Volume 22 Number 2
The HERMES Study – blue ligHt photobiomodulation thErapy on neuRoischeMic patiEntS – Experimental Design and Study Protocol
Alberto Piaggesi, Alessia Scatena, Sara Sandroni, Stefano Gasperini
Keywords diabetic foot, ischemia, blue light, photo-biomodulation, therapy
DOI 10.35279/jowm202107.07
Abstract
Aims To evaluate the safety and effectiveness of photobiomodulation1 therapy in addition to the standard of care (SoC) for managing diabetic foot ulceration (DFU), we designed a prospective randomised double-blind trial for neuro-ischemic patients (HERMES study), whose design and study protocol we describe in this paper.
Patients and methods All patients with a chronic neuro-ischemic DFU wider than 1 cm2 attending the S. Donato Hospital DF Clinic in Arezzo, Italy (I), will be screened for enrolment.
After two weeks, while patients are treated with SoC, those whose lesions have not decreased by 50% or more in size will be randomised into two groups: the control group will be managed with SoC, while the study group will be treated with photobiomodulation + SoC twice weekly for 20 weeks, or until healing. Both groups will be managed in the community by visiting nurses. The outcomes [healing rates at 24 weeks (primary endpoint), healing times, speed of area reduction, pain, quality of life, adverse events] will be blinded to the treatment.
Results As a pilot study, we cannot anticipate results, but we expect a positive difference of at least 15% in the study group’s primary outcomes, compared to controls, with no worsened safety profile.
Conclusions and implications for clinical practice The interest of the HERMES study, beyond the findings related to the efficacy and safety of photobiomodulation, lies in the characteristics of this low-cost, no-waste technology and its integration in specialist and community-based care.
Background The complications of diabetes mellitus (DM) in the lower limbs, generically known as ‘diabetic foot’ (DF), affect one in three patients with DM at least once in their life and represent the most prevalent cause of lower extremity amputation (LEA) in the world, such that it is estimated that a limb is lost every 20 seconds because of DF.1,2
The pathology of DF is multifactorial, involving in its aetiology both endogenous [neuropathy, peripheral arterial disease] and exogenous (infection, repetitive traumas) components, and it is characterised by the frequent and recurrent development of ulcers (DFU), typically marking the acute phases of the disease, which represent the most peculiar and clinically relevant aspect of the pathology.3
The pathogenesis of DFU is complex and conditioned by the many components of the disease; if the occurrence of the lesion can actually be attributed to the increased pressure in an insensate and deformed neuropathic foot, the progression and the delay of healing is mostly conditioned by local ischemia, which limits the possibility of tissue repair progression and freezes the lesion in a low-intensity, chronic inflammatory state, prolonging the ulcerative condition far beyond the physiological healing time.4
How the duration of a DFU directly correlates with the likelihood of developing an infection, which in turn increases the risk of LEA up to 300%,5 has been demonstrated.
Since LEAs are preceded in 85% of cases by a DFU, it is evident that fast and effective in healing of DFUs will mean a reduction in the amputation risk for a vast majority of patients with DF.6
The management of DFUs is, per se, a complex issue, since there are many different components of the therapy that must be implemented to adequately accomplish the task, as clearly outlined in the international guidelines released every four years by the International Working Group on Diabetic Foot (IWGDF).7
Relief of pressure, debridement, treatment of infection and restoration of blood supply are the cornerstones for managing DFU and are considered the standard of care (SoC) for this pathology.7
In recent years, revascularisation procedures, technically adequate offloading, specialised surgery and better local care have led to a positive change in the prognoses of DF patients, leading to a significant decrease in the number of LEAs in Europe and the USA.8,9
This important achievement, although changing the outcomes of DFU in the medium term, have not significantly affected long-term prognoses, since frequent recurrences transform an acute problem in a chronic, recurrent and worsening ulcerative condition.3
These new clinical patterns have drastically changed the paradigm of care for the disease, from a perspective in which the aim was to cure the DFU to heal the patient, to a situation in which the cure of the DFU would be finalised by giving the patients as many ulcer-free days as possible.10
In this situation, the possibility for managing the ulcerations in an effective and timely manner becomes central to the project of caring for DF patients, not only to give them a maximum chance of living a good quality life, but also for decreasing the possibility of complications such as infection or gangrene, which would lead the patient to a much more likely LEA.11
Blue Light Therapy
The application of physical treatments to cure many forms of chronic ulcers dates back a number of years, to when many experiments were done to test a variety of options.12,13 The advantages of physical therapies for the management of chronic ulcerations, when compared with the more traditional bio-chemical approach, lie in the different relationships between the therapeutic mean and the biology of the lesion; while in the latter the effects are mediated by a direct interaction, in the former the interaction is indirect and the consequences are the results of the physical modifications of the environment of the ulcers, which can be affected in many ways at multiple levels and with a single application.14
Electricity and magnetism, positive and negative pressure, ultrasounds, a range of ionising radiations and many other means have all been tested with varying effects on chronic wounds; a summary of these experience has recently been published in an EWMA Document on new technologies in wound management.14
Phototherapy, using either coherent (laser) or non-coherent light, has found application in many different clinical models with generally good results, although the rationale, selection of patients, mode of application and outcomes are highly variable from one study to another.15–17
More recently, a blue LED light (420 nm) was tested in wound repair, initially as a haemostatic mean, and then in a wider range of ulcerative conditions, testing and demonstrating a number of different effects in the process of wound repair and tissue regeneration.18
The choice of this range of light frequency is related to the observation that both haemoglobin and protoporphirin IX absorb light of this frequency. In the case of the absorption from haemoglobin, the effects are related to the heat generated (the photothermal effect), while in the case of protoporphirin IX, the effect is direct (the photochemical effect) and interferes with many different biological processes involved in wound healing.19–22
The cellular target of the blue light’s application is Cytochrome C, a component of the mitochondrial electron transport chain responsible for ATP synthesis. The light absorption increases the formation of ATP molecules, giving the cell extra energy, which is important, especially during the reparative phase of tissue regeneration.24–26
Moreover, the application of blue light has been associated with the increase of reactive oxygen species (ROS), and in the activation of macrophages, both of which are important during the inflammatory phase of wound repair.23
A pro-angiogenetic action has also been demonstrated after the application of blue light in animal models of chronic ulcers, which can be beneficial in all the stages of tissue repair.27
From these promising findings, a class IIa medical device was designed, engineered and manufactured by an Italian company,2 which patented the technology and the device, named it and tested it in a number of clinical applications involving pilot trials on both venous leg ulcers and difficult-to-heal lesions. These yielded positive, although anecdotal, results.28–31
This is a portable device that employs LED sources emitting non-coherent blue light with a wavelength between 410–435 nm and an optical power density of 120 mW/cm2, powered by a rechargeable lithium battery (Fig. 1). The portability and non-invasiveness of the device make it suitable for its use both in hospital and community settings, and for the management of chronic ulcerative pathologies.
