Volume 5 Number 1
Securing cannulas in combat: an in vitro study of three intravenous securement techniques
Benjamin R Mackie, Darwin Alvarenga, Christopher Williams, Gabor Mihala, Joseph Dawson, Benjamin Tribe, Guy Williams, Jessica Schults, Gillian Ray-Barruel, Nicole Marsh
Keywords catheterisation, intravenous, tactical combat casualty care, occlusive dressings, securement, peripheral intravenous, Ranger
For referencing Mackie BR et al. Securing cannulas in combat: an in vitro study of three intravenous securement techniques. JHTAM 2023; 5(1):3-9.
DOI
https://doi.org/10.33235/JHTAM.5.1.3-9
Submitted 16 December 2022
Accepted 18 January 2023
Abstract
Introduction Haemorrhage is the leading cause of preventable death in combat settings. Pre‑hospital practice guidelines for shock advocate for intravenous (IV) access to enable the rapid delivery of blood products and adjunct treatments. However, peripheral intravenous catheter (PIVC) insertion and securement is challenging in the austere setting, and catheter failure is common. This study investigated dressing and securement methods to prevent PIVC dislodgement within an Australian Defence Force (ADF) tactical combat casualty care (TCCC) training course.
Methods A pragmatic in vitro design was used to compare the proportion of dislodged PIVCs using three securement techniques – the ruggedised field IV method (Ranger), the S‑Wrap technique, and standard care (a TegadermTM dressing). The study was undertaken from February to May 2022 at the Australian Army School of Health (ASH) in Victoria, Australia. Pull-out force was simulated using a purpose-designed pulley system and measured with a digital forge gauge. The null hypothesis of no difference in average force between products was tested using one-way analysis of variance, followed by a post‑hoc Bonferroni test. Statistical significance was determined at p≤0.05. This study is registered with the Australian New Zealand Clinical Trials Registry CTRN12621000314820.
Results Of the three methods of PIVC securement tested in this study (standard, Ranger, S‑Wrap), the S‑Wrap method demonstrated a significantly higher average pull-out force (66.1N; SD 7.1), and the lowest average pull-out force was associated with TegadermTM (9.7N; SD 2.2). The securements provided greater resistance against the pull-out force at 0˚ compared to 30˚. The three Bonferroni-adjusted p values for the three null hypotheses between the three products were all <0.001.
Conclusion PIVC securement is challenging in the austere or combat setting, and failure is common. The S‑Wrap technique is an uncomplicated, feasible and safe PIVC securement dressing for use in high threat/austere pre‑hospital settings.
Background
The insertion of a peripheral intravenous catheter (PIVC, also called a cannula) is one of the most common clinical procedures performed1. About 30 million are used in Australia each year, with up to 70% of hospitalised patients requiring a PIVC2. However, it is well documented that PIVCs often fail before the completion of intravenous (IV) treatment3, with an incidence as high as 69%4. Researchers have shown that this failure is, in part, a result of inadequate stabilisation or securement of the catheter to the skin3. Such failure is multifactorial; it may be due to poor initial cannula securement, failure of dressings to remain adherent due to diaphoresis or, more commonly in a dynamic pre‑hospital environment, due to inadvertent traction on an attached IV line. In addition to interruption of IV therapy, poor securement technique increases the risk of phlebitis and infection, and leads to patient pain, dislodgement of the catheter, occlusion or blockage of the catheter2.
Intra-osseous (IO) access has become a popular alternative to repeated PIVC access attempts, particularly in the shocked pre‑hospital patient5. In the pre‑hospital or austere setting, IO should be at the forefront of vascular access options because it provides rapid and high technical success, with the ability to administer any drugs and fluids that are traditionally administered via IV6. However, whilst success rates and repeatability in IO access is sound, comparison of flow rates between IV and IO is discrepant, with several studies showing that IO flow rates may be limited to approximately 60–100mls/hr, even with pressure applied7,8. Comparatively, flow rates for an 18G PIVC averaged approximately 200mls/hr, with up to 450mls/hr with a 16G PIVC9. Additionally, while generally safe, IO it is not without limitations, such as pain, inability to aspirate blood, and is associated with small risks of extravasation, compartment syndrome, technical difficulties, displacement and osteomyelitis10. Therefore, if rapid IV access is achievable in the pre‑hospital environment, this is preferable and facilitates the rapid escalation of resuscitative methods once the patient reaches the next level of care.
