Volume 32 Number 1
Changes in the integument across the lifespan
Keryln Carville
Keywords skin, neonatal, childhood, adolescence, adult
For referencing Carville K. Changes in the integument across the lifespan. Wound Practice and Research. 2024;32(1):11-16.
DOI
10.33235/wpr.32.1.11-16
Submitted 2 March 2024
Accepted 12 March 2024
Abstract
The integumentary system is the largest and most visible body organ and is composed of the skin, hair, nails, sebaceous glands, eccrine and apocrine sweat glands. Integumentary assessment, prevention of its injury and management of the injury or other defect, should it occur, is a paramount goal for all clinicians across all health domains. Furthermore, it is a fundamental requirement for ensuring quality care outcomes. However, in order to conduct a comprehensive and informative assessment the clinician needs to recognise and consider the normal skin characteristics that present across the lifespan in order to identify any pathological presentations. The major lifespan periods that influence the integumentary system changes are: neonatal, childhood, adolescence, pregnancy, adulthood and elderly time periods.
Introduction
The integumentary system is the largest and most visible body organ and is composed of the skin, hair, nails, sebaceous glands, eccrine and apocrine sweat glands.1−3 The integument reflects the individual’s age, physical health status, race and ethnic diversity. The integument or ‘body suit’ is largely taken for granted until it is injured or demonstrates evidence of disease, trauma or ageing. However, the physiological changes in the integument and especially in the skin, that evolve across the life span are noticeable and need to be recognised and considered when performing an assessment of the integument.
The skin
The skin measures approximately 7,600 sq cm on the average adult and weights 15% of body weight and has a third of the circulating blood volume.2 The skin surrounds the body and forms a physical barrier against the extracorporeal environment and protects against desiccation and invasion by pathogens and toxins. The skin fulfils many functions which include: homeostasis; immunological and pathogenic defense; sensory perception; temperature and pH regulation; protection against ultraviolet radiation; regulation of transepidermal water loss (TEWL) and skin water content; synthesis of vitamin D and cosmesis.2,3 The skin is divided into three layers: the epidermis, dermis and hypodermis.
However, it is the epidermis and specifically the stratum corneum or the outer ‘horny’ layer, which is on view to the world and thus, the focus of skin inspection and primarily this review. The keratinocytes that form in the stratum germinativum or basal layer migrate up through the stratum spinosum, stratum granulosum, stratum lucidum (absent in some anatomical skin locations) into the stratum corneum where they are transformed into non-viable, anucleate, protein-dense corneocytes.3,4 These are flat hexagonal cells that comprise 15–25 layers of compressed corneocytes.4 The construction of the stratum corneum is said to resemble a brick wall structure.4,5 The corneocytes are the bricks which are linked together by corneodesmosomes and the ‘mortar’ that surrounds these junctions is comprised of the natural moisturising factor (NMF) and lipids.4,5 The NMF is derived from filaggrin proteolysis and is composed of amino acids, amino acid derivatives, lactic acids, urea and salts.4 The NMF is essential for stratum corneum hydration, desquamation, and plasticity.6,7 While the lipids consist of ceramides, acylceramides, cholesterol, cholesterol esters and free fatty acids.5 However, lipid composition in the stratum corneum can vary according to anatomical site, age of the individual, sebaceous gland density and UV exposure.5,8 The lipids are important for the skin barrier function (also referred to as the epidermal permeability barrier), which regulates TEWL and aids defense against pathogenic and toxic agents.9 The epidermis is labile tissue and regeneration of the epidermis in the healthy adult takes approximately 28 days and is aided by regulated desquamation or shedding of old corneocytes in the stratum corneum.1,10,11
Skin colour
Assessment of the integument involves: a clinical, familial and habitual history, inspection, palpation and possible microscopy, mycology and histopathology investigations. Inspection ascertains anatomical colour, xerosis, moisture, presence of primary, secondary and vascular lesions and oedema.