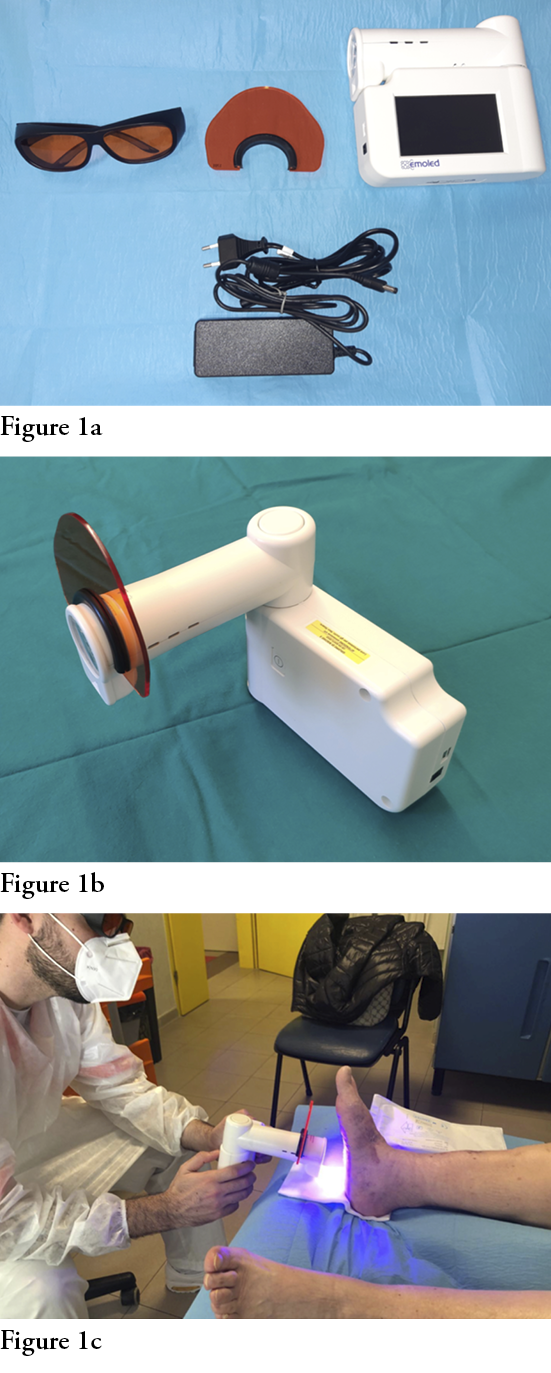
Aims of the study
With the aim of testing the safety and efficacy of blue light photo-biomodulation in addition to SoC for the management of chronic DFUs, in comparison with SoC alone, we designed a prospective double-blinded randomised study (HERMES) to be conducted with outpatients, followed by specialised centre and treatment in a community setting in Tuscany. The study was registered with clintrials.gov (# NCT04831606).