Optimal dressing and securement of PIVCs in the pre‑hospital setting is a crucial practice strategy to reduce complications and failure; however, variations in practice are widespread11. PIVC dressing and securement are two inter-related interventions4. A PIVC dressing should cover the insertion site, keeping it clean and dry, be comfortable for the patient and offer protection from external contamination or trauma12. Further, PIVCs should be secured to the skin and stabilise the PIVC hub to minimise catheter movement. PIVC dressings should be cost-effective and easy to remove13. A recent, secondary analysis of over 40,000 PIVCs revealed that dressing and two-step securement combinations (tape dressing combined with tape or a tubular bandage) – termed ‘methods’ in this study – were significantly associated with decreased catheter site complications compared with a reference combination (simple polyurethane plus non‑sterile tape over the dressing14. Specifically, these two-step securement approaches were associated with fewer phlebitis symptoms, less bruising, and reduced ‘micro-movement’ of the device within the vein12.
PIVC are essential during pre‑hospital trauma resuscitation because both civilian and military guidelines recommend administration of blood products, IV fluids and medications15,16. However, patient outcomes can be negatively impacted if the PIVC fails before treatment completion17,18. Disaster or high threat conditions such as treating a trauma patient during a domestic terrorist event or in combat pose an even greater risk of accidental PIVC dislodgement (e.g., during extraction via helicopter or movement over rough terrain)19. Further, loss of vascular access in these austere settings may delay crucial fluid resuscitation or lead to missed treatment as limited opportunity or limited catheters or trained medics may prevent repeated PIVC insertion attempts20.
One way in which the United States (US) military has addressed the dislodgement risk in combat is to develop a securement intervention known as the ruggedised field (Ranger) IV method21. Army medics in the Australian Defence Force (ADF) have also developed a novel two-part securement method termed the S‑Wrap to reduce the chance of PIVCs being dislodged during casualty evacuation. However, the effectiveness of combat PIVC securement methods remains unclear, and we could find no studies that evaluated cannula securement in the austere, combat or disaster context.
Researching in a disaster or combat setting has numerous inherent risks, including physical harm to researchers, breach of confidentiality, legal action, or psychological discomfort22. When real-life situations preclude research opportunities, high fidelity simulations present an alternative option. High fidelity simulations are defined as a healthcare education methodology that involves sophisticated life-like manikins23. The ADF conducts high fidelity simulated combat casualty care training, termed tactical combat casualty care (TCCC). The TCCC training is mandated in the ADF for all healthcare professionals (HCPs), including defence medics, paramedics, nurses and physicians; thus, simulated TCCC training in the ADF affords a unique opportunity to evaluate the effectiveness of dressing and securement practices in the austere setting. The aim of this study was to address cannula failure due to inadvertent traction on an attached IV line by comparing the pull-out force of three PIVC securement methods and inform future securement practices.
Methods
Study design
A pragmatic in vitro design was utilised to compare the dislodgements with the Ranger technique, the S‑Wrap technique, and standard PIVC dressings on an attached IV line during TCCC simulations24. The assessment of pull-out force was used to determine the effectiveness of each PIVC securement method to prevent cannula dislodgement in vitro against a control. This study formed part of the larger multi-method S2C study, and the protocol has previously been published25.
Setting
The study was undertaken at the Australian Army School of Health (ASH) in Victoria, Australia. The ASH has world-class facilities, particularly simulation that affords realistic medical training to support military operations using simulated casualties.
Intervention (PIVC securement methods)
Control method
A standard PIVC securement method involved the use of a PIVC (Becton Dickinson [BD] InsyteTM Autoguard 18 GA, 1.16 IN 1.3x30mm) and connected IV saline lock (Baxter ClearlinkTM Luer Activated Valve) covered with a bordered IV transparent film dressing (3M TegadermTM IV 7x8.5cm) (Figure 1).