Skin colour is a predominant feature and is largely determined by race, but skin colour changes are noted in relation to anatomical location (sun exposed or sun protected); genetics (albinism, vitiligo); amount of ultraviolet (UV) radiation exposure; impaired arterial perfusion (ischaemia); chronic venous insufficiency (haemosiderosis) or respiratory disorders (hypoxia, cyanosis); obstruction of the hepatobiliary system or inhibited hepatic clearance of bilirubin (jaundice); thermal injury or inflammation (erythema); medication induced pigmentation (minocycline, amiodarone); ingestion of certain foods or agents in excess (carotene enriched foods or agyria from colloidal silver); artefact henna use; or tattooing.1−3
Hemoglobin, oxyhemoglobin, melanin and carotenoids influence cutaneous colour associated with different races. Pink-toned Caucasian skin is influenced by the hemoglobin and oxyhemoglobin which reflects back as red tones due to the absorption of certain wavelengths of light.12 The yellow skin tones of Asian races is subject to the amount of carotene in the skin and dark skin tones are dependent upon the amount of melanin.12 All races have the same amount of melanocytes, which synthesise melanin, and in the integument they are found in the stratum germinativum in the epidermis, hair and irises of the eyes.12 Melanin protects from UV radiation and the darker skin toned populations predominately live closer to the equator where UV rates are higher. Dark skin doesn’t demonstrate evident blanching on pressure nor is reactive erythema visible on relief of pressure.1 Furthermore, erythema due to inflammatory responses or infection is very difficult to detect and the affected skin may appear as blue/purple tones.13 Skin colour assessment should be performed using a validated tool such as Fitzpatrick Skin Type Scale.14

Figure 1. Asian, dark and light skin tones. © istock yacobchuk
More recent evidence highlights certain other skin physiological characteristics pertinent to skin colour.5,12 Lighter skin tones, such as Caucasian skin, are subject to earlier photoaging and wrinkling, as well as having the weakest stratum corneum barrier function. Asian skin has the lowest TEWL and stratum corneum barrier function, but greater water content and amount of stratum corneum lipids.5,12 While dark skin has a stronger stratum corneum barrier function, higher UV protection, increased sebum, more layers of compressed corneocytes, higher TEWL and less water content.5,12
However, skin appearance and characteristics primarily reflect the changes that occur across the lifespan during infancy, childhood, adolescence, adulthood and with chronological ageing.
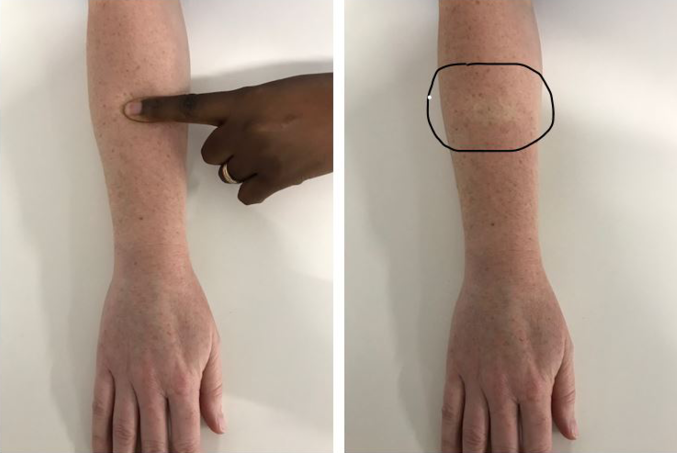
Figure 2. Blanching of light skin on pressure
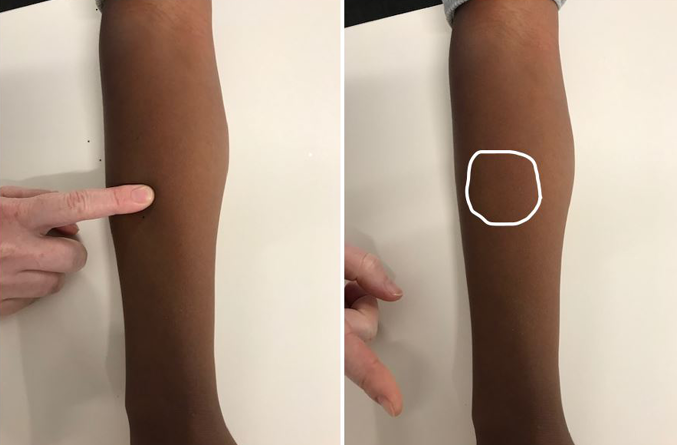
Figure 3. Non-blanching of dark skin tone on pressure
Neonatal integumentary changes
Neonatal skin is thinner and has less sweat and fewer sebaceous glands than more mature skin. It demonstrates unique characteristics that are subject to the gestation period of the baby at delivery and are as follows.
