Volume 22 Number 3
Multi-centre study demonstrating safety and efficacy of an upgraded sNPWT system on closed surgical wounds
Sudheer L Karlakki, Jessica R Evans, Simon P Booth, Jason KF Wong, Lyn Wilson,, Steven LA Jeffrey, Rohit Makhija, Yves Harder, Iain McNamara, Mike Reed, David E Barnes, Sarah Megginson, Brian Gilchrist,
Keywords Closed surgical incisions; PICO 7; single-use negative pressure wound therapy; skin flaps; split-thickness skin grafts; surgical site complications
DOI 110.35279/jowm202110.07
Abstract
Background Surgical site complications (SSCs) pose a major postoperative challenge. Single-use negative pressure wound therapy (sNPWT) systems have been shown to be effective for reducing SSCs. An upgraded sNPWT system (PICOTM 7) with improved functional performance and ease-of-use was assessed in a prospective, single-arm study. The study aimed to assess if the system was a safe and effective therapy for delivering consistent negative pressure.
Methods Between November 2018 and May 2019, eligible patients across 11 sites with a closed abdominal or knee arthroplasty incision, skin graft or flap, were enrolled. Postoperatively, patients received sNPWT for up to 7 days with follow-up until 30 days. Endpoints included the ability to maintain a consistent negative pressure for the intended duration, complication rates, clinician and patient usability and satisfaction and serious adverse events.
Results The device was capable of delivering consistent negative pressure across all assessed surgical wounds and was statistically equivalent to the reference negative pressure (-80.2 mmHg) (n=46; p=0.002). Low complication rates were observed within 30 days post-surgery. High levels of clinician usability (>90%) and patient satisfaction (95%) were reported. No serious device-related adverse events were reported.
Conclusions The study demonstrated the functional performance, favourable safety profile and clinician- and patient-reported satisfaction of this upgraded PICO 7 sNPWT device across multiple closed surgical wound types.
Implications Application of an sNPWT system can lead to improved wound healing outcomes within closed surgical wounds while also demonstrating high levels of clinician and patient satisfaction.
INTRODUCTION
Postoperative wound complications are a major challenge following surgery. Surgical site complications (SSCs), such as surgical site infection (SSI), dehiscence, seroma and haematoma, have been reported following arthroplasty,1 skin grafts2,3 and flaps.4 The wound complication rate following knee arthroplasty is reportedly 12%,5 although it can be as high as 34% for closed abdominal incisions.6 Skin grafts are used to treat trauma-related wounds and chronic ulceration,2,3,7,8 but graft failure can occur in up to one-third of patients.3 Split-thickness skin grafts (STSGs), with a variable thickness of dermis, have various applications, such as resurfacing large wounds, burns and muscle flaps.8,9 Unlike skin grafts, flaps have their own blood supply,9 but complications (e.g., flap failure due to factors such as infection and vascular occlusion) can adversely impact patient morbidity and quality of life.4,10,11,12 Wound-related complications can result in prolonged hospital stays and additional treatments (such as antibiotics), which may impose resource and financial burdens on healthcare providers10,13,14 and affect patients’ treatment outcomes.10,14
Single-use negative pressure wound therapy (sNPWT) systems have been shown to reduce complications while increasing patients’ freedom of movement and comfort.15,16 One such system is a canister-free, disposable device (PICOTM; Smith + Nephew, Hull, UK) that generates an effective nominal negative pressure of -80 mmHg.17 Several studies have demonstrated its efficacy for various surgical wounds,18,19 including SSC reduction in closed incisions16,20,21,22 and the rapid successful take of grafts and flaps with limited complications.23,24 This system is indicated for low- to moderately exuding wounds. It consists of a vacuum pump that is connected to a dressing and which incorporates a silicone wound contact layer, minimising skin trauma.25 The delivery of negative pressure wound therapy (NPWT) from the pump across the wound bed or closed incision26 allows evacuation of exudate into the dressing, where 80% is evaporated through the film top layer, while the remainder is retained within the absorbent layer of the dressing.25
An upgraded version of this device (PICO 7; Smith + Nephew, Hull, UK) was launched in 2018, with a number of improved technical features (Figure 1a). The pump has a higher air leak tolerance and is quieter than the previous version. When the dressing is full, a visual indicator signals the need for dressing replacement. For improved portability, a clip is supplied with the pump (Figure 1b), enabling attachment to the patient’s belt. This may be particularly convenient for acute or ambulatory care settings.
A prospective, post-market, multi-centre study was undertaken to confirm the capability of the upgraded PICO 7 sNPWT system to deliver negative pressure as expected and to assess whether it is a safe and effective therapy for surgically closed incisions and skin flaps. Additionally, the acceptability of this system to clinicians and patients was assessed.
Figure 1.
(a) Pump and dressing of upgraded sNPWT device (PICO 7, Smith and Nephew, Hull, UK).
(b) Clip for attachment to patient’s belt.
METHODS
Study Design
Between November 2018 and May 2019, investigators at 11 sites (UK (n=10) and Switzerland (n=1)) enrolled patients in this prospective, post-market, non-randomised, non-blinded, single-arm study. Patients were eligible if they were ≥ 18 years of age and had a suitable closed abdominal or knee surgery incision, STSG (meshed or unmeshed), or a skin flap that would fit under the PICO 7 sNPWT device dressing. Exclusion criteria were contraindications or hypersensitivity to the investigational product, extremely fragile skin, skin features that may interfere with the study’s assessment (e.g., tattoos), wounds that were bleeding or infected at the time of surgery, skin grafts to correct pressure ulcers, undergoing palliative care, previous poor compliance with medical treatment and/or a medical or physical condition that precluded safe study participation.
Eligible patients were treated with the advanced PICO 7 sNPWT device, applied by the investigators at the conclusion of the operative visit (Day 0), and continued for up to seven days or until the patient exited from study participation.
The study was performed in compliance with the ethical principles of the Declaration of Helsinki, Good Clinical Practices (GCP) and International Organization for Standardization (ISO) 14155:2011. The study protocol was reviewed and approved by the relevant local independent ethics committees. All patients provided informed consent. The study was registered with ClinicalTrials.gov (NCT03698968). Relevant patient demographics and medical histories were recorded preoperatively.
Primary, secondary and exploratory endpoints were measured using a number of post-baseline assessments (Figure 2). Assessments were undertaken at Day 4 and at the end of the seven-day treatment, with follow-up visits at Days 14 and 30 to assess healing progress and complications.