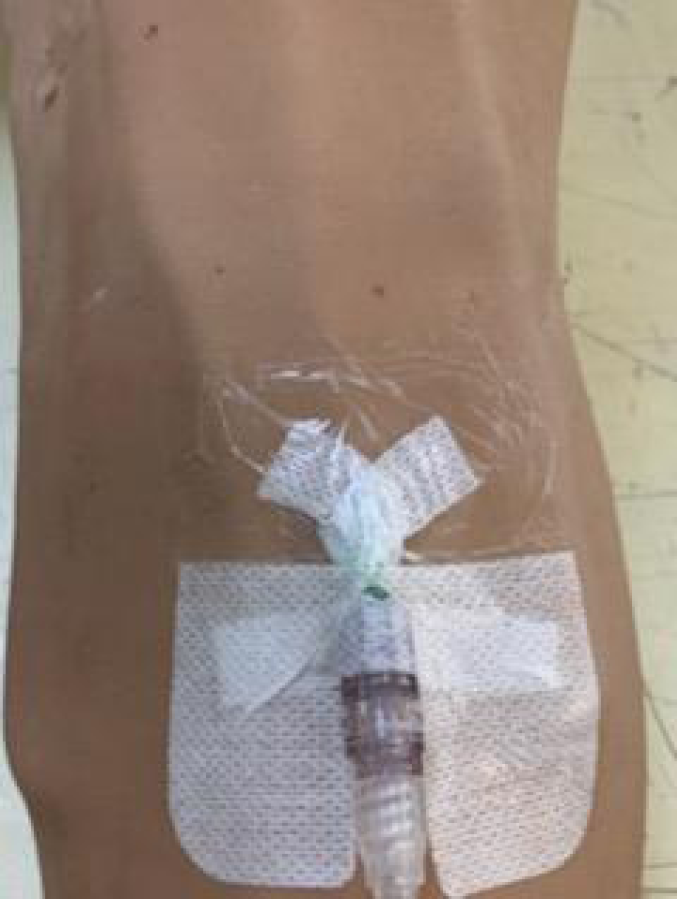
Figure 1. Standard IV securement technique
Ranger method
This securement method involved the use of PIVC (BD InsyteTM Autoguard 18 GA, 1.16 IN 1.3x30mm) and connected IV saline lock (Baxter IV Bung InterlinkTM Injection Site) that is established and covered with a bordered polyurethane dressing (Plastod HYPOR Film® 10x12.5cm). The subsequent step used the IV catheter (BD InsyteTM Autoguard 18 GA, 1.16 IN 1.3x30mm) inserted through the polyurethane dressing into the Baxter IV Bung InterlinkTM Injection Site, leaving the plastic cannula insitu and connecting the IV administration tubing (Baxter ClearlinkTM Solution Set) to the cannula and securing the line with the Raptor™ IV Securing Device (NA Rescue®). The Raptor IV securing device is a tapeless, secondary securement device that holds IV tubing in place with a secured hook and loop velcro closure (Figure 2).
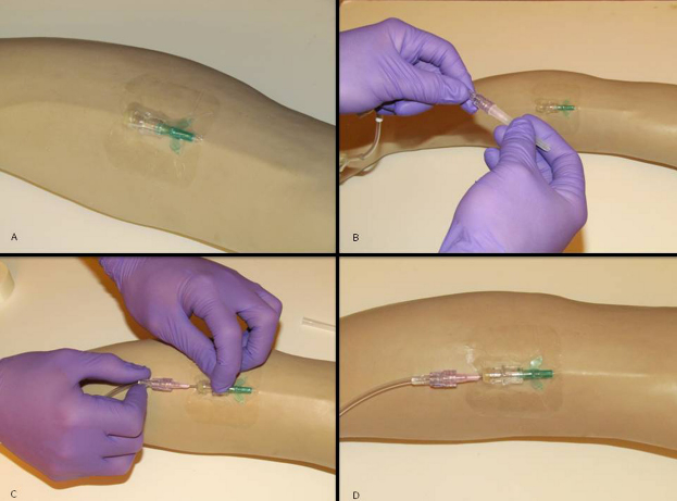
Figure 2. Ranger IV securement technique – to preview a video of this technique, go to:
https://www.youtube.com/watch?v=VP8QMhMGxe8
S‑Wrap method
A PIVC was inserted using the previously described method for the Ranger. The IV administration tubing (Baxter ClearlinkTM Solution Set) was connected to the cannula and then looped in an S shape between several layers of non‑elastic cotton crepe bandage (BSN Handy Crepe® 10cmx1.5m) secured with 3M Transpore™ Tape 2.5cm (Figure 3).