Patients, materials and methods
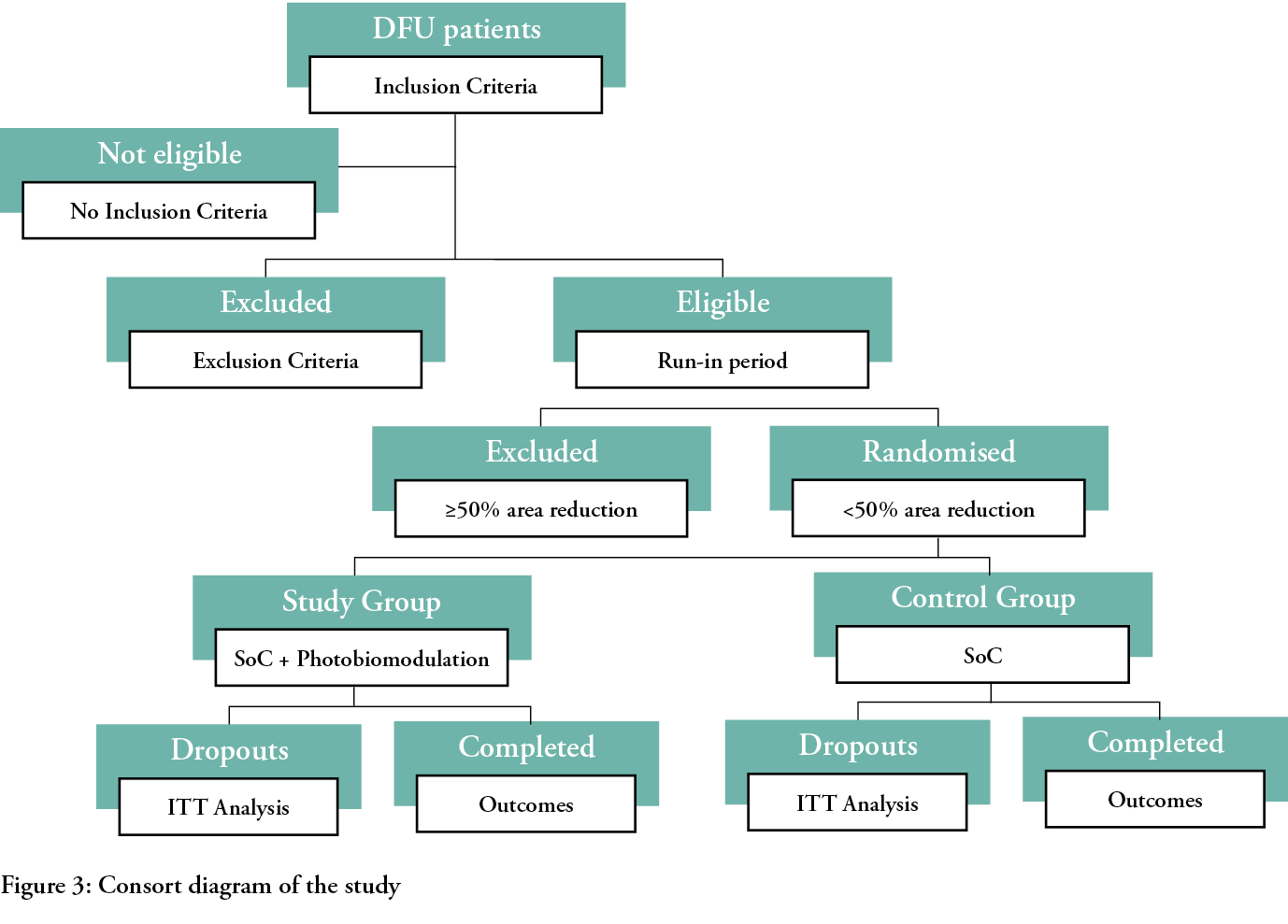
All new patients consecutively presenting at the investigator site, a specialised centre for DF (DF Clinic of the Hospital S. Donato, Arezzo, Italy) will be screened according the following inclusion criteria: they should be over the age of 18; affected by T1DM or T2DM with a glycated haemoglobin ≤10% (86 mmol/mol); peripheral neuropathy confirmed with Semmes-Weinstein monofilament (5.07/10g/cm2); an ankle/brachial pressure index (ABPI) of 0.7–0.9; a DFU (IC–IIC according to the Texas University Scoring System - 32) wider than 1 cm2 located on the toes, on the plantar side, on the margins or on the dorsum of the foot lasting from 1–24 months. In cases of more than one lesion present on the same foot, only one will be selected for the sake of the study, based on the judgement of the investigator, and then identified as the index lesion.
Patients could be either ambulatory or admitted to the hospital at the moment of the screening, but then they should be followed on an outpatient basis in the community by visiting nurses; patients should be willing to be included in the study and agree to follow all the prescriptions, including wearing the offloading devices. They should also sign a written informed consent document before entering the study. The nurses who will carry out the dressing changes and treatments are specialist experts in wound care who belong to the skin lesions network.
Exclusion criteria will be: location of the ulcer on the heel or pressure ulcers, dialysis treatment, being bedridden or not ambulatory, having a life expectancy of less than one year, being pregnant or breastfeeding, the presence of infection or osteomyelitis according to the criteria of the Infectious Disease Societies of America,33 having a diagnosis of Charcot’s disease, being affected by cancer or any chronic pathology potentially interfering with tissue repair, being treated with high doses of steroids or immuno-suppressants, psychiatric disturbances that might interfere with the course of the treatment, photosensitisation, participation in a clinical study in the three months preceding the enrolment, not being able to understand the scope of the study and being incapable of providing adequately informed consent.
Once patients are screened and sign the informed consent document, they will be treated for two weeks with SoC and then re-evaluated. If the ulcer will be reduced in size ≤50%, they will be randomised into one of two groups: the control group will be treated with the SoC according to IWGDF guidelines. A synthetic anamnesis and demographics of the patients, alongside with their systemic and local conditions, will be recorded; the eventual presence of pain will be evaluated with a VAS scale. The lesion will be surgically debrided, removing all necrotic and non-viable tissue, cleaned with saline solution and then measured with a dedicated electronic device.3 The lesions will then be dressed with sucrose octasulfate-impregnated gauzes and the offloading device4 will be applied according to the instructions of manufacturer. These will be rendered irremovable by the patients, according to indications in the IWGDF guidelines.7,34,35
After the first visit, patients will be followed twice weekly by visiting nurses who, at the patient’s home, will remove the offloading device and dressing; check for eventual adverse events; clean the lesion with saline solution; and reapply the dressing and offloading device, securing it so that it is not removable by the patient.