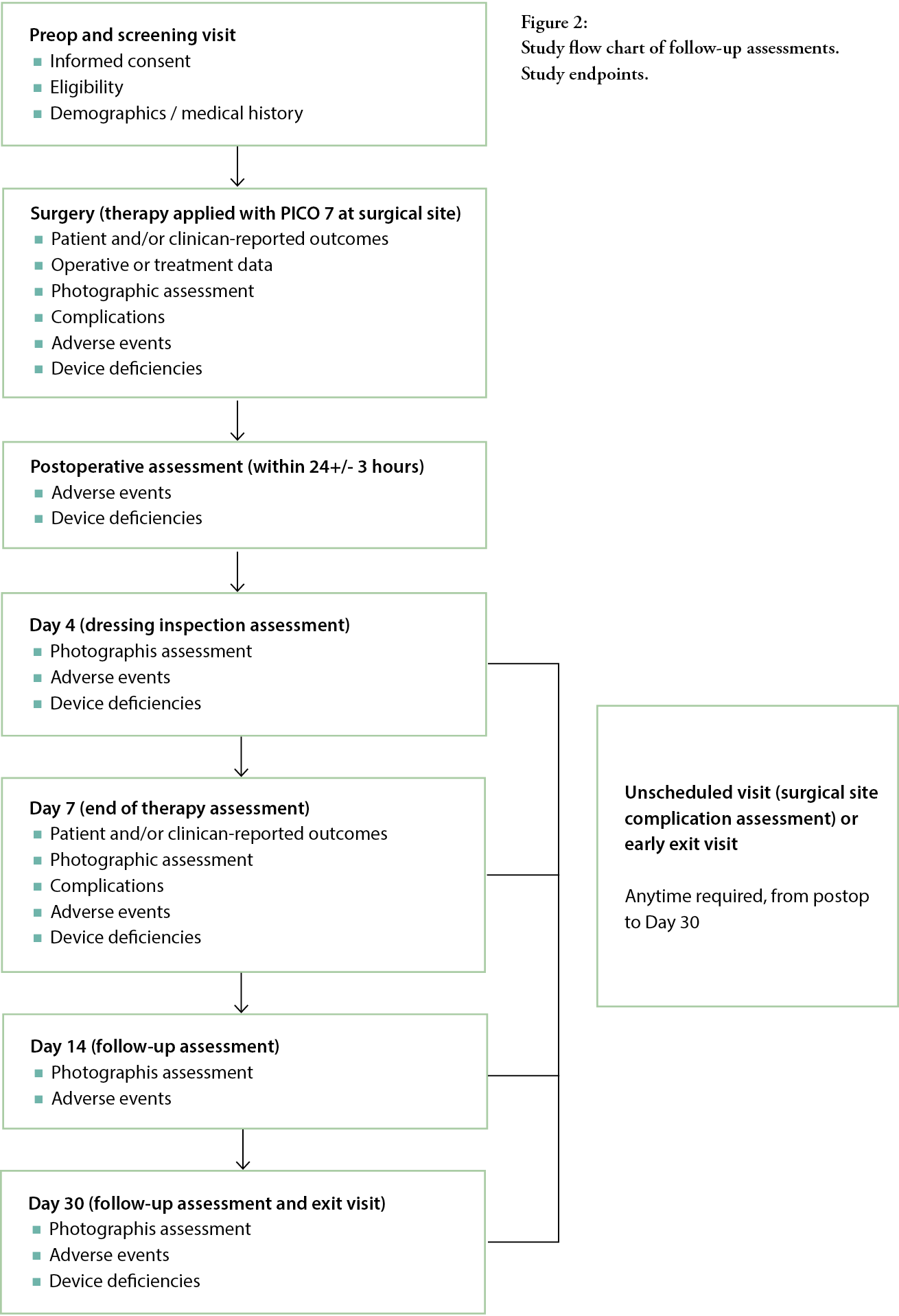
The primary study endpoint was the assessment of the average negative pressure value, delivered over the treatment period. The PICO 7 sNPWT device (pump) contained a microchip that regularly recorded the delivered negative pressure, at least every 30 seconds, indicative of functional clinical performance over the duration of therapy. The difference between mean negative pressure (mmHg) and the reference negative pressure of -80.2 mmHg (extracted from a sNPWT device) was calculated for each patient.
The following secondary endpoints were measured: incidence of SSCs, including SSIs within 30 days of surgery; dressing wear time; skin graft or flap take at end of therapy and at Days 14 and 30, assessed by visual assessment; percentage of successful STSG or flap take/survival at end of therapy and at Days 14 and 30; condition of peri-wound skin assessed by visual inspection at Days 7, 14 and 30; level of pain during wear, removal and at application, assessed by visual analogue scale (VAS) between 0 and 100 (with 0 reflecting no pain experienced), recorded at Day 4 of therapy, end of therapy and additional dressing changes.
The following exploratory endpoints relating to clinician and patient satisfaction were also investigated: clinician acceptability of the upgraded sNPWT device (clinician-reported satisfaction and comparison with previously used NPWT); patient acceptability (patient-reported performance, comfort during wear and portability) at discontinuation of therapy; and ease of dressing application and removal. These were obtained using ‘yes/no’ or ‘better/same/worse’ questions, with respondents given the opportunity to provide further context in free text replies.
Safety was assessed in all patients who received the study device, based on the incidence and severity of device-related adverse events (AEs) and device deficiencies.
Statistical analysis
Based on data and assumptions used for sample size estimation (mean operating pressure = 80.2 mmHg; SD=14.8), a minimum of 30 patients were required to achieve 80% power to detect equivalence within 7 mmHg at the significance level of α=0.025 (one-sided), using a two-one-sided t-test approach. Assuming 25% of patients would be lost to follow-up by the end of the study and allowing for varying losses across the selected indications, a sample size of 50 patients was required.
For continuous variables, the number of observations, mean, standard deviation (SD), minimum
and maximum are presented, while for categorical variables the number of observations, frequency and percentages are reported. Where appropriate, 95% confidence intervals (CI) are reported. Statistical analyses were undertaken using SAS for Windows version 9.4 or later.
RESULTS
Patient population
In total, 53 patients agreed to participate in the study, but three patients failed the screening, resulting in 50 patients receiving treatment with the sNPWT device. A further two patients left the study early, due to difficulties in obtaining a seal. The remaining 48 patients, all of whom received sNPWT for up to seven days, were included in primary, secondary and exploratory endpoint analyses. At the end of therapy, two of the pumps failed to be recovered, therefore device data were available from only 46 patients for primary endpoint analysis.
