Volume 22 Number 1
Is it safe to compress venous leg ulcers without an ankle brachial pressure index?
Bernadette M Hannon, Caitriona M Canning, Collette B Fahy, Mary-Paula Colgan
Keywords venous ulcers, pedal pulses, ABPI and compression
DOI 10.35279/jowm202104.08
Abstract
Background Most wound guidelines recommend the measurement of an ankle brachial pressure index (ABPI) prior to the application of compression therapy. However, several studies have shown that this is not happening in reality, and the proper management of venous leg ulcers is delayed. Our study explored the safety of compression therapy for managing venous leg ulcers in the absence of an ABPI where pedal pulses are palpable.
Materials and Methods This study included 149 consecutive new patients referred to a vascular medicine clinic with lower extremity ulceration. Full histories were recorded and a physical examination was performed at the initial visit, and a provisional diagnosis of ulcer aetiology was made. ABPIs were only performed if pedal pulses were not palpable. Patients diagnosed with venous ulceration were treated with compression therapy and followed-up at 12 weekly intervals for up to one year, per our clinic’s protocol.
Results The study included 60 males and 89 females (mean age 71 years, range: 24–100 years). The mean ulcer history was nine months (1 week–10 years); 101 ulcers (68%) were venous in aetiology. ABPIs were only performed on four patients (4%), when pedal pulses were not palpable. The remaining 97 patients were treated with full compression (ankle pressure 30–40 mmHg) in the absence of ABPIs. No adverse events occurred during follow-up, and 65 patients had fully healed at four months.
Conclusion In our patient cohort, it was safe to compress venous ulcers in the absence of ABPIs when pedal pulses were palpable.
INTRODUCTION
The introduction of the Charing Cross four-layer bandage in the 1980s1 had a major impact on the management of lower extremity venous ulcers in Western Europe. Since then, the role of compression has been widely accepted, with a Cochrane review2 confirming the superiority of multi-layer compression in the management of venous leg ulcers. In spite of this compelling evidence, a large percentage of ulcer patients are not managed using compression bandaging. There are many reasons for this, one of which is that an ankle brachial pressure index (ABPI) is mandated prior to commencing compression, and this is not being performed.3 The absolute need for an ABPI has been perpetuated in most guidelines4–8; however, few vascular specialists perform ABPIs if pedal pulses are palpable. Additionally, as the population ages and diabetes and renal disease become more prevalent, ABPIs are often misleading, due to arterial calcification. In these instances, toe pressures are required for making a treatment decision. Surely, it is time to return to our clinical skills and use ABPIs selectively?
The aim of this study was to determine if it is safe to compress venous ulcers in the absence of an ABPI if pedal pulses are palpable. Patients were followed at 12 weekly intervals to document ulcer healing rates and possible complications of the compression therapy.
MATERIALS AND METHODS
Over a nine-month period, all consecutive patients referred to a vascular medical service with new lower extremity ulcers were enrolled in this study, which was a simple observational study of our normal clinical practice. Data were collected on referral diagnosis, the performance of ABPIs in the community and/or at their hospital visit and treatment following the referral. In keeping with our normal departmental protocol, all patients received a provisional clinical diagnosis upon completion of their first hospital visit. If the ulcer was considered venous in aetiology, patients were treated with one of three compression regimes, an Unna boot, Coban 2® or Actico®. Patients’ wounds were dressed and compression was applied once or twice weekly by their public health nurse. Patients were reviewed at 12 weekly intervals, as per our clinic’s practice, to document healing, in addition to any possible side effects from the compression therapy. Patients were followed until they were fully healed, or for up to one year. This study was conducted prior to the study published by Gohel et al.9, and no patient was considered for surgery at their initial visit. Ethical approval was not sought, as this was an observational study of our normal clinical practice.
RESULTS
There were a total of 149 patients (89 females, 60 males) with a mean age of 72 years (range: 24–100). All were referred with lower extremity ulceration that had been present for a mean of 10 months (range: 1 week–156 months). Thirteen patients (9%) had documented ABPIs on referral. Eleven of the 13 had palpable pedal pulses, but only three were in compression.
Fifty of the total patient cohort (33%) had a diagnosis included on their referral letter, and 40 of these were considered venous in aetiology. Of the 40 patients referred with a diagnosis of a venous ulcer, only five had a documented ABPI. Of these five, only two were in compression. An additional three patients were in full compression, with a further four in reduced compression.