- Generalised erythema and acrocyanosis of the hands and feet is evident at birth. Acrocyanosis is caused by vasomotor instability, capillary stasis and increase in circulating haemoglobin. The erythema and acrocyanosis usually fades within 24 hours, although, mottling skin colour can occur because of transient temperature decreases.15
- Vernix caseosa is a white, ‘cheesy’, odourless substance that is composed of sebum and desquamated epithelial cells that covers and protects the skin of the foetus in utero from amniotic fluid and its components.16 Vernix caseosa is composed of water, proteins sebum lipids and antimicrobial peptides.17 Vernix caseosa is more evident on premature infants at delivery, but is absorbed or removed by friction usually within 24−48 hours after birth.
- Lanugo is fine, soft hair that may be evident on the forehead, buttocks, pinnas of ears and back. It is more obvious in premature infants and is replaced by vellus and terminal hair as the infant develops. In utero lanugo assists in binding the vernix caseosa to the skin of the fetus.16
- Desquamation is the flaking of skin commonly on the hands and feet of neonates, particularly those born post-term.15
- Jaundice or physiological neonatal jaundice or hyperbilirubinemia presents as yellowing of the skin and sclera and occurs when a baby has elevated levels of bilirubin in the blood due to an increase in the number of red blood cells haemolysing following birth. Jaundice affects approximately 60−80% of neonates and is usually more obvious on the third or fourth day post delivery, but it generally fades rapidly as the liver clears the excess bilirubin.19,20 Phototherapy may be employed to facilitate the conversion of bilirubin molecules into water-soluble isomers that can be excreted by the body.19,20

Figure 4. Jaundice treatment with phototherapy in a neonate
However, there are two other conditions that cause jaundice in the neonate and they are breast milk jaundice and breast feeding jaundice. Breast milk jaundice was first described by Arias in 196321 and usually appears during the second or later weeks post-delivery in otherwise healthy breastfed neonates. It has been reported in 20−30% of neonates in the US.22 Although the aetiology of breast milk jaundice is uncertain, some reports suggest proteins and enzymes in human breast milk inhibit the conjugation of bilirubin, and thus its excretion.19 Breast milk is known to contain beta-glucuronidase, which in the intestine, decouples glucuronic acid from conjugated bilirubin and allows bilirubin to be reabsorbed by the enterohepatic circulation.23 Breast milk jaundice first becomes visible on the face and progresses to the trunk and then the extremities around the first weeks of life and may last up to 3 months.19−21 The jaundice is not actively treated unless the total serum bilirubin level of the infant is greater than 20mg/dL, when phototherapy may be employed. In extreme cases a temporary rest from breast feeding in favour of formula feeds may be instigated.20
Breast feeding jaundice on the other hand is related to inadequate lactation and milk intake by the infant leading to dehydration, which impairs clearance of bilirubin from the body.19 It usually becomes evident in the first weeks of breast feeding and is usually associated with weight loss.19 The treatment is to increase the number and frequency of breast feeds. However, regardless of the jaundice aetiology, marked or prolonged jaundice requires medical review and intervention.
Milia are benign and transient subepidermal keratin cysts which present primarily on the baby’s face, particularly the nose, and are due to clogged sebaceous glands.24 They occur in the first weeks following birth in 40−50% of neonates and resolve usually within months.24
‘Harlequin’ colour changes may be observed when the infant is lying on its side. The lower half of the body tends to be pink and the upper half has a paler hue. The colour change develops suddenly and can last for 30 seconds to 20 minutes. Harlequin colour change affects approximately 10% of infants and usually during the second to fifth day of life and may continue for up to three weeks. It is thought to be caused by immaturity of the hypothalamic center that controls the dilation of peripheral blood vessels. It resolves with increased muscle activity or crying.25
Mongolian spots present as patches of blue/purple discolouration over the sacrogluteal region in babies of dark skin and Asian races. They are due to benign dermal melanocytosis or a concentration of pigmented cells which take 3−5 years to fade.15
‘Stork bites’ (naevus simplex) are pink discoloured patches on the back of a baby’s neck and are due to a concentration of immature and dilated blood vessels. The name is derived from the myth that babies are delivered by storks and the marks result from the stork’s bill when carrying the baby. They may be more evident when the baby is crying, and they generally fade completely.26
Birthmarks may be due to vascular alterations or abnormal pigmentation and they can be permanent or temporary. Permanent birthmarks include portwine stains which are flat, pink, red, or purple colored birthmarks.27 They are due to a concentration of dilated capillaries usually found on the head or neck.27 Port-wine stains do not blanch. Capillary haemangioma (synonymous terms are infantile haemangioma, strawberry naevae or strawberry haemangioma) are temporary birthmarks. They are dark red, flat lesions that occur in the first weeks of life, increase in size up to 9 months and gradually reduce at a rate of 10% each year.28
Childhood integumentary changes
During childhood changes in children’s skin are usually associated with sun exposure, trauma or infectious diseases (measles, rubella, varicella). The colour of vellus and terminal head hair may change or darken as the child nears puberty.