Fifty patients were treated with the sNPWT device for up to seven days, and 40 (80%) patients reached the end of the seven-day treatment period. Reasons for not reaching the full seven days included: dressing seal could no longer be obtained (n=1), failure of the pump (n=1) and eight other reasons that were unrelated to the device’s performance or usability.
Patients who received sNPWT post-surgery underwent the following surgical procedures: 20 (41.7%) patients had a closed surgical incision following open abdominal surgery (n=10) or knee arthroplasty (n=10), whilst 28 (58.3%) patients received an STSG. The mean incision lengths of the abdominal and knee arthroplasty procedures were 16.7 and 13.7 cm, respectively. Patients’ demographic characteristics and co-morbidities are presented in Table 1.
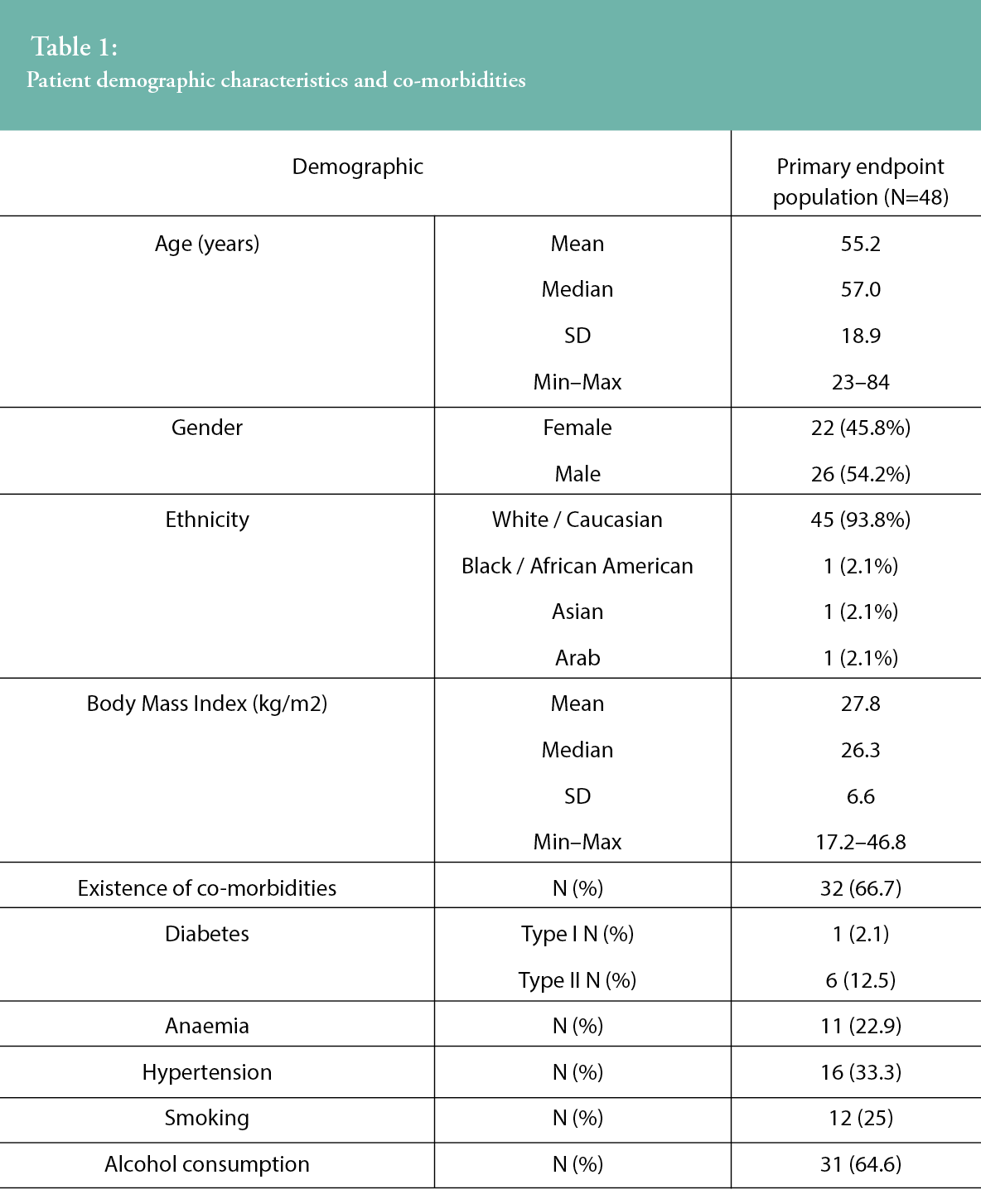
Thirty-two (66.7%) patients had at least one co-morbidity at baseline, including seven (14.6%) with diabetes; among diabetics, there was a higher prevalence of Type II (85.7%). Further to the presence of diabetes, anaemia and hypertension were also present in the study population, at 22.9% and 33.3%, respectively. In addition to the co-morbidities reported, the incidence of smoking and alcohol consumption at baseline was also captured, with 12 (25%) patients actively smoking and more than 61% of the study sample consuming alcohol, at a mean of 6.6 units per week (SD=11.2, range 0–60). The reported co-morbidities of alcohol consumption and smoking are both known to impact the progression of wound healing.
Primary endpoint: Assessment of average negative pressure level
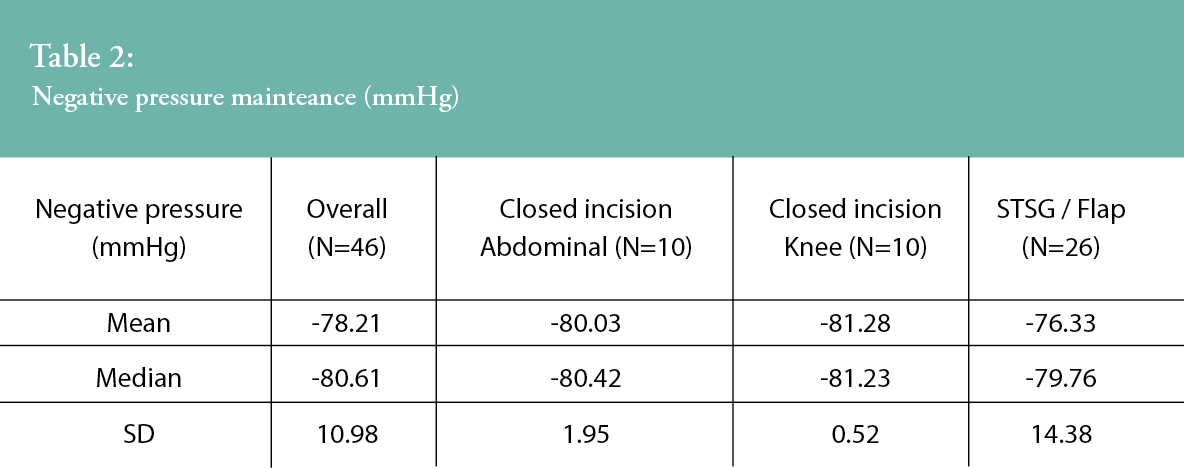
Negative pressure measurement data from 46 patients with available microchips are presented in Table 2. The overall mean pressure was 78.21 mmHg throughout the treatment period. The ¬mean difference between each patient’s average pressure and the reference negative pressure of -80.2 mmHg was 1.99 mmHg (95% CI around the¬ mean: -1.27; 5.25). As the 95% CI lies within the equivalence test boundaries (-7; 7), then statistical equivalence (to the reference negative pressure) was demonstrated (p=0.002).