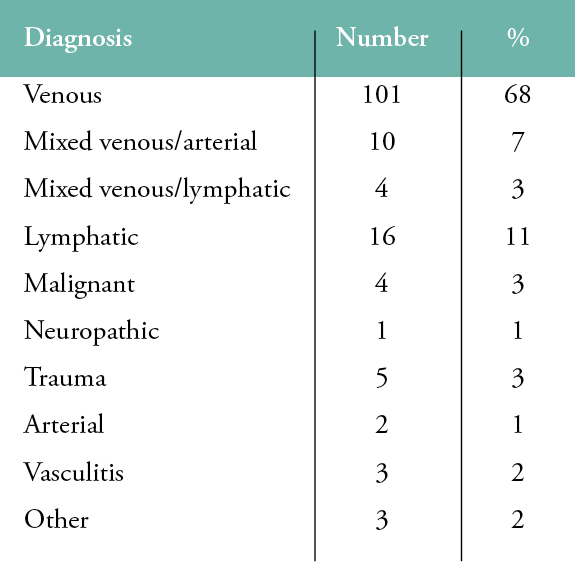
A provisional diagnosis based on the patient’s history and a physical examination was made for all patients during their first hospital visit, as illustrated in Table 1. A total of 101 patients (68%) were considered to be suffering from a venous ulcer at presentation, and these formed the population of this study. There were 58 females and 43 males with a mean age of 71 years (range: 24–97). The mean ulcer size was 12cm2, and the mean ulcer history was seven months (range: 1 week–120 months). Ninety seven patients (97%) had palpable pedal pulses and were treated with full compression without ABPIs. We performed ABPIs on the remaining four patients. Two had calcified tibial arteries, and two had mildly reduced ABPIs, in keeping with mild peripheral arterial disease. Toe pressures in all four cases were well above ischaemic range, and all were treated with compression.
Two patients died of unrelated causes during the follow-up period, and 11 patients (11%) were lost to follow-up. Sixty one percent of the venous ulcer patients were fully healed at four months, and 78% were at six months. There were no complications related to compression noted during the study.
Table 1: Provisional diagnoses during the first hospital visit
DISCUSSION
Lower extremity ulceration remains a huge drain on health resources.10–13 The annual cost incurred by the National Health Service in the UK has been estimated to be £5.3 billion, or 4% of the total expenditure of the publicly funded NHS budget.10 In 2017, Guest et al. retrospectively reviewed 2,000 patient records and found that 12% of treated wounds lacked a specific diagnosis; the annual growth rate of this group of wounds was 13%.11 They emphasised the importance of patient-specific treatment plans and the need for greater senior clinical involvement in dealing with chronic wounds. A 2018 publication reported that the average annual cost of treating a venous leg ulcer is £7600.00, but this increased 4–5 times for unhealed ulcers, or £2981 per healed venous ulcer, compared to £13,455 per unhealed ulcer.3 They concluded that progressive integrated pathways of care were needed, as were dedicated wound clinics to improve care and help reduce costs.
Over the last three decades, and in spite of improved technology and advances in wound care products, we are failing to make a significant impact on healing rates. Reported healing rates vary from 40% to >70% at 12 weeks.14–16 Lazareth et al.14, in a European randomised controlled trial of two compression systems, reported healing rates of 38–48% at 12 weeks. Others have reported healing rates of 70% at 24 weeks, while more recently a 53% healing rate at 52 weeks was reported in a large retrospective review. One of the most striking issues is the continued delay in the initiation of compression bandaging. Guest et al.3 reported in 2018 that only 12% of lower extremity ulcer patients had ABPIs documented in their records within the first three months of diagnosis. An additional 10% underwent ABPI measurements after the first three months. In our patient population, only 12.5% of those patients with presumed venous leg ulcers had documented ABPIs performed prior to referral, with only two of the five in compression. An additional seven patients without ABPIs (20%) were in either modified or full compression, despite a working diagnosis of venous ulceration; this suggests the presence of a significant barrier to standard care in the community management of venous ulcers.
In our initial clinical assessment, 95% of venous ulcer patients were found to have palpable pulses, did not require ABPIs and were compressed without any further arterial testing. Assessment facilitating the earlier commencement of compression therapy is a key strategy for improving ulcer healing rates in this patient cohort.
The recommendation for mandatory ABPIs delays the commencement of compression bandaging in venous leg ulcer patients with normal pedal pulses. The other downside to ABPIs is that they may be difficult to interpret, particularly without a full arterial assessment of the patient. In one study of patients with critical limb ischaemia, 30% were found to have an ABPI between 0.7 and 1.4.16 As an ABPI within normal limits (0.9–1.2) does not necessarily equate with the absence of arterial disease, the overreliance on ABPIs must be questioned. A history, physical examination and an ABPI if pedal pulses are not palpable, interpreted in the context of the clinical findings, is a more accurate pathway for diagnosing the aetiology of the ulcer and deciding if the patient is a suitable candidate for compression.