In the case of a non-severe adverse event (i.e., a mild or moderate infection), this would be recorded and the physician responsible for the patient will be informed so a timely decision can be made on how to deal with it and whether or not the patient may continue with the study or be removed.
In the case of a severe adverse event (i.e., a severe infection) the patient will be withdrawn from the study and the communication sent for monitoring within 24 hrs, together with all information regarding his or her condition and actions taken.
The patients in the study group will be managed in the same way, with the adjunct of application of EmoLED® after the dressing removal and cleaning with saline solution. The application will consist of irradiation for 2 minutes for every 5 cm2 of the lesion, until the entire surface of the lesion is covered. Then, the dressing and offloading will be re-applied.
The duration of treatment will be 20 weeks, for a total of 40 home visits, but the patients will be followed up to 24 weeks.
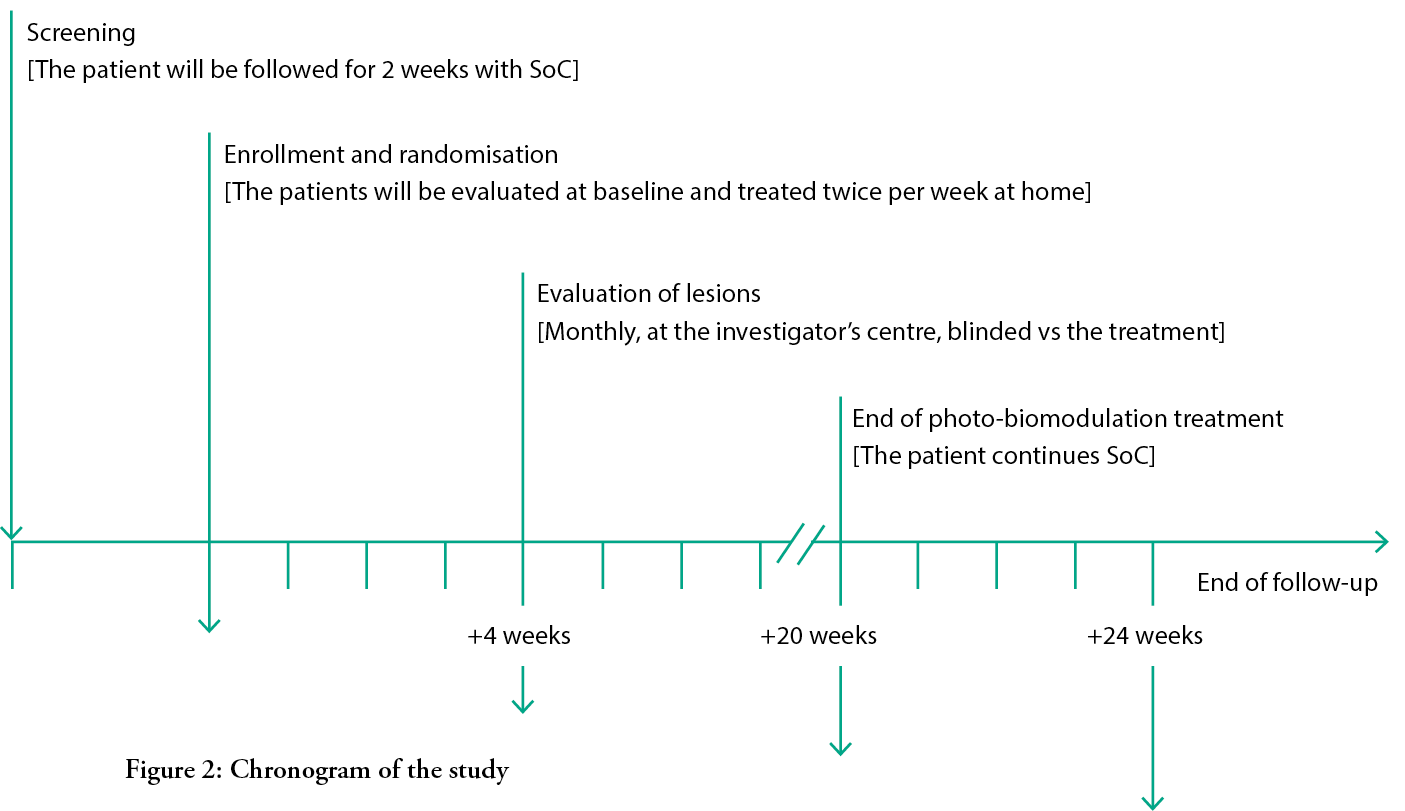
Once per month, patients will visit the specialised centre, where the investigator will measure the lesion area and score it, making a clinical evaluation of both the lesion and the patient, looking for possible adverse events and evaluating their pain using the VAS scale. Compliance with the treatment and offloading device will also be monitored.
After the visit on the 20th week, if the lesion is not yet healed, patients in both groups will receive only SoC, with the same frequency and in the same way they were treated in the previous 20 weeks, but without light exposure.