Secondary endpoints
All surgical site complications reported within 30 days are described in Table 3. No SSIs were reported within the 30-day follow up period following treatment with sNPWT in the knee arthroplasty group. One superficial infection and one deep infection were reported in closed incisions following abdominal surgery, and there was one superficial infection in the STSG group. Three SSCs were reported in two knee patients, including oedema and discoloration. While 18 SSCs were reported in four abdominal patients, six of the complications were recorded for a single patient and appeared to be related to the presence of an infection. Reported SSCs included: discharge, wound breakdown, delayed healing, unexpected pain or tenderness, superficial dehiscence, deep dehiscence, seroma and abnormal smell. In patients with STSGs, 10 SSCs reported were from six patients, including superficial infection, seroma, wound breakdown, unexpected pain or tenderness and ‘other’. There were no reports of organ space SSI, haematoma, abscess or severe bruising.
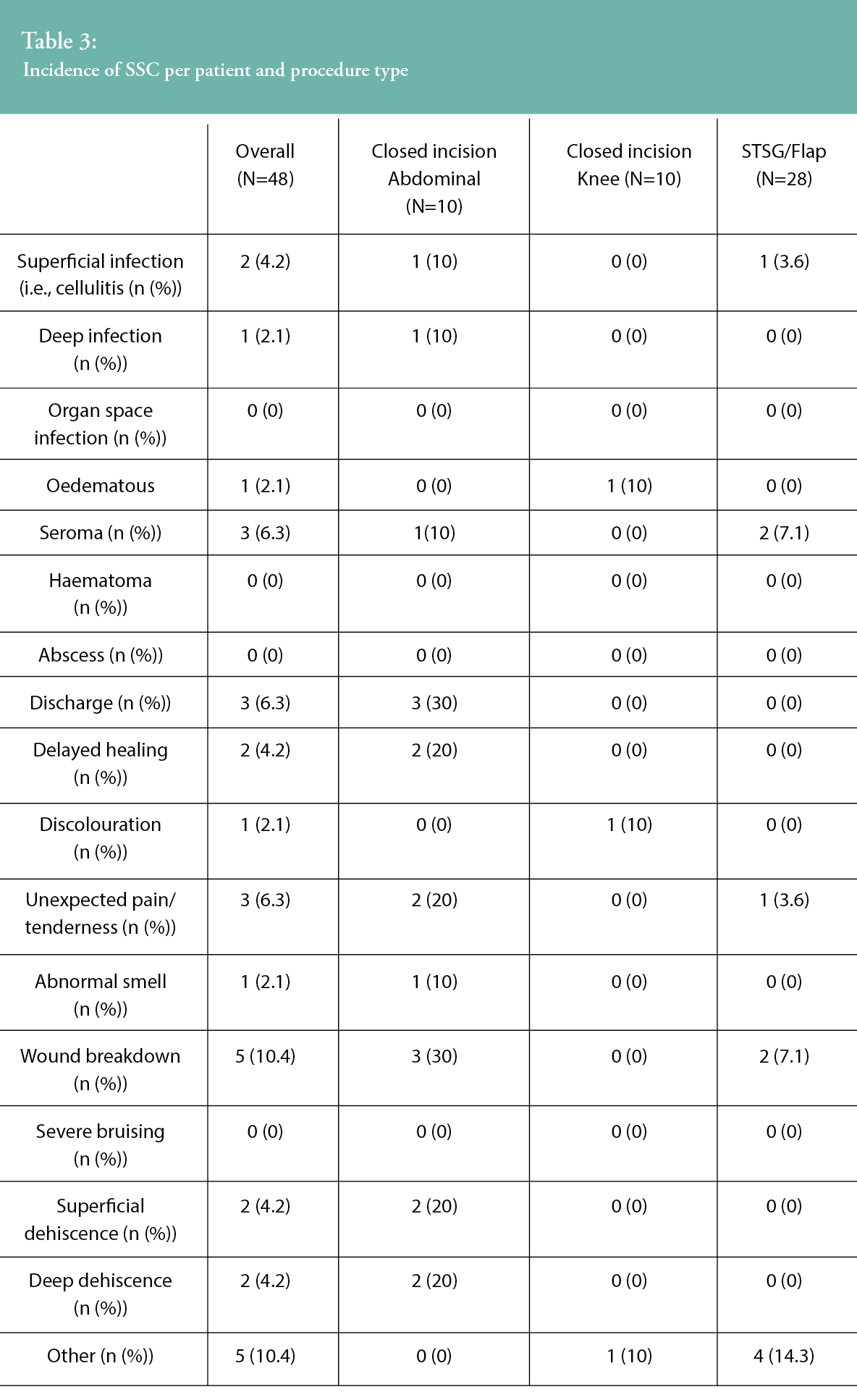
The mean dressing wear time of the updated sNPWT device was 4.8 days across all subjects. When specifically examining dressing wear time among the different surgical groups, the mean dressing wear time was 5.2 days following knee arthroplasty and 4.8 days for both abdominal closed incisions and STSGs. The mean number of dressings used over the seven-day treatment period was 1.3 (SD=0.47, range 1–2) across all surgical groups, with 30.2% of patients overall requiring a single dressing for the seven-day treatment period.
Table 4 shows the percentages of patients who achieved complete incision closure or graft take at the end of therapy, at Day 14 follow-up and at Day 30 follow-up. All sNPWT-treated incisions (n=10, 100%) in patients who underwent knee arthroplasty were closed at study completion (30 days post-surgery), with 80% (8/10) of abdominal incisions fully closed. STSG/flap success was recorded as a percentage of successful graft take, whereby the mean percentage of STSG/flap take was 83.3% (SD, 28.9; range, 2–100) at end of therapy (Day 7), 78.4% (SD, 31.1; range, 0–100) at Day 14, and 80.9% (SD, 33.3; range, 0–100) at Day 30. Furthermore, 35.7% of sNPWT STSGs/flaps were fully healed (100% take) at the end of the 30-day study period.