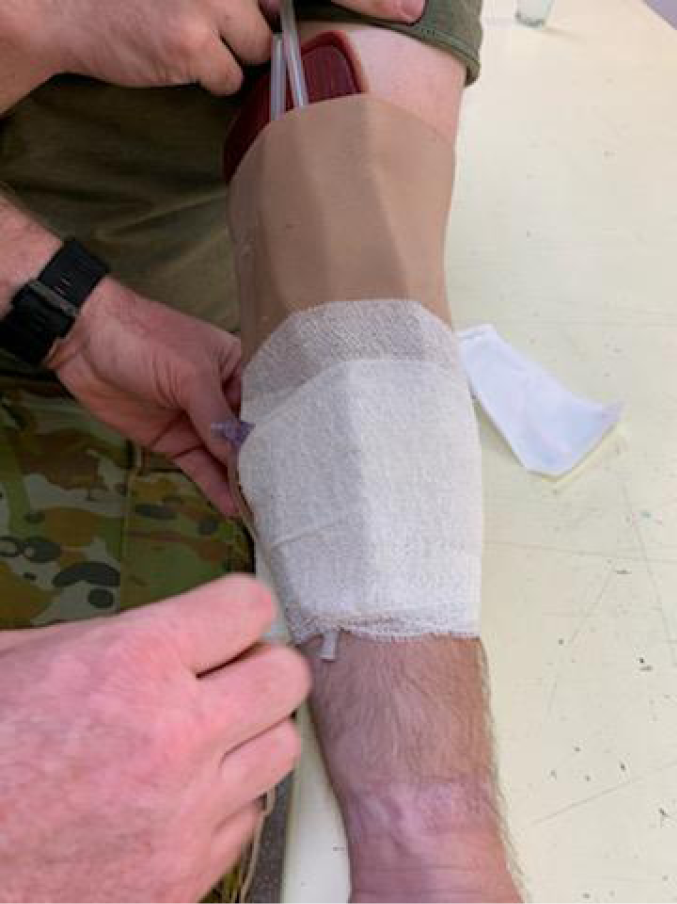
Figure 3. S-Wrap IV securement technique – to preview a full video of this technique, go to: https://vimeo.com/754161768
Study procedures
The study investigated the pull-out force in vitro of two PIVC securement methods against the control (standard care) method. Pull-out force was defined in this study as the tensile force measured in Newtons (N) required to produce PIVC dislodgement or PIVC equipment failure against the different securement methods. PIVC dislodgement was defined as the IV cannulae being partially removed from the vessel. Previous studies and the protocol have recommended pigskin as a surrogate for human skin; however, for pragmatic reasons in this study for comparative testing, each securement method was completed using a MEDIQUIP® Surgical Cut Suit component – sleeve. The Hyper-Realistic™ interchangeable sleeve is used routinely at the ASH in simulation because it benefits training by replicating the look and feel of skin or other wounds in Moulage (mock injuries) to practise actual procedures in the application of combat healthcare.
Under the sleeve was a padded plastic and rubber replica of a muscle. The design team inserted two 0.5mm clear plastic tubing between the muscle and the Hyper-Realistic™ interchangeable sleeve to simulate vessels that were used for the cannulation. This setup was then positioned on a study team member’s upper arm in the cubital fossa area.
Following PIVC securement, the IV administration tubing (Baxter ClearlinkTM Solution Set) was connected to the cannula according to the type of securement method. The IV administration tubing was then passed through a purpose-designed and built pulley system that achieved constant angles of 0˚ or 30˚ to the positive x-axis (Figure 4). These angles were selected because they attempt to replicate how IV administration tubing may become caught on a fixed object from any angle (e.g., tree branch, stretcher, body armour) in an austere combat setting.

Figure 4. The digital force gauge and pulley system
The IV administration tubing was attached to a calibrated handheld digital force gauge (ADM InstrumentsTM Lutron FG). This is a measuring instrument used across industries to measure the force during a push or pull test and aligns with previous studies examining PIVC dressing securement17.
Sixteen PIVCs using the control method were inserted and tested at angles of 0˚ (eight times) and 30˚ (eight times) until PIVC dislodgment. Sixteen (eight at each angle) were replicated for the S‑Wrap method and again for the Ranger method until PIVC dislodgment or IV equipment failure. The peak force occurring before dislodgment or equipment failure was measured on the digital force gauge and recorded.
A study team member (CW) experienced in cannulation performed the control and the two different securement methods, while another member (BM/BT) of the study team observed their practice to ensure consistency and accuracy. A third study member (DA) performed the pull-out force tests by continuously increasing tension on the catheter securement method at the designated angle through the pulley system. Each item utilised for cannulation and the different securement methods were in date, used only once, and correctly applied as per the manufacturer’s instructions.