At the 24-week visit in the centre, the patient will undergo the same procedures and evaluations conducted in the other monthly controls at the specialist centre. If the lesion has healed before the end of the follow up period, the patient will have one extra visit at the centre within one week from the supposed healing; if the investigator confirms the healing, the final visit protocol is then performed, and the healing time is set as the previous visit. Otherwise, the patient will continue with the study until healing is achieved.
To standardise the procedures and evaluations, all investigators and visiting nurses will be trained in a dedicated session held before the start of the screening period at the DF clinic of the Hospital of S. Donato, in Arezzo.
All investigators and visiting nurses will wear personal protection devices during the visits and evaluations of patients and lesions, and rules and procedures for preventing COVID-19 dissemination will be adopted and implemented according to the indications of local health authorities.
In the case of a possible COVID-19 infection, the rules for preventative isolation, contact-tracing and viral screening will be put in place, as per Italian Ministry of Health indications; patients will be suspended from the study until the results of the screening are released. In the case of a negative result, the patient will re-enter the study, and the symptoms will be considered an adverse event and managed as such. In the case of a positive result, it would be considered a severe adverse event, recorded and reported as such, and the patient will be removed from the study.
At the first visit and at the follow-up visit at 24 weeks (or at the last visit, in cases where the lesion heals before the end of the follow up period), patients will be asked to complete two questionnaires evaluating their quality of life (EuroQuol 5.0 and WoundQuol); moreover, they will be asked to express their appreciation for and level of satisfaction with the treatment received, by answering questions targeted these items.
To secure the double-blindness of the study, both the investigators in the hospital centre (AS) and the patients will be unaware of the randomisation, which will be performed using freeware (www.randomization.com) managed by an external technician not involved in the study (DR) and communicated via mail only to the responsible visiting nurses (SS), who will organise the home visits and allocate patients into the groups according to the results of the randomisation.
Patients will be blinded to the treatment by the use of opaque plastic glasses of the same kind used in phototherapy settings to protect the eyes from actinic damage; the nurses will irradiate the lesion in the patients belonging to the study group, but they will turn the lamp to the on the other side in the cases of the patients in the control group, although they will complete all the same operations otherwise.
All measurements and clinical evaluations will be performed by the investigators at the hospital centre, who will be blinded to the treatments the patients have actually received.
Dimension of the sample
Being the first prospective trial to use blue light therapy in DFUs, there are no previous studies to refer to calculate the power and dimension of the sample.
This study should be considered a pilot trial, and from this perspective, in consideration of the real possibility of captioning the study centre, we aim to include 40 patients during an 18-month enrolment period.
To select these patients, we forecast screening 200 consecutive patients, which will represent roughly 2/3 of the patients seen in the centre over 18 months.
Calculating a 15–20% drop-out rate, we would end the study with 32–34 patients who have completed the treatment; the results will then be analysed according to the intention to treat model, and so referred to the 40 patients enrolled.
The duration of the study, considering the 18-month enrolment period, the 2-week pre-randomisation and the 24-week follow-up, will be 24 months.
Outcomes
The following outcomes will be evaluated: the number of adverse events; the number of severe adverse events, with special attention to deaths, amputations, infections and recurrences, to define the techniques’ safety; healing rate at 24 weeks; healing time; speed of area reduction; pain intensity, to define the effectiveness of the treatment and patients’ quality of life; and satisfaction of the patients and nurses with the treatment.
Cost-effectiveness will be evaluated by measuring all direct and indirect costs related to the care provided during the whole treatment process.
Healing will be considered to be the complete and durable re-epithelisation of the ulcer, with no secretions or fistulae of sinuses, confirmed at two visits within one week. The healing date will be reported as the first finding of re-epithelisation. Though there are no published clinical data to which to refer to anticipate, being a superiority study, we can expect a difference of at least 15% in favour of the blue light treatment in addition SoC, compared to SoC alone.
Statistical analysis
Data will be reported as continuing or categorical variables; the first will be expressed as mean ± standard deviation, while the second as percentages; continuous data will be analysed with the student’s t-test, analysis of variance and a Mann-Witney U test, in the case of non-parametric distribution. Categorical variables will be compared using the Chi2 test with Fisher’s correction.
Survival analyses will be performed on the healing and reduction of lesional areas via the Kaplan-Meier test. An α-error of less than 5% will be considered significant; the analysis will be performed by a professional statistician who is unaware of the details of the study (for the sake of statistical elaboration, the two groups will be identified as Group A and Group B), using an open source statistical package (R Commander; an open source statistical software developed on Linux by John Fox) running on a personal computer.
Discussion and implications for clinical practice
The HERMES study (blue ligHt photobiomodulation thErapy on neuRoischeMic patiEntS) represents the first prospective randomised trial of this kind in DFUs and can be considered a pilot study, since blue light has never been tested in this model of chronic ulceration.
The interests of the study lie not only in the novelty of the therapeutic approach, which is based on the multiform interaction between non-coherent light and the cellular biology, but also in the peculiar subset of the patients selected and for the design of the study, which is shared between the hospital and the community.