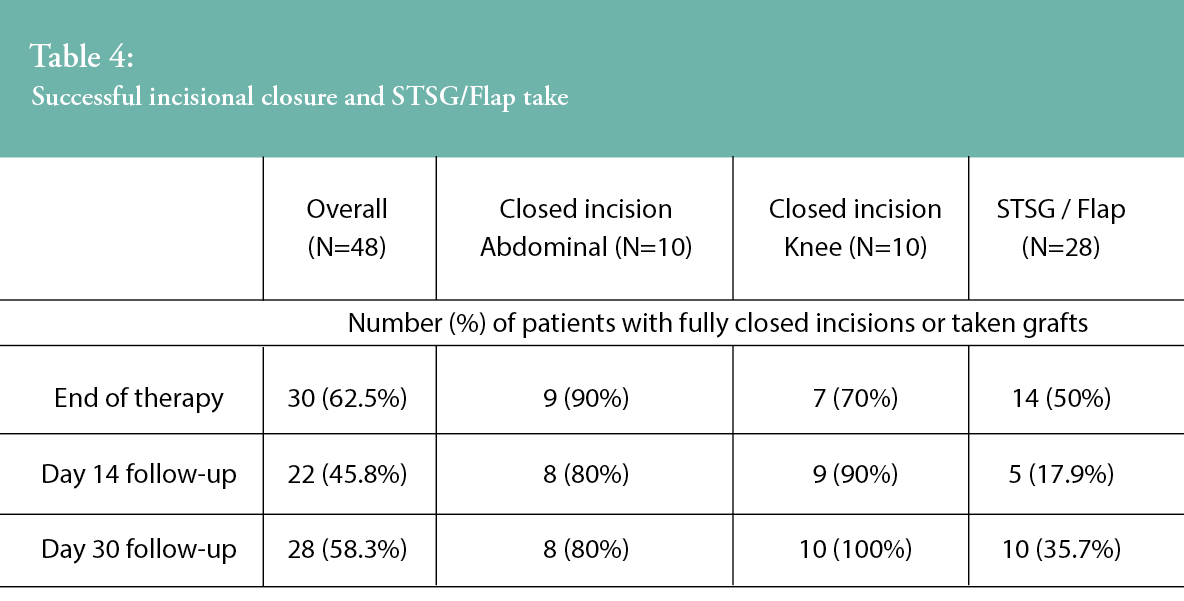
Peri-wound skin assessments were undertaken throughout the study period by the study investigators, and the proportion of patients in which the peri-wound skin condition was described as ‘healthy’ is presented in Table 5. Investigators were asked to confirm if, in their clinician opinion, the peri-wound area was deemed healthy in appearance at the study timepoints. Across all wound types, there was an improvement in peri-wound skin health from the end of therapy to the 30-day visit. For the closed incision groups, all patients (n=20, 100%) were assessed as having healthy peri-wound skin at the end of the study. Within the STSG/flap group, an improvement in peri-wound skin health was observed from the operative visit (n=20, 71.4% patients) through to the study’s completion at Day 30 (n=24, 85.7% of patients assessed as having healthy peri-wound skin).
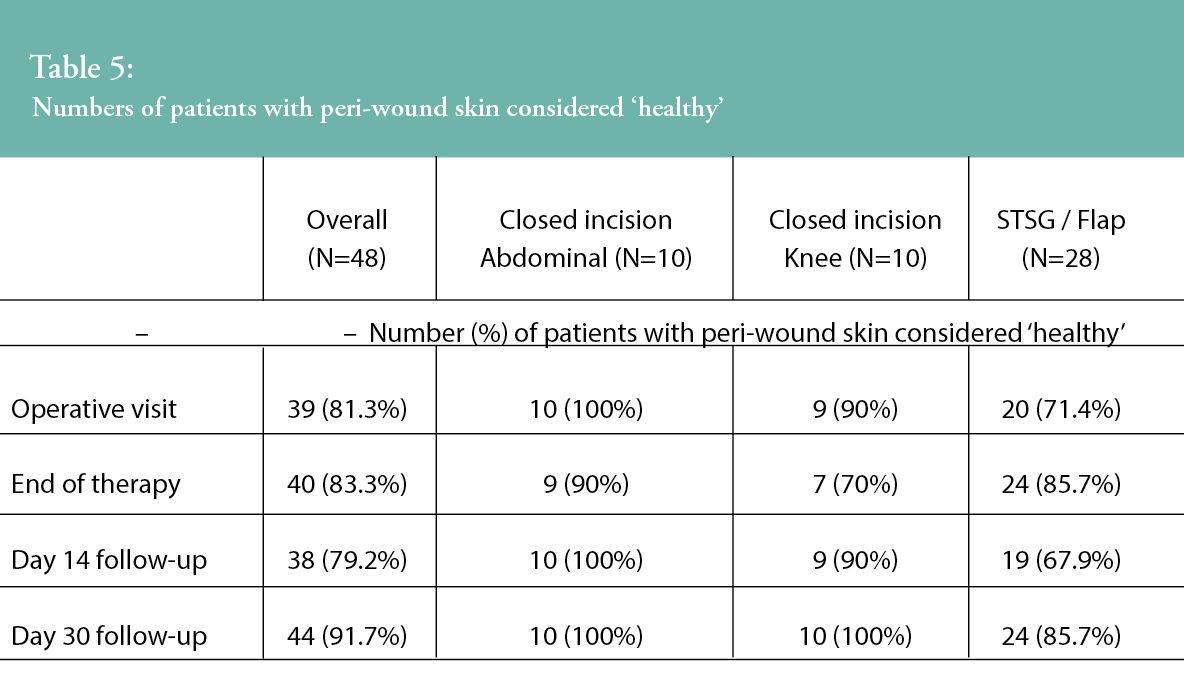
Patient pain scores were captured during dressing wear (N=107) and at the point of dressing removal (N=57) using a Visual Analogue Scale (VAS) scale of 0–100 (with 0 representing no pain). Overall, mean VAS pain scores during wear were 20.5 (SD, 27.4; range 0–100). Mean VAS pain levels during wear were 29.5 (SD, 29.4; range 0–70) for abdominal incisions, 41.2 (SD, 35.8; range 0–100) for knee incisions and 9.9 (SD, 15.8; range 0–80) for STSGs/flaps.