Figure 5. Unblemished Caucasian childhood skin
Adolescent integumentary changes
Adolescence is the period between childhood and adulthood and it is heralded by pubescence, which is a time of great physical and psychological development. Adult anatomical characteristics such as enlargement of breasts in females and genitalia in both genders become apparent. Apocrine sweat glands are stimulated by sex hormones and are associated with pubic and axilla hair and this sweat can become malodourous due to contact with skin flora.28 Facial and chest hair appears on the male and hair on the legs of both genders darkens. Sebaceous gland activity increases and the skin, and in particular on the face, becomes a focus of attention in response to these developments. Acne vulgaris is a relatively common concern among teenagers and is categorised29 as:
- Grade I or comedones (blackheads and whiteheads)
- Grade II comedones and pustules
- Grade III comedones, pustules and cysts
- Grade IV widespread cysts with scarring

Figure 6. Adolescent integumentary changes may impact on alterations in body image perceptions
Pregnancy integumentary changes
Besides the obvious anatomical changes in body size and shape due to fetal growth and breast enlargement, during pregnancy the skin in particular undergoes changes that relate primarily to colour and are as follows.
- Linea nigra or pigmentation of the linea alba which appears as a dark thin line between the two rectus abdominal muscles on the abdomen, extending from the umbilicus to the symphysis pubis.30
- Areola pigmentation of the areola and enlargement of the breasts.30,31
- Striae gravidarum, or stretch marks may appear on the abdomen, breasts and thighs of primipara and multipara women during pregnancy. Striae usually occur in the third trimester when weight gain is increased, and they appear initially as reddish streaks which fade to pale lines about 6 months post-delivery.30−32
- Chloasma gravidarum or hyperpigmentation appears as a ‘mask of pregnancy’ on the face, which tends to fade within months post-pregnancy.31,32
- Hair and nail growth is reported to either be faster and stronger by some pregnant women and the reverse by others.33

Figure 7. Skin changes during pregnancy include the linea nigra https://www.pexels.com/search/PREGNANT
Adult integumentary changes
Mature adult skin continues to fulfil the roles of homeostasis, protection, thermoregulation, immunologic defense, sensory perception, regulation of TEWL and skin lipids, communication, synthesis of vitamin D and cosmesis as does the skin of other lifespan eras.1−3 Changes in adult skin reflect influences of health status and habitual behaviours, diet and medication intake, environmental and UV exposure and lifestyle choices. Primary, secondary and vascular lesions may become apparent, as can sinister skin lesions. Changes in hair pattern growth or alopecia can occur in both genders. Greying of hair can occur.