Data analysis
The pull-out forces by product and direction of force were presented with means and standard deviations (SD) and on a scatter plots. The null hypothesis of no difference in average force between products was tested using one-way analysis of variance, followed by a post‑hoc Bonferroni test. Statistical significance was determined at p≤0.05. Data was using Stata 16 (StataCorp LLC, College Station, Texas, USA).
Ethics
This study was endorsed by the Australian Army Health Services Working Group, and reviewed by the Departments of Defence and Veterans’ Affairs Human Research Ethics Committee (DDVA HREC) and deemed to be an evaluation activity. This study is registered with the Australian New Zealand Clinical Trials Registry, CTRN12621000314820.
Results
The average pull-out force was lowest using TegadermTM (9.7N; SD 2.2) and highest with S‑Wrap (66.1N; SD 7.1). The securements provided greater resistance against the pull-out force at 0˚ compared to 30˚ (Table 1; Figure 5). The three Bonferroni-adjusted p values for the three null hypotheses between the three products were all <0.001. Dislodgment of the PIVC occurred using TegadermTM, while equipment failure occurred consistently with S‑Wrap (i.e., line breaking) and Ranger (i.e., second line dislodged) in achieving peak force measurement.
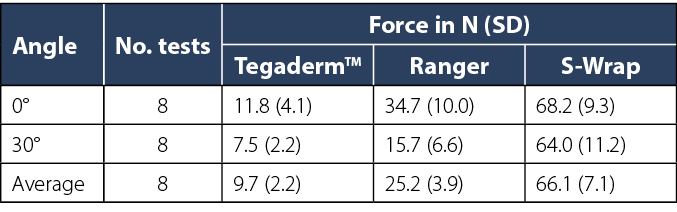
Table 1. Descriptive statistics of pull-out force outcomes by product
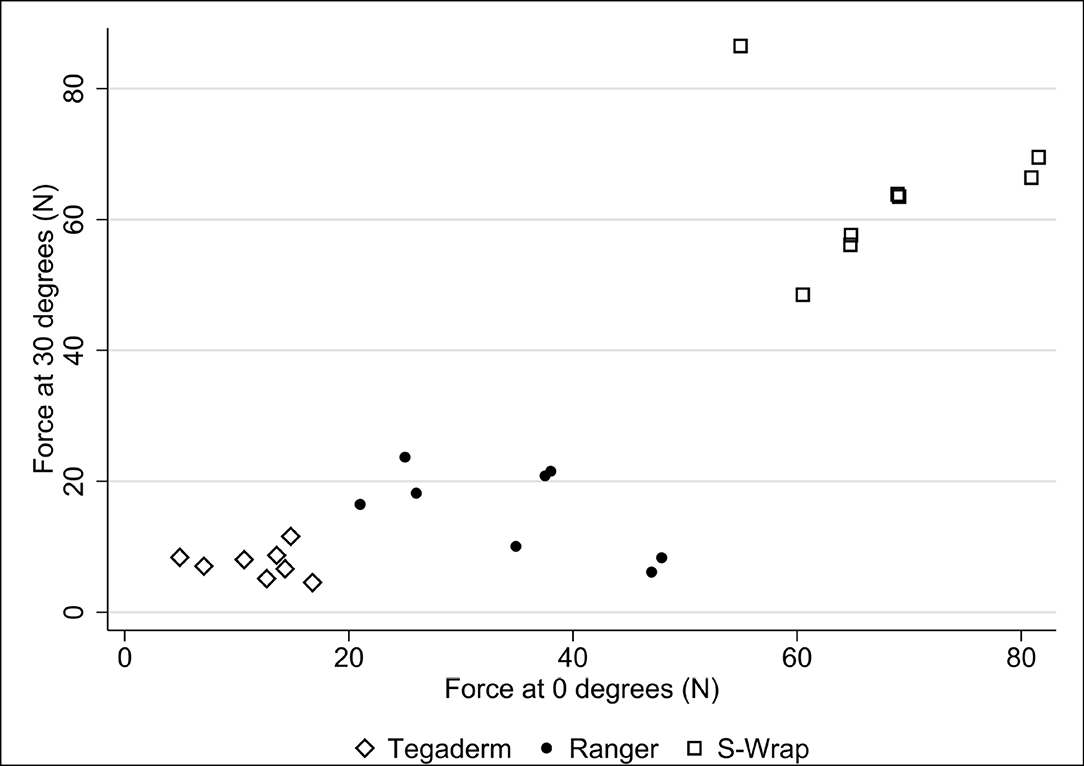
Figure 5. Results of pull-out forces by product
Discussion
This is the first study to evaluate PIVC securement in a simulated austere, combat context. Of the three methods of securement tested in this study (standard, Ranger, S‑Wrap), the S‑Wrap method demonstrated a significantly higher pull-out force. Further, our study found that the S‑Wrap is reliable, safe and provides a greater securement benefit than the next best alternative, the Ranger approach. Figure 5 demonstrates that, across all angles, the peak force measurement for S‑Wrap was over twice as high when compared to Ranger.