Overall, patients reported a mean VAS pain level of 12.7 (SD, 16.1; range 0–60) at dressing removal, with little difference across the wound types. Mean VAS pain levels during dressing removal were 14.5 (SD, 18.1; range 0–60) for abdominal incisions (11 assessments), 14.6 (SD, 17.7; range 0–60) for knee incisions (13 assessments) and 11.3 (SD, 15.1; range 0–50) for STSGs/flaps (33 assessments).
Exploratory endpoints:
Clinician and patient acceptability
A majority of clinicians reported being satisfied overall with the sNPWT system for 41 (93.2%) of study participants, its ability to manage the incision/skin graft site (95.5%) and its dressing retention (93.2%). For those with experience with previously used sNPWT systems, this system was rated as ‘better’ in 29 subjects (65.9%), ‘the same’ in 9 (20.5%) and ‘worse’ in 1 (2.3%) case. For the case rated as ‘worse’, this was an STSG, where the clinician noted the reason for the rating being due to the ‘pump stopped working’. This was recorded as a device deficiency.
At the end of sNPWT therapy, patients were asked by the investigator how satisfied they were with the sNPWT system by answering ‘yes’ or ‘no’ to a range of questions, such as comfort, noise and portability. Patient satisfaction with the device is reported in Figure 3. Of the 44 patients asked, the majority (n=43, 95.6%) were satisfied with the overall performance of the sNPWT device. The majority of patients also reported satisfaction with the device in terms of interference with daily living activities, such as sleep and socialising (82.2% (n=37) and 82.2% (n=37) respectively), whilst just over half were satisfied with the impact on their ability to shower (n=25, 55.6%).
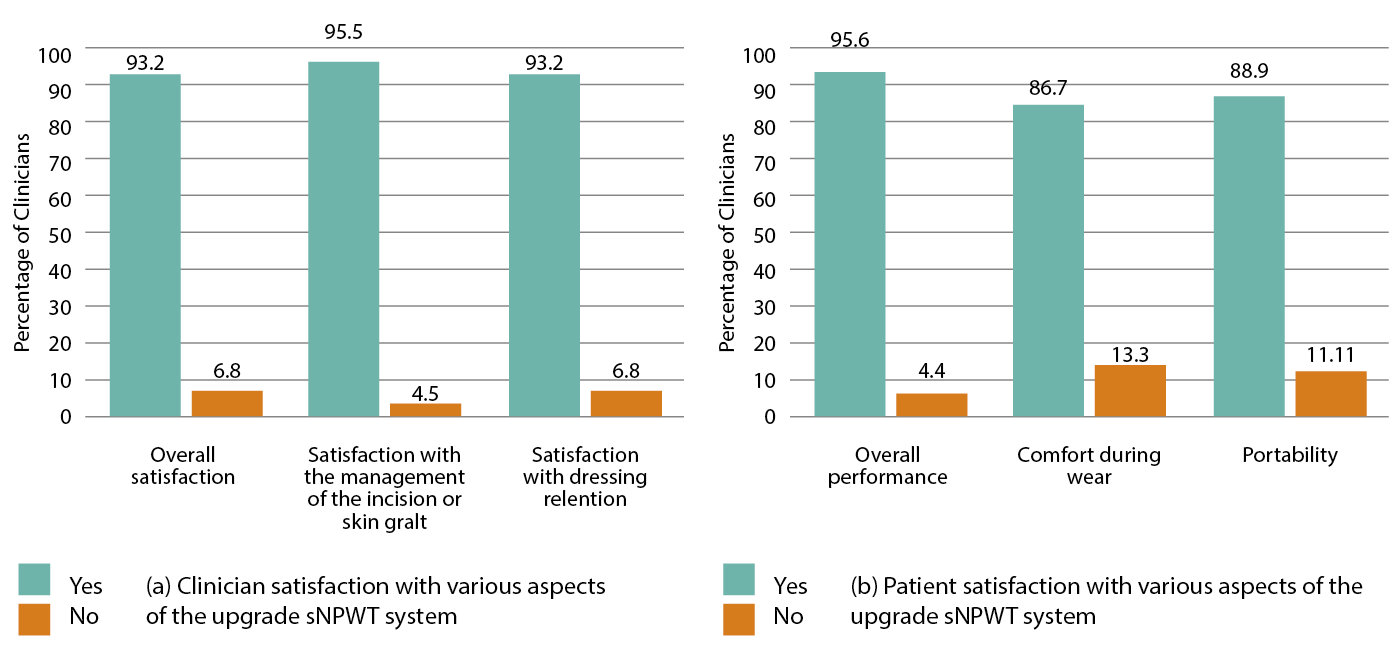
Figure 3: Clinician- and patient-rated satisfaction with various aspects of the sNPWT system during treatment
Investigators were asked to assess the application and removal of the upgraded sNPWT device on a 5-point Likert type scale ranging from ‘very easy’ to ‘very difficult’. Figure 4 details the clinicians’ reported scoring on the devices’ application and removal. Dressing application, in particular the ease of forming a seal and delivering negative pressure, was reported for 63 sNPWT applications, with 52 (82.5%) being rated by the investigator as ‘very easy’ or ‘easy’. Two occurrences (3.2%) were reported as being ‘difficult’, due to ‘being in the natal cleft’ and ‘being unable to achieve a seal’. The majority of dressing removals were reported as ‘very easy’ or ‘easy’ (n=53 91.4%). One (1.7%) occurrence of dressing removal was rated as ‘very difficult’, as the investigator reported the need to use ‘lots of adhesive remover’. The challenges reported with application and removal are common with sNPWT dressings, due to challenging anatomical areas and the need to use additional adhesive strips to ensure a seal is achieved. Nevertheless, the overall results illustrate a >80% application and >90% removal acceptance rate of the upgraded sNPWT device.