The subset of DFUs chosen for this first experience with blue light therapy is the neuro-ischemic ulcer, in a range of ischemia that is not yet so critical as to constitute an indication to revascularisation, but which actually negatively affects the progression towards wound repair.36
These kinds of lesions are not only the most recalcitrant to healing, they are also the most frequent among those affecting DF patients. The EURODIALE study demonstrated how DFUs with an ischemic component accounted for 49% of all ulcers in the whole group of 1300 DFU patients followed for one year in 14 specialised centres in 10 European countries.37
Since then, revascularisation practices, which in recent years have changed the prognoses of patients with critical limb ischemia, have saved limbs from amputation and actually transformed a significant number of critical patients to patients with a neuro-ischemic DFU, thus inflating this particular subset of DFU which now can be estimated to be roughly 60% of those followed in the specialised DF clinic in Italy.38
According to the most recent edition of international guidelines on the management of diabetic foot, the SoC for these DFUs is represented by debridement, offloading and sucrose-octasulfate dressing, which has been demonstrated as more effective than standard dressings for promoting healing in this context; this is the SoC we adopted in the study, on top of which we will test to what extent blue light can further improve the efficacy of local treatment.35
In this model, blue light therapy should not be considered an alternative therapy, but rather a complementary one, for a very difficult-to-heal ulceration; this is also in the indications of the guidelines, which recommend adjunctive treatments in this context.7
Another novelty of the HERMES study is its bi-dimensional design; patients will be screened, enrolled and characterised in a highly specialised DF clinic inside the hospital and then treated in the community by a pool of specially trained visiting nurses.
This is the first time, to our knowledge, that shared care is part of an interventional study on DFUs; beyond the evaluation of the efficiency of such a model, the organisational and econometrical aspects are of specific interest, since the containment of management-related costs is expected to be an indirect consequence of this strategy.
These aspects will be evaluated in a spin-off of the HERMES study after completion of the clinical portion: all direct and indirect costs generated by the treatments of patients will be analysed in a systematic way, in order to determine the cost-effectiveness of the two different treatments and, more generally, to compare it with the standard approach, in which patients are followed up by the DF clinic only.
The limitations of this study are that it is a single-centre one, which might introduce a selection bias, since outcomes may be influenced by local practices, and another selection bias, since the centre is a referral centre for south-eastern Tuscany, which could limit the generalisability of the results. The very specific selection of patients who do not reflect the heterogeneity of Diabetic Foot Syndrome could also be considered a limitation.
Another risk of bias is related to the nature of the lesions treated, as all belong to the Diabetic Foot Syndrome spectrum of ulceration; this would, of course, limit the extendibility of the results to other kinds of ulcers, such as venous leg ulcers or pressure ulcers.
Despite these limits, the study is of extreme interest because it will bring new and important information on both the clinical and organisational aspects of the management of DFU.
IMPLICATIONS FOR CLINICAL PRACTICE
- This is the first prospective, double-blind RCT on photobiomodulation for the management of neuroischemic DFUs.
- If the results meet expectations, photobiomodulation could be proposed as an adjunctive therapy in the home care follow-up management of this subset of DFUs.
- Being a low-cost technology based on the application of blue light, with no consumption waste and no limitations for the use in home care, this therapy is potentially cost-effective for this indication.
- Further research will evaluate cost-effectiveness of this therapy, and its other possible indications for use in the field of chronic ulcers.
Disclosure
The study has been commissioned and will be fully financed by EmoLED SrL, which will only sponsor the study and supply the materials for conducting it, without interfering with data collection, analysis or interpretation. This study will be carried out according to the European Rules of Management of Clinical Trials during COVID-19.39 AP and SG designed the study; AS and SS will perform the clinical study and collect the data; AP will analyse the data and write the paper; AP, AS, SS and SG will interpret the results and contribute to the writing of the paper, AP will revise and edit the paper and is the grantor of the study.
The study has been designed and will be carried out according to the Declaration of Helsinki’s principles and the ethical standards of the NIH.40
Acknowledgements
We acknowledge the expertise of Duccio Rossi PhD, who will independently take care of the randomisation list’s generation and implementation.
Key messages
- We describe the design and study protocol of a prospective RCT to be carried out in combination between a specialised DF clinic and home care service.
- The aim of this study is to compare photobiomodulation to the standard of care for the management of neuro-ischemic diabetic foot ulceration.
- We expect a superior outcome in the healing rates at 24 weeks of 15% in favour of photobiomodulation, with no worsened safety profile.
- In addition to these outcomes, the interest of the study lies both in the novelty of the therapy, which is a low-cost, no-waste technology, and in the integration of specialist in-hospital care and home care.
Author(s)
Alberto Piaggesi MD1, Alessia Scatena MD2, Sara Sandroni RN3, Stefano Gasperini4
1Diabetic Foot Section, Department of Medicine – University Hospital of Pisa, Italy
2Diabetology Unit – S. Donato Hospital, Arezzo, Italy
3Skin Lesion Network Hospital, Home Care – South East Tuscany Health Service, Arezzo, Italy
4Medical Advisor – Pisa, Italy
Correspondence: alberto.piaggesi@med.unipi.it
Conflict of interests: None
References
- Zhang Y, Lazzarini PA, McPhail SM, van Netten JJ, Armstrong DG, Pacella RE. Global disability burdens of diabetes-related lower-extremity complications in 1990 and 2016. Diabetes Care. 2020 May; 43(5):964–74. doi:10.2337/dc19-1614.
- Rastogi A, Goyal G, Kesavan R, Bal A, Kumar H, Mangalanadanam, Kamath P, et al. Long term outcomes after incident diabetic foot ulcer: Multicenter large cohort prospective study (EDI-FOCUS investigators) epidemiology of diabetic foot complications study: Epidemiology of diabetic foot complications study. Diabetes Res Clin Pract. 2020 Apr; 162:108113.