Elderly integumentary changes
Skin changes in the elderly are related to intrinsic and extrinsic factors. Intrinsic factors encompass chronological ageing and/or physiological factors, such as genetics and hormone alterations.35 Whilst extrinsic factors include environmental exposure to UV radiation, pollutants and toxic chemicals, and lifestyle choices, such as smoking.36,37 Skin ageing impacts on skin cosmesis, structural integrity and functionality.36,37
Structural changes present as thinning and flattening of the dermo-epidermal junction and atrophy and contraction of the dermis.1,35,36 Skin turgor is decreased due to a reduction in collagen and elastic fibres. In addition, the collagen present becomes thinner and when combined with a decrease in adipose tissue, the skin support structure is compromised and skin wrinkling and loss of elasticity is evident.1,35,36 The skin on the back of the hands may become thin and transparent, while the skin on sun exposed sites demonstrates signs of solar elastosis and photoageing.37,38 Solar elastosis on the back of neck, especially in the male photoaged skin, has a furrowed appearance, which is referred to as cutis rhomboidalis nuchae.39 Dermatoporosis or chronic skin fragility may be associated with senile purpura and stellate pseudoscars and affected skin is subject to friction and shearing trauma, leading to wounds, such as skin tears.39
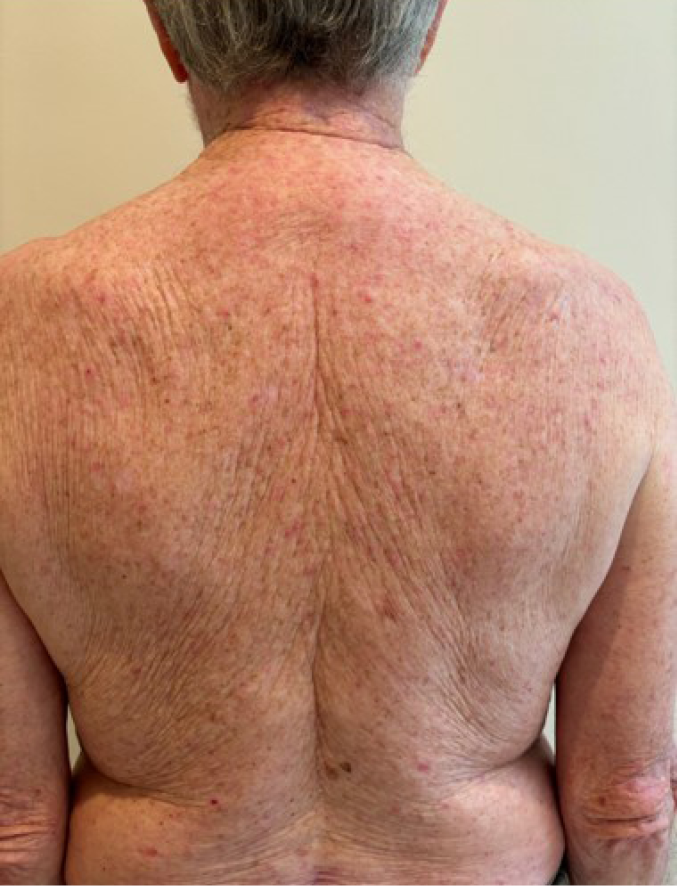
Figure 8. Integumentary changes in the elderly include: skin wrinkling, increase in lesions, rhomboidalis nuchae on the neck and greying of hair
Ageing skin demonstrates an increase in benign skin lesions such as seborrheic keratosis, skin tags, naevi and an increase in vascular lesions such as purpura and angiomas. There is also an increased risk of pre-malignant lesions, such as actinic keratosis and malignant non-melanoma skin cancers.1,36,40
Skin colour changes can be related to hyperpigmentation (naevi, lentigines); vascular lesions (telangiectasis, ecchymosis and purpura), hypoxia or impaired peripheral circulatory changes. The latter can be evident as pallor or dependent rubor in the extremities affected by severe arterial disease or haemosiderosis (blue/brown/black staining of the skin) in the lower leg when chronic venous insufficiency presents.1,35,36 Sebaceous and sweat gland activity decreases and, therefore, the elderly sweat less and the skin becomes drier and less supple.1,36,40 Facial hair in the male decreases and increases in the female in response to alternations in hormones. Pubic and axillary hair thins, straightens, greys and lessens in amount. Scalp hair greys and alopecia may be more evident in both male and females.1,41 Nails lose their lustre and toenails thicken, particularly if arterial insufficiency is present.42
Conclusion
The integumentary system is a complex dynamic structure that undergoes changes in appearance and functionality across the lifespan. The skin and its appendages, are also highly adaptive with specialised functions in different body sites. Integumentary assessment is a fundamental precursor to determining health status and the need for preventive and treatment interventions. Furthermore, the assessor needs to be familiar with the normal anatomical and physiological changes that occur over the lifespan in order to be able to identify abnormal pathological changes that require further investigation and targeted treatment.
Conflict of interest
The author declares no conflicts of interest.
Ethics statement
An ethics statement is not applicable.
Funding
The authors received no funding for this study.
Author(s)
Keryln Carville
Silver Chain Group Inc, Perth, Australia
Curtin University School of Nursing, Perth, Australia
Curtin Health Innovation Research Institute (CHIRI), Perth, Australia
Curtin Wound Healing and Management Collaboration, Perth, Australia
*Corresponding author email Keryln.Carville@silverchain.org.au
References
- Carville K. Wound care manual. Osborne Park, WA: Silverchain Foundation; 2023.