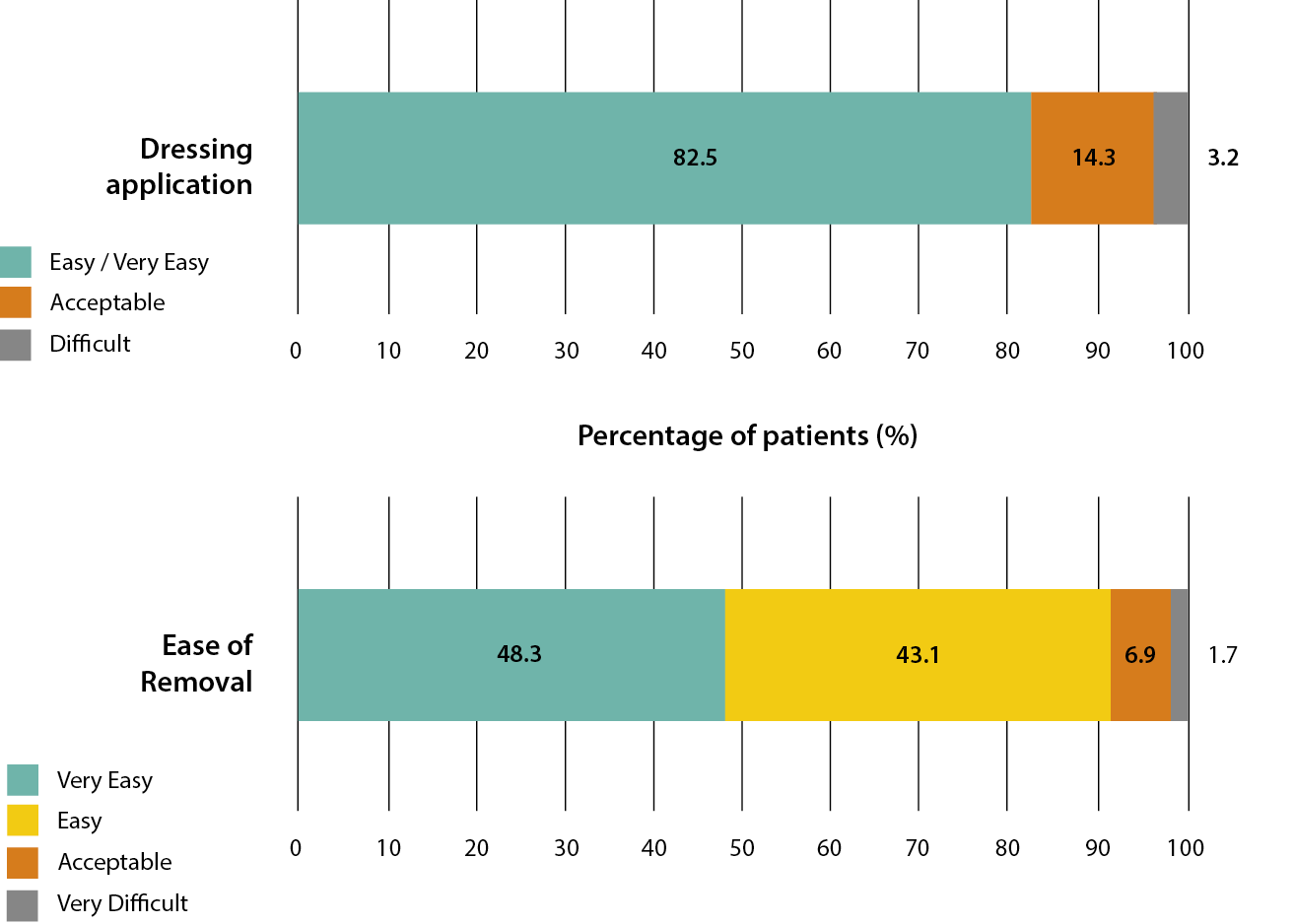
Figure 4: Clinicians’ reported ease of dressing application (ease of forming a seal and delivering negative pressure) and removal
Safety evaluation
The mean number of treatment days for the safety population was 6.1 days (SD=1.84; range: 0–8). In this population, there were three device-related AEs in three (6%) patients (1 abdominal incision and 2 knee incisions), all non-serious (presence of 1–2 small blisters, in all cases), two of which recovered by the end of the study.
DISCUSSION
The published literature consistently reports the clinical and economic benefits of NPWT for wound management, both for open wounds and closed surgical incisions.21,30,31,32 The study reported here focused on the functional and clinical performance of the upgraded PICO 7 sNPWT system in patients with closed surgical incisions and STSGs/flaps. The device was found to maintain negative pressure levels within a safe and acceptable range throughout the seven-day period of therapy and led to positive healing outcomes. Moreover, the system’s overall safety was supported by a lack of serious device-related AEs.
A key design improvement of the PICO 7 sNPWT system is its improved air leak tolerance, with a two-fold increase in maximum leak rate tolerance over previous predecessor systems,27 thus enabling more challenging anatomical areas to benefit from PICO 7, given its new ability to tolerate higher air leaks. NPWT requires an airtight, durable seal that can be compromised by air leakage, with increased potential for loss of the negative pressure, exudate leakage and increased frequency of dressing changes, all of which can impact therapy delivery, the fundamental mode of action and thus the benefit to the wound.28 Furthermore, the inability to maintain an airtight seal has cost implications both for healthcare professionals’ (HCP) resources and device costs. An sNPWT device that is unable to maintain an airtight seal would require more attention by HCPs to either re-seal the device or replace it with another device, as an additional dressing change. Therefore, any unnecessary replacement due to seal issues would be costly to the HCP.
The observed functional outcome and favourable safety profile indicates that the improved pump is capable of safe, consistent negative pressure performance in clinical practice. A broad mixture of clinical applications was purposefully selected to measure the efficacy of the device, given that it is more difficult to achieve a consistent seal around a dressing with STSGs/flaps, as opposed to closed surgical incisions such as knee replacements and abdominal closure wounds, due to differences in wounds’ shapes and patients’ anatomy. Equally, knee incisions are located in a highly mobile and curved area of the body and are subject to both lateral and longitudinal tensional forces as the knee bends; therefore, they present a significant challenge for sNPWT devices in maintaining a seal and delivering an effective level of pressure. Maintenance of negative pressure was slightly higher and more consistent (lower SD) in closed incisions compared with STSGs or flaps. Additionally, the increased presence of exudate in STSGs can compromise dressing and seal integrity; hence, the need for enhanced pump performance and consistency of negative pressure over its active period of seven days. The management of exudate by the PICO 7 sNPWT is key within routine clinical practice, as consistent delivery of the negative pressure over the period will actively remove exudate away from the wound, reducing incidence of maceration and unnecessary dressing changes.20,21,33-36 Allowing the PICO 7 sNPWT to remain in situ over the wound not only delivers constant negative pressure (when the pump is active) but also provides a bacterial barrier, enabling the patient to carry out their activities of daily living without concern of exudate leakage or need for multiple dressing changes throughout the seven-day wear time.