Vascular access in the austere or disaster environment is often complex as the environment can be volatile and/or uncertain, which may require prolonged care in the field26,27. The integrity of the IV dressing at the site is therefore paramount, regardless of line securement technique. Dressing integrity may be impacted by diaphoresis, haemoserous ooze or leaking IV fluid from the insertion site, as well as rolling or lifting of the dressing edges. A major contributing factor to device failure is inadequate fixation of the PIVC to the patient’s skin, causing not just dislodgement but also micromotion, leading to vein irritation (phlebitis or occlusion) and entry of skin bacteria into the entry site (infection)3.
Establishing the ideal PIVC securement technique is essential to guide formal training for military health practitioners, particularly as consumables (e.g., catheter and dressings), and equipment (e.g., administration sets and IV fluids) are often limited in this setting compared to civilian pre‑hospital settings28. Military clinicians must optimise their available resources, and our findings demonstrated the S‑Wrap technique required minimal stock and was therefore more effective at preventing PIVC dislodgement compared to a transparent dressing (standard care) and PIVCs secured with the Ranger approach.
In the austere/tactical and pre‑hospital setting there has been support to secure PIVC using the Ranger approach29. However, in 2014 an observational study highlighted that using the Ranger securement system can significantly delay fluid bolus administration rates, which could potentially delay resuscitation efforts21. Haemorrhage from the injured extremity is the primary cause of preventable death in military settings, and TCCC guidelines advocate for the rapid delivery of IV fluid for the hypovolaemic trauma patient in the tactical environment21,30. In these dynamic environments, IO access provides an effective vascular access alternative to PIVC6. However, with discordant flow rates between IV and IO access, if IV access is rapidly achieved it is preferable for resuscitation, and requires appropriate securement7,31–33.
In the austere/tactical setting military medics, nurses and physicians must feel confident that the PIVC securement approach they employ can withstand the additional demands that these dynamic environments realise28. The findings from this study will inform military medical training and pre‑hospital emergency care, and ensure best PIVC dressing and securement practices are embedded into future training to reduce catheter failure and any resultant patient harm.
Strengths and limitations
A limitation to this study was that the use of a simulated setting may not adequately represent a real-life disaster or combat setting. However, high-fidelity simulations afford a pragmatic alternative option. In addition, the pragmatic nature of this study meant that dressings and securements were only attached for a short period of time before the pulling force was applied. Therefore, the PIVC site (e.g., cubital fossa, hand) and the effects of soiling, sweat, blood or moisture were not taken into account. Further, the impact of different dressing options on IV flow rates was beyond the scope of this study. The acceptability of the S‑Wrap method was also not explored; however, this study formed part of larger mixed method study, and phase two aims to explore the real-world experiences of PIVC insertion and securement practices of ADF members during TCCC25.
Conclusion
PIVC securement is challenging in the austere or combat setting. The increasing use of damage control resuscitation in combat and austere settings has mandated the need for reliable, secure IV access. The initial step of a secure adherent dressing on the skin at the cannula entry site remains paramount. Results from this study support the use of the S‑Wrap technique to augment the initial dressing and prevent PIVC dislodgement. Further research, including clinical trials, is needed to establish the benefit of the S‑Wrap for preventing other PIVC complications including occlusion and infiltration.
Author contribution
BM, CW, DA, BT: manuscript write-up and collection of data. GM, JD, GW: manuscript write-up and data synthesis. GM, JD, GW: manuscript write-up and study supervisors.