- Wyscoki A. In Bryant R, Nix D. editors. Acute and chronic wounds: current management concepts (3rd ed.). Philadelphia: Mosby Elsevier; 2007. 39−55.
- Kim JY, Dao H. Physiology, Integument. 2023 May 1. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan. Available from: https://www.ncbi.nlm.nih.gov/books/NBK554386/
- Lee T, Friedman A. Skin barrier health: regulation and repair of the stratum corneum and the role of over-the-counter skin care. J Drugs Dermatol. 2016;15(9):1047−1051.
- Knox S, O’Boyle NM. Skin lipids in health and disease: A review. Chem Phys Lipids. 2021 May;236:105055. doi: 10.1016/j.chemphyslip.2021.105055
- Robinson M, Visscher M, Laruffa A, Wickett R. Natural moisturizing factors (NMF) in the stratum corneum (SC). I. Effects of lipid extraction and soaking. J Cosmet Sci. 2010;61(1):13−22.
- Fowler J. Understanding the role of natural moisturizing factor in skin hydration. July, 2012. Practical Dermatology. 36−40. https://practicaldermatology.com/articles/2012-jul/understanding-the-role-of-natural-moisturizing-factor-in-skin-hydration/pdf
- Ludovici M, Kozul N, Materazzi S, Risoluti R, Picardo M, Camera E. Influence of the sebaceous gland density on the stratum corneum lipidome. Sci Rep. 2018;8(1):11500. DOI:10.1038/s41598-018-29742-7
- Pappas A. Epidermal surface lipids. Dermatoendocrinol. 2009 Mar;1(2):72−6. DOI:10.4161/derm.1.2.7811
- Abdo J, Sopko N, Milner S. The applied anatomy of human skin: a model for regeneration, Wound Medicine. 2020; (28):100179. https://doi.org/10.1016/j.wndm.2020.100179
- Egelrud T. Desquamation in the stratum corneum. Acta Derm Venereol Suppl (Stockh). 2000;208:44-5. DOI: 10.1080/000155500750012513
- Rawlings A. Ethnic skin types: are there differences in skin structure and function? Inter J of Cosmetic Sc. 2006;28:79−93.
- Sonenblum SE, Patel R, Phrasavath S, Xu S, Bates-Jensen BM. Using technology to detect erythema across skin tones. Adv Skin Wound Care. 2023 Oct 1;36(10):524−533. DOI: 10.1097/ASW.0000000000000043
- Fitzpatrick T. The validity and practicality of sun reactive skin types I to IV. Arch Derm. 1988;124:869−871.
- Kutlubay Z, Tanakol A, Engýn B, Onel C, Sýmsek E, Serdaroglu S, Tuzun Y, Yilmaz E, Eren B. Newborn skin: Common skin problems. Maedica (Bucur). 2017 Jan;12(1):42−47.
- Verhave BL, Nassereddin A, Lappin SL. Embryology, lanugo. [Updated 2022 Oct 10]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK526092/
- Rissman R, Groenink HW, Weerheim AM, et al. New insights into ultrastructure, lipid composition and organization of vernix caseosa. J Invest Dermatol. 2006;126:1823–1833.
- Oranges T, Dini V, Romanelli M. Skin physiology of the neonate and infant: clinical implications. Adv Wound Care (New Rochelle).. 2015 Oct 1;4(10):587−595.
- Shihab RAH, Samman AMB, Aqeel FA, Ghabrah ZT, Ghabrah OT, Ghannam AN, et al. Difference between breast milk jaundice and breast-feeding jaundice: literature review. Int J Community Med Public Health. 2021;8:2559−2563.
- Bratton S, Cantu RM, Stern M. Breast milk jaundice. [Updated 2023 Jan 17]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024. Available from: https://www.ncbi.nlm.nih.gov/books/NBK537334/
- Arias IM, Gartner LM, Seifter S, Furman M. Prolonged neonatal unconjugated hyperbilirubinemia associated with breast feeding and a steroid, pregnane-3(alpha), 20(beta)-diol, in maternal milk that inhibits glucuronide formation in vitro. J Clin Invest. 1964;43(11):2037−47.
- Maisels MJ, Clune S, Coleman K, Gendelman B, Kendall A, McManus S, et al. The natural history of jaundice in predominantly breastfed infants. Pediatrics. 2014;134(2):340−345.