- Armstrong DG, Boulton AJM, Bus SA. diabetic foot ulcers and their recurrence. N Engl J Med. 2017 Jun 15; 376(24):2367–75.
- Toki F, Nanba D, Nishimura EK, Matsuzaki K. Evaluation of the proliferative potential of skin keratinocytes and fibroblasts isolated from critical limb ischemia patients. Regen Ther. 2020 May 15; 14:222–6.
- Prompers L, Schaper N, Apelqvist J, Edmonds M, Jude E, Mauricio D, et al. Prediction of outcome in individuals with diabetic foot ulcers: Focus on the differences between individuals with and without peripheral arterial disease. The EURODIALE study. Diabetologia. 2008 May; 51(5):747–55.
- Reiber GE, Vileikyte L, Boyko EJ, del Aguila M, Smith DG, Lavery LA, et al. Causal pathways for incident lower-extremity ulcers in patients with diabetes from two settings. Diabetes Care. 1999 Jan; 22(1):157–62.
- Hinchliffe RJ, Forsythe RO, Apelqvist J, Boyko EJ, Fitridge R, Hong JP, et al. Guidelines on diagnosis, prognosis, and management of peripheral artery disease in patients with foot ulcers and diabetes (IWGDF 2019 update). Diabetes Metab Res Rev. 2020 Mar; 36 Suppl 1:e3276.
- Lombardo FL, Maggini M, De Bellis A, Seghieri G, Anichini R. Lower extremity amputations in persons with and without diabetes in Italy: 2001–2010. PLoS One. 2014 Jan 28; 9(1):e86405.
- Gregg EW, Li Y, Wang J, Burrows NR, Ali MK, Rolka D, et al. Changes in diabetes-related complications in the United States, 1990–2010. N Engl J Med. 2014 Apr 17; 370(16):1514–23.
- Khan T, Armstrong DG. Ulcer-free, hospital-free and activity-rich days: Three key metrics for the diabetic foot in remission. J Wound Care. 2018 Apr 1; 27(Sup4):S3–4.
- Jia L, Parker CN, Parker TJ, Kinnear EM, Derhy PH, Alvarado AM, et al. Incidence and risk factors for developing infection in patients presenting with uninfected diabetic foot ulcers. PLoS One. 2017 May 17; 12(5):e0177916.
- Ferroni L, Gardin C, De Pieri A, Sambataro M, Seganfreddo E, Goretti C, et al. Treatment of diabetic foot ulcers with Therapeutic Magnetic Resonance (TMR®) improves the quality of granulation tissue. Eur J Histochem. 2017 Aug 7; 61(3):2800.
- Romanelli M, Piaggesi A, Scapagnini G, Dini V, Janowska A, Iacopi E, et al. EUREKA study - the evaluation of real-life use of a biophotonic system in chronic wound management: an interim analysis. Drug Des Devel Ther. 2017 Dec 11; 11:3551–8.
- Piaggesi A, Låuchli S, Bassetto F, Biedermann T, Marques A, Najafi B, et al. Advanced therapies in wound management: Cell and tissue based therapies, physical and bio-physical therapies smart and IT based technologies. J Wound Care. 2018 Jun 1; 27(Sup6a):S1–137.
- Zhou Y, Chia HWA, Tang HWK, Lim SYJ, Toh WY, Lim XL, et al. Efficacy of low-level light therapy for improving healing of diabetic foot ulcers: A systematic review and meta-analysis of randomized controlled trials. Wound Repair Regen. 2021 Jan; 29(1):34–44.
- Santos CMD, Rocha RBD, Hazime FA, Cardoso VS. A systematic review and meta-analysis of the effects of low-level laser therapy in the treatment of diabetic foot ulcers. Int J Low Extrem Wounds. 2020 May 12; doi:10.1177/1534734620914439.
- Dos Santos Mendes-Costa L, de Lima VG, Barbosa MPR, Dos Santos LE, de Siqueira Rodrigues Fleury Rosa S, Tatmatsu-Rocha JC. Photobiomodulation: Systematic review and meta-analysis of the most used parameters in the resolution diabetic foot ulcers. Lasers Med Sci. 2020 Nov 15; doi:10.1007/s10103-020-03192-y. Online ahead of print.
- Hamblin MR, Mechanisms and mitochondrial redox signaling in photobiomodulation. Photochem Photobiol. 2018 Mar; 94(2): 199–212. doi:10.1111/php.12864.
- Rossi F, Pini R, De Siena G, Massi D, Pavone FS, Alfieri D, et al. A blue-LED-based device for selective photocoagulation of superficial abrasions: Theoretical modeling and in vivo validation. Photonic Ther Diagnostics. 2010; 7548 (Proceedings of SPIE).
- Alfieri D, Bacci S, Cicchi R, De Siena G, Lotti V, Pavone F, et al. Blue LED treatment of superficial abrasions. Proceed SPIE. 2013; 8565:85650H-85650H-6. doi:10.1117/12.2003933.
- Cicchi R, Rossi F, Alfieri D, Bacci S, Tatini F, De Siena G, et al. Observation of an improved healing process in superficial skin wounds after irradiation with a blue-LED haemostatic device. J Biophotonics. 2016; 9(6):645–55. doi:10.1002/jbio.201500191.