We suggest the following two-pronged approach. First, all leg ulcers should have a working diagnosis of underlying aetiology, rather than being considered simply as wounds. In all other fields of medicine, treatment is always directed at a specific cause (e.g., one would never prescribe a treatment for ‘abdominal pain’ without knowing or suspecting its underlying cause). We know there are a number of aetiologies of leg ulcers, including venous disease, arterial disease, lymphatic disease, infection, trauma and malignancy, to name but a few. Each aetiology is managed differently, but this can only be done if we have a working diagnosis, so this must become part and parcel of our initial assessment. Without a diagnosis, the patient will receive sub-optimal care.
Second, we must encourage competent clinical skills, including the routine palpation of pedal pulses. Practitioners need to move away from a complete reliance on ABPI measurements and be more comfortable commencing compression bandaging at the initial presentation if arterial circulation is intact. The development of clinical skills is an integral part of medical and nursing education. We never question the ability to palpate radial or carotid pulses, yet we seem to believe that the palpation of pedal pulses is a redundant skill for medical and nursing professionals.
This paper shows that, in our patient cohort, it was safe to compress venous ulcers in the absence of ABPIs when pedal pulses were palpable.
Key messages
The aim of the study was to determine if it is safe to compress venous ulcers in the absence of ABPIs if pedal pulses are palpable.
Our results shows that the vast majority of venous ulcer patients had palpable pedal pulses and support the use of compression therapy in the absence of ABPIs when pedal pulses are palpable.
Author(s)
Bernadette M Hannon, MA, Caitriona M Canning, MB Collette B Fahy, Dip Appl Science, Mary-Paula Colgan, MD
Department of Vascular Medicine, St. James’s Hospital
Correspondence: mpcolgan@stjames.ie
Conflicts of Interest: None
References
- Thomson B, Hooper P, Powell R, Warin AP. Four-layer bandaging and healing rates of venous leg ulcers. J Wound Care. 1996; 5(5): –.213-216.
- O’Meara S, Cullum N, Nelson EA. Dumville JC. Compression for venous leg ulcers. Cochrane Database Syst Rev. 2012; 11:CD000265.
- Guest JL, Fuller GW, Vowden P. Venous leg ulcer management in clinical practice in the UK: Costs and outcomes. Int Wound J. 2018; 15(1):29–37.
- Health Service Executive. HSE national wound management guidelines. Dublin: HSE Clinical Strategy and programmes Division2018.
- EWMA. Management of patients with venous leg ulcers. Challenges and current best practice. A joint EWMA document. J Wound Care. 2016; 25:S1–S67.
- O’Donnell TF, Passman MA, Marston WA, Ennis WJ, Dalsing M, Kistner RL, et al. Management of venous leg ulcers: Clinical practice guidelines of the Society for Vascular Surgery and the American Venous Forum. J Vasc Surg. 2014; 60:3S–59S.
- Australian and New Zealand clinical practice guideline for prevention and management of venous leg ulcers. Sydney Cambridge Publishing; 2011.
- Royal Society of Medicine, Venous Forum. Management of patients with leg ulcer. London: Royal Society of Medicine; 2019.
- Gohel MS, Heatley F, Liu X, Bradbury A, Bulvulia R, Cullum N et al. A randomised trial of early endovenous ablation in venous ulceration. New Engl J Med 2018; 378(22):2105–14.
- Guest JL, Ayoub N, McIlwraith T, et al. Health economic burden that wounds impose on the National Health Service in the UK. BMJ Open. 2015; 5:e009283.
- Guest JL, Vowden K, Vowden P. The health economic burden that acute and chronic wounds impose on an average clinical commissioning group/health board in the UK. J Wound Care. 2017; 26 (6):292–303.
- Gillespie P, Carter L, McIntosh C, Gethin G. Estimating the health-care costs of wound care in Ireland. J Wound Care. 2019; 28(6):324-30..
- Phillips CJ, Humphreys I, Thayer D, Elmessary M, Collins H, Roberts C,et al. Cost of managing patients with venous leg ulcers. Int Wound J. 2020;17(4):1074-82.
- Lazareth I, Moffatt C, Dissemond J, Lesnie Padieu AS, Truchetet S, Beissert S, et al. Efficacy of two compression systems in the management of VLUs: Results of a European RCT. J Wound Care. 2012; 21(11):553–61.
- Vowden KR, Mason A, Wilkinson D, Vowden P. Comparison of the healing rates and complications of three four-layer bandage regimes. J Wound Care. 2000; 9(6):269–72.
- Guest JL, Fuller GW, Vowden P. Clinical outcomes and cost-effectiveness of three different compression systems in newly-diagnosed venous leg ulcers in the UK. J Wound Care. 2017; 26:244–54.
- Bunte MC, Jacob J, Nudelman B, Shishehbor MH. Validation of the relationship between ankle–brachial and toe–brachial indices and infragenicular arterial patency in critical limb ischemia. Vasc Med. 2015; 20(1):23–9.