- Ma XW, Fan WQ. Earlier nutrient fortification of breastmilk fed LBW infants improves jaundice related outcomes. Nutrients. 2020 Jul 17;12(7):2116. DOI: 10.3390/nu12072116
- Berk DR, Bayliss SJ. Milia: a review and classification. J Am Acad Dermatol. 2008 Dec;59(6):1050−1063.
- Tang J, Bergman J, Lam JM. Harlequin colour change: unilateral erythema in a newborn. CMAJ. 2010 Nov 23;182(17):E801. DOI: 10.1503/cmaj.092038
- Wu W. DermNet Naevus simplex, 2019. https://dermnetnz.org/topics/naevus-simplex
- Liu L, Li X, Zhao Q, Yang L, Jiang X. Pathogenesis of port-wine stains: directions for future therapies. Int J Mol Sci. 2022 Oct 12;23(20):12139. doi: 10.3390/ijms232012139. PMID: 36292993; PMCID: PMC9603382.
- Roberts N. Infantile haemangioma: harmless ‘strawberry’ or life-threatening vascular anomaly? Clin Med (Lond). 2009;9(4):385−389.
- Hodge BD, Sanvictores T, Brodell RT. Anatomy, Skin sweat glands. [Updated 2022 Oct 10]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024. Available from: https://www.ncbi.nlm.nih.gov/books/NBK482278/
- Witkowski JA, Parish LC. The assessment of acne: an evaluation of grading and lesion counting in the measurement of acne. Clin Dermatol 2004;22:394-7.
- Vora RV, Gupta R, Mehta MJ, Chaudhari AH, Pilani AP, Patel N. Pregnancy and skin. J Family Med Prim Care. 2014 Oct-Dec;3(4):318−24. DOI: 10.4103/2249-4863.148099
- Akkoca A, Ozdemir Z, Kurt R, Sen B, Yengil E, Karatepe C, Karapinar O, Ozer C. The physiological changes in pregnancy and their distribution according to trimester. J Gynecology and Obstetrics. 2014; 2(6):86−90.
- Brennan M, Clarke M, Devane D. The use of anti stretch marks’ products by women in pregnancy: a descriptive, cross-sectional survey. BMC Pregnancy Childbirth. 2016 Sep 21;16(1):276.
- Matushansky J, Wang Y, Chang M, Thomas C Hockstein S, Lipner S. Nail changes during pregnancy: a cross-sectional survey of patients at an academic center, Skin Appendage Disord. 2023;9:27–29. DOI: 10.1159/000526870
- Blume-Peytavi U, Kottner J, Sterry W. Age-associated skin conditions and diseases: current perspectives and future options. Gerontologist. 2016;56(S2):S230-S242.
- Wong QYA, Chew FT. Defining skin aging and its risk factors: a systematic review and meta-analysis. Sci Rep. 2021;11:22075 DOI: https://doi.org/10.1038/s41598-021-01573-z
- Ayer J. Skin ageing. DermNet, June 2018. Available from: https://dermnetnz.org/topics/ageing-skin
- Selheyer K. Pathogenesis of solar elastosis: synthesis or degradation? J Cutaneous Pathology. 2003;30(2):123−127.
- Wollina U, Lotti T, Vojvotic A, Nowak A. Dermatoporosis:The chronic cutaneous fragility syndrome. Open Access Maced J Med Sci. 2019 Aug 30;7(18):3046−3049. DOI: 10.3889/oamjms.2019.766
- Bonkevitch F, Souza PR. Cutis rhomboidalis protects skin from malignant epithelial tumors. Med Hypotheses. 2014 Jun;82(6):652−653. DOI: 10.1016/j.mehy.2014.02.006.
- Farage MA, Miller KW, Elsner P, Maibach HI. Characteristics of the aging skin. Adv Wound Care (New Rochelle). 2013 Feb;2(1):5-10. DOI: 10.1089/wound.2011.0356
- Villani A, Ferrillo M, Fabbrocini G, Ocampo-Garza SS, Scalvenzi M, Ruggiero A. Hair aging and hair disorders in elderly patients. Int J Trichology. 2022 Nov-Dec;14(6):191−196. doi: 10.4103/ijt.ijt_90_21
- Abdullah L, Abbas O. Common nail changes and disorders in older people: diagnosis and management. Can Fam Physician. 2011 Feb;57(2):173−181.



