/jps.2600570633
- Peacock EE. Wound repair. Philadelphia: W.B. Saunders; 1984. p. 39.
- Abdullahi A, Amini-Nik S, Jeschke MG. Animal models in burn research. Cell Mol Life Sci 2014;71(17):3241–3255.
- Kotade K, Mohammed A. Wound healing activity of Sesamum indicum L seed and oil in rats. Indian J Exp Biol 2008;46:777–782.
- Meena K, Mohan AV, Sharath B, Somayaji SN, Bairy KL. Effect of topical phenytoin on burn wound healing in rats. Indian J Exp Biol 2011;49:56–59.
- Chandra S, Chatterjee P, Dey P, Bhattacharya S. Evaluation of anti-inflammatory effect of Ashwagandha: a preliminary study in vitro. Pharmacognosy J 2012;4(29):47–9.
- Alam N, Hossain M, Mottalib MA, Sulaiman SA, Gan SH, Khalil MI. Methanolic extracts of Withania somnifera leaves, fruits and roots possess antioxidant properties and antibacterial activities. BMC Complement Altern Med 2012 Oct 7;12:175. doi:10.1186/1472-6882-12-175
- Singh G, Kumar P. Evaluation of antimicrobial efficacy of flavonoids of Withania somnifera L. Indian J Pharm Sci 2011;73(4):473–8.
- Acharya YT, editor. Commentary Nibandha sangraha of Dalhanacharya on Sushruta Samhita of Shushruta, Sutrasthana. Chapter 37, verse 24. Varanasi: Chaukhamba Surbharati Prakshan; 2014. p. 162.
- Bisht P, Rawat V. Antibacterial activity of Withania somnifera against Gram-positive isolates from pus samples. Ayu 2014;35(3):330–2.
- Mahesh B, Satish S. Antimicrobial activity of some important medicinal plant against plant and human pathogens. World J Agricult Sci 2008;4(5):839–843.
- Sundaram S, Dwivedi P, Purwar S. Withania somnifera (Ashwagandha) to bacterial pathogens. Asian J Biotechnol 2011;3(2):194–199.
- Beena S. Health awareness in women with the use of traditional medicinal plants in district Raipur of Chhattisgarh. J Ecobiotech 2011;3:15–17.
- Dey A, Chatterjee SS, Kumar V. Analgesic activity of a Withania somnifera extract in stressed mice. Orient Pharm Exp Med 2016;16:295–302.
- Frankel E. Nutritional benefits of flavonoids. International conference on food factors: chemistry and cancer prevention, Hamamatsu, Japan, 1995. Abstracts C6-2.