Disclaimer
The views expressed in this presentation are those of the author(s) and do not necessarily reflect the official policy of the ADF or ASH. The use of trademark names do not imply endorsement by the ADF but is intended only to assist in the identification of a specific product.
Acknowledgements
The authors gratefully acknowledge the efforts of CPL Adrian Lee for designing and building the force test platform used in this study.
Orcid numbers
Benjamin R Mackie 0000-0002-7662-3357
Gabor Mihala 0000-0001-6654-405X
Joseph Dawson 0000-0001-9229-9545
Jessica Schults 0000-0002-5406-9519
Gillian Ray-Barruel 0000-0001-8875-7523
Nicole Marsh 0000-0002-5779-130
Conflict of interest
NM: Griffith University and the University of Queensland have received on her behalf speaker fees from 3M and investigator-initiated research grants from 3M, Becton Dickinson, Eloquest and Cardinal Health, unrelated to the current project.
GRB: reports consultancy payments provided to her previous employer (Griffith University) by product manufacturers (3M, Becton Dickinson) and education providers (Ausmed, Wolters Kluwer, Continulus), unrelated to this project.
Funding
The authors received no funding for this study.
Author(s)
Benjamin R Mackie*1,2, Darwin Alvarenga1, Christopher Williams1, Gabor Mihala3, Joseph Dawson4,5, Benjamin Tribe1, Guy Williams1,6, Jessica Schults8,9, Gillian Ray-Barruel7,9, Nicole Marsh11
1 Army School of Health, Australian Army, Latchford Barracks, Victoria
2 School of Nursing and Midwifery, Menzies Health Institute, Griffith University, Queensland
3 Centre for Applied Health Economics, Menzies Health Institute, Griffith University, Queensland
4 Department of Vascular and Endovascular Surgery, University of Adelaide, Royal Adelaide Hospital, South Australia
5 3rd Health Battalion, Australian Army
6 Albury Wodonga Health, New South Wales
7 School of Nursing, Midwifery and Social Work, University of Queensland
8 Herston Infectious Disease Institute, Metro North Hospital and Health Service, Queensland
9 UQ Centre for Clinical Research, Metro North Hospital and Health Service, Queensland
10 Nursing and Midwifery Research Centre, Royal Brisbane and Women’s Hospital, Queensland
11 School of Nursing and Midwifery, Griffith University, Queensland
*Corresponding author email benjamin.mackie@defence.gov.au
References
- Rickard C, Ray-Barruel G. Peripheral intravenous catheter assessment: beyond phlebitis. Lancet Heamatol 2017;4(9):e402–e03.
- Keogh S, Mathew S, Alexandrou E, et al. Peripheral intravenous catheters: a review of guidelines and research. Sydney: ACSQHC; 2019.
- Marsh N, Webster J, Mihala G, et al. Devices and dressings to secure peripheral venous catheters to prevent complications. Cochrane Database System Rev 2015;12(6). doi:10.1002/14651858.CD011070.pub2
- Marsh N, Larsen E, Genzel J, et al. A novel integrated dressing to secure peripheral intravenous catheters in an adult acute hospital: a pilot randomised controlled trial. Trials 2018;19(1):596. doi:10.1186/s13063-018-2985-9
- Granfeldt A, Avis S, Lind P, et al. Intravenous vs. intraosseous administration of drugs during cardiac arrest: a systematic review. Resuscitation 2020(Apr):150–57.
- Dornhofer P, Kellar JZ. Intraosseous vascular access. Treasure Island: StatPearls; 2022.
- Ngo AS-Y, Oh JJ, Chen Y, et al. Intraosseous vascular access in adults using the EZ-IO in an emergency department. Int J Emerg Med 2009;2(3):155–60. doi:10.1007/s12245-009-0116-9
- Pasley J, Miller C, DuBose J, et al. Intraosseous infusion rates under high pressure: a cadaveric comparison of anatomic sites. J Trauma Acute Care Surg 2015;78(2):295–99. doi:10.1097/TA.0000000000000516
- Wrenn E, Wohlers R, Montgomery M, et al. Comparison of flow dynamics of peripherally and centrally inserted intravenous catheters using a rapid infusion system (ThermaCor 1200). J Am Assoc Nurse Anaesth 2017;85(4):256–60.