- Rossi F, Cicchi R, Magni G, Tatini F, Bacci S, Paroli G, et al. In-vivo wound healing modulation after irradiation with a blue LED photocoagulator. Proceed. 2017; 10417, 104:1041706. doi:10.1117/12.2286053.
- Rossi F, Cicchi R, Magni G, Tatini F, Bacci S, Paroli G, et al. Blue LED induced thermal effects in wound healing: Experimental evidence in an in vivo model of superficial abrasions. Proceed SPIE. 2017; 10066 1006:100660B. doi:10.1117/12.2251947.
- Magni G, Tatini F, Bacci S, Paroli G, De Siena G, Cicchi R, et al. Blue LED light modulates inflammatory infiltrate and improves the healing of superficial wounds. Photodermatol Photoimmunol Photomed. 2019 July 5; 1–3. doi:10.1111/phpp.12527.
- Magni G, Banchelli M, Cherchi F, Coppi E, Fraccalvieri M, Pugliesi AM, et al. Human keloid cultured fibroblasts irradiated with blue LED light: Evidence from an in vitro study. Proceed SPIE. 2019 July 31; doi:10.1117/12.2527084.
- Magni G, Cherchi F, Coppi E, Fraccalvieri M, Tatini F, Fusco I, et al. Blue light effects in human keloid fibroblasts. Proceed SPIE. 2019; 1086107(March):6. doi:10.1117/12.2509504.
- Yang K, Li D, Wang M, Xu Z, Chen X. Exposure to blue light stimulates the proangiogenic capability of exosomes derived from human umbilical cord mesenchymal stem cells. Stem Cell Res Ther. 2019 Nov 28; 10(1):358. doi:10.1186/s13287-019-1472-x.
- Magni G, Tatini F, Bacci S, Paroli G, De Siena G, Cicchi R, et al. Blue LED light modulates inflammatory infiltrate and improves the healing of superficial wounds. Photodermatol Photoimmunol Photomed. 2020 Mar; 36(2):166–8.
- Dini V, Romanelli M, Oranges T, Davini G, Janowska A. Blue light emission in the management of hard to heal wounds: A case series. G Ital Dermatol Venereol. 2020 Jul 28; doi:10.23736/S0392-0488.20.06691-2. Online ahead of print.
- Mosti G, Gasperini S, Fraccalvieri M, Tripodi C [Internet]. Apporto della luce blu nel processo di guarigione: Casi di studio su lesioni croniche. In: Atti XIV Congresso Nazionale 201, AIUC. Available from: https://bit.ly/2LYi9yw.
- Mosti G, Gasperini S. Observations made on three patients suffering from ulcers of the lower limbs treated with Blue Light. Chronic Wound Manag Res. 2018: 5: 23–8.
- Armstrong DG, Lavery LA, Harkless LB. Validation of a diabetic wound classification system. The contribution of depth, infection, and ischemia to risk of amputation. Diabetes Care. 1998 May; 21(5):855–9.
- Lipsky BA, Berendt AR, Cornia PB, Pile JC, Peters EJ, Armstrong DG, et al. Infectious Diseases Society of America clinical practice guideline for the diagnosis and treatment of diabetic foot infections. Clin Infect Dis. 2012 Jun; 54(12):e132–73.
- Piaggesi A, Macchiarini S, Rizzo L, Palumbo F, Tedeschi A, Ambrosini Nobili L, et al. An off-the-shelf instant contact casting device for the management of diabetic foot ulcers: A randomized prospective trial versus traditional fiberglass cast. Diabetes Care 2007 Mar; 30(3):586–90.
- Edmonds M, Lázaro-Martínez JL, Alfayate-García JM, Martini J, Petit JM, Rayman G, et al. Sucrose octasulfate dressing versus control dressing in patients with neuroischaemic diabetic foot ulcers (Explorer): An international, multicentre, double-blind, randomised, controlled trial. Lancet Diabetes Endocrinol. 2018 Mar; 6(3):186–96.
- Yotsu RR, Pham NM, Oe M, Nagase T, Sanada H, Hara H, et al. Comparison of characteristics and healing course of diabetic foot ulcers by etiological classification: Neuropathic, ischemic, and neuro-ischemic type. J Diabetes Complications. 2014 Jul-Aug; 28(4):528–35.
- Prompers L, Huijberts M, Apelqvist J, Jude E, Piaggesi A, Bakker K, et al. High prevalence of ischaemia, infection and serious comorbidity in patients with diabetic foot disease in Europe. Baseline results from the Eurodiale study. Diabetologia. 2007 Jan; 50(1):18–25.
- Faglia E, Clerici G, Scatena A, Caminiti M, Curci V, Prisco M, et al. Severity of demographic and clinical characteristics, revascularization feasibility, major amputation, and mortality rate in diabetic patients admitted to a tertiary diabetic foot center for critical limb ischemia: Comparison of 2 cohorts recruited at a 10-year distance. Ann Vasc Surg. 2014 Oct; 28(7):1729–36.
- European Medicine Agency. Guidance on the management of clinical trials during the Covid-19(coronavirus) pandemic, version 4. Brussels: European Commission; 2021 April 2.
- NIH Clinical Center [Internet]. Patient recruitment: Ethics in clinical research. Bethesda, MD: NIH; [2021 March 30; 2021 May 21]. Available at: www.clinicalcenter.nih.gov/recruit/ethics.html,