- Fowler R, Lippmann M. Benefits vs. risks of intraosseous vascular access; 2014 [cited 2022 Nov 21]. Available from: https://psnet.ahrq.gov/web-mm/benefits-vs-risks-intaosseous-vascular-access
- Mason MF, Wallis M, Lord B, et al. Prehospital use of peripheral intravenous catheters and intraosseous devices: an integrative literature review of current practices and issues. Australas Emerg Care 2020;23(3):196–202. doi:10.1016/j.auec.2020.06.004
- Rickard CM, Ullman A, Kleidon T, et al. Ten tips for dressing and securement for IV device wounds. Aust Nurs Midwif J 2017;24(10):32.
- Campbell C, Bowden T. Peripheral vascular access devices: care and maintenance. Br J Cardiac Nurs 2011;6(3):132–40.
- Corley A, Ullman AJ, Mihala G, et al. Peripheral intravenous catheter dressing and securement practice is associated with site complications and suboptimal dressing integrity: a secondary analysis of 40,637 catheters. Int J Nurs Stud 2019;100:1–10. doi:10.1016/j.ijnurstu.2019.103409
- Avery P, Morton S, Tucker H, et al. Whole blood transfusion versus component therapy in adult trauma patients with acute major haemorrhage. Emerg Med J 2020;37(6):370–78.
- Ghosh S. Handbook of intravenous fluids. Singapore: Springer; 2022.
- Schmutz A, Menz L, Schumann S, et al. Dislodgement forces and cost effectiveness of dressings and securement for peripheral intravenous catheters: a randomized controlled trial. J Clin Med 2020;9(10). doi:10.3390/jcm9103192
- Bester BH, Sobuwa S. Utilisation of prehospital intravenous access. S Afr Med J 2014;104(9):615–8. doi:10.7196/samj.7969
- Quaile A. Manchester stands united after terrorist attack. J Paramed Pract 2018;10(7):300.
- Oliver G, Jones M. ECG or x-ray as the ‘gold standard’ for establishing PICC-tip location? Br J Nurs 2014;23(19):S10–6. doi:10.12968/bjon.2014.23.Sup19.S10
- Morgan TR. Evaluation of fluid bolus administration rates using ruggedized field intravenous systems. Wilderness Environ Med 2014;25(2):204–9. doi:10.1016/j.wem.2013.12.026
- Collogan LK, Tuma F, Dolan-Sewell R, et al. Ethical issues pertaining to research in the aftermath of disaster. J Trauma Stress 2004;17(5):363–72.
- Maran NJ, Glavin RJ. Low- to high-fidelity simulation – a continuum of medical education? Med Education 2003;37(1):22–28. doi:10.1046/j.1365-2923.37.s1.9.x
- Teddlie C, Tashakkori A. A general typology of research designs featuring mixed methods. Res in School 2006;13:12–28.
- Mackie B, Williams C, Tribe B, et al. The S2C study – securing cannulas in combat: a simulation-based mixed-method study. Vasc Access 2021;7(2):12–18.
- Schmid J, Pannell D. The origin, evolution, and future of prolonged field care in the Canadian Special Operations Forces Command. J Milit Vet Family Hlth 2022;1(8):97–103.
- Keenan S, Riesberg J. Prolonged field care: beyond the “golden hour”. Wilderness Environ Med 2017;1(28):S135–39.
- Evans N, Sekkarie M. Allocating scarce medical resources during armed conflict. Disaster Milit Med 2017;3(5).
- Hensley J. Ranger IVs decrease flow: life in the fast lane; 2021 [cited 2022 Nov 21]. Available from: https://litfl.com/ranger-ivs-decrease-flow/
- Unlu A, Kaya E, Guvenc I, et al. An evaluation of combat application tourniquets on training military personnel: changes in application times and success rates in three successive phases. J Royal Army Med Corps 2015;161(4):332–35. doi:10.1136/jramc-2014-000339
- Hammer N, Möbius R, Gries A, et al. Comparison of the fluid resuscitation rate with and without external pressure using two intraosseous infusion systems for adult emergencies, the citrin (comparison of intraosseous infusion systems in emergency medicine) study. PLoS One 2015;10(12).
- Deaton T, Auten J, Betzold R, et al. Fluid resuscitation in tactical combat casualty care; TCCC guidelines change 21-01. 4 November 2021. J Spec Operat Med 2021;21(4):126–37.
- Butler F. Fluid resuscitation in tactical combat casualty care: yesterday and today. Wilderness Environ Med 2017;28(2S):S74-S81.