Exudate management is particularly important in the successful take of skin grafts and flaps, as is effective for bolstering the graft over the recipient site during graft integration. The use of NPWT is a well-established wound management practise to ensure skin graft/flap take.30 The successful skin graft/flap take rate reported in this study, following treatment with the upgraded sNPWT device, illustrates the benefits of managing wound exudate to prevent maceration within the wound bed and peri-wound area while also providing compression through the action of negative pressure, thus immobilizing the STSG while promoting the primary intention healing.
Another key clinical performance outcome of this study was the incidence of surgical site complications, including infection. The benefits of sNPWT on the reduction of SSC is well established.16 However, it is important to note that patient risk factors can contribute to the occurrence of SSC and SSI, as well as surgical procedure risks. Abdominal surgery, including colorectal surgeries, are classified as clean/contaminated or contaminated surgeries and carry a higher procedural risk for SSI when compared to clean knee arthroplasty surgery. The knee arthroplasty group reported no SSIs. Within the STSG/flap group, one SSI was reported, which resolved by study completion. The impact of SSC on a skin graft/flap could result in graft loss, meaning the graft/flap procedure will need to be repeated. This can be costly to the HCP and a painful experience for the patient.
Technical complexity and intrusion on daily activities have been identified as contributors to a lack of patient adherence with NPWT.29 Thus, design improvements amenable to patients’ and clinicians’ ease-of-use may lead to increased adherence. The results of the clinician and patient acceptability analysis in the current study offer promising evidence that this upgraded sNPWT device is highly satisfactory to both of these groups. Clinician satisfaction rates were in excess of 90% for all questions asked, while patients rated their overall experience in treatment with the upgraded sNPWT at higher than 95% during their post-operative experience. This level of patient satisfaction is important to note, especially concerning a patient’s overall wellbeing and quality of life, since post-procedure recovery can be painful and limit mobility.
There are limitations to the current analysis that should be noted. The primary endpoint required the analysis of the microchip within the sNPWT device, which for two patients was not possible due to non-return of the device, therefore reducing the sample size of the primary endpoint. However, as the lack of microchip data had no bearing on other study endpoints, the full cohort of 48 patients was maintained for secondary and exploratory analyses. Furthermore, 10 patients did not receive a full seven days of NPWT for various clinical reasons. Benefits can still be derived from the application of this sNPWT device over briefer durations, yet this nonetheless reduces the population of patients for whom we have data over seven days of wear.
CONCLUSION
Results from this prospective multi-centre study indicate the PICO 7 sNPWT system can maintain negative pressure at a nominal -80 mmHg in clinical practice for up to seven days with no serious device-related AEs reported. This finding is highly relevant, as this study is the first since Hudson et al.20 to measure and report the real-time delivery of NPWT in situ at a therapeutic level of -80 mmHg throughout clinical use. Furthermore, the findings from the exploratory analysis indicate that the device is favourable to clinicians and patients alike, who reported high levels of satisfaction and usability. The absence of SSIs in the knee arthroplasty closed incision group, and the overall low rate of complications, provides further evidence of the safe and effective use of this PICO 7 sNPWT system for reducing SSCs in closed surgical incisions.
IMPLICATIONS FOR CLINICAL PRACTICE
Technologies for sNPWT have advanced greatly in recent years. The upgraded PICO 7 sNPWT device investigated in this study has proven the delivery of therapeutic negative pressure over seven days and shown new design features that are amenable to ease-of-use by patients and clinicians for the treatment of closed surgical incisions and STSGs/flaps. These are important factors for improving patient concordance, while also enabling patients to receive an accepted standard of care for closed incision management across multiple surgical procedures and aligned to the current NICE guidance.16
Acknowledgements
The authors wish to dedicate this manuscript to our colleague Brian Gilchrist, who supported this study and manuscript but sadly passed away at the time of its submission. Furthermore, the authors would like to extend their thanks to Sherwin Barretto, John Watson, Elizabeth Huddleston and Julie Murdoch for their assistance in the preparation and reviewing of this manuscript.
Disclaimer
This study was sponsored and funded by Smith + Nephew. Sarah Megginson and Brian Gilchrist were employees of Smith + Nephew at the time of this manuscript’s preparation and submission.
KEY MESSAGES
Surgical site infections and complications can result in major post-operative challenges; single-use negative pressure wound therapy (sNPWT) can have a positive prophylactic role.
The use of an upgraded PICO 7 sNPWT device to manage surgical incisions, skin grafts and flaps was investigated in a prospective single-arm study.
The PICO 7 sNPWT device delivered consistent negative pressure, with low complication rates, a favourable safety profile and high rates of clinician- and patient-reported satisfaction.
Author(s)
Sudheer L Karlakki, FRCS (Orth), MSc,The Robert Jones and Agnes Hunt Orthopaedic NHS Foundation Trust, Oswestry, UK;
Jessica R Evans, MBBS, FRCS Dept. of Research and Innovation and General Surgery, Queen Elizabeth the Queen Mother Hospital, Margate, UK
Simon P Booth, RN (Dip), MScQueen Victoria Hospital, East Grinstead, UK
Jason KF Wong, PhD,FRCS (Plast), Blond McIndoe Laboratories, Division of Cell Matrix and Regenerative Medicine, University of Manchester, Manchester, UK; Department of Burns and Plastic Surgery, Manchester University Foundation Trust, Wythenshawe Hospital, Manchester Academic Health Science Centre, Manchester, UK
Lyn Wilson,, Pinderfields Hospital, Wakefield, UK
Steven LA Jeffrey, FRCS (Plast), Wound Healing Practice Development Unit, Birmingham City University, Birmingham, UK
Rohit Makhija, FRCS (Glasg, GenSurg) Peterborough City Hospital, Peterborough, UK
Yves Harder, MD, Department of Plastic, Reconstructive and Plastic Aesthetic Surgery, Ente Ospedaliero Cantonale (EOC), Lugano, Switzerland; Faculty of Biomedical Sciences, Università della Svizzera Italiana (USI), Lugano, Switzerland
Iain McNamara, FRCS (Tr and Orth) MD, Norfolk and Norwich University Hospital, Norwich, UK; University of East Anglia, Norwich, UK
Mike Reed, MD, FRCS, Northumbria Healthcare NHS Foundation Trust, North Tyneside General Hospital, Ashington, UK
David E Barnes, MSc, FRCS, Plast Edin St Andrews Centre for Burns and Plastic Surgery, Broomfield Hospital, Chelmsford, UK
Sarah Megginson, MSc, Biostatistics, Smith+Nephew, Hull, UK
Brian Gilchrist, MSc, RN, Global Clinical Strategy, Smith+Nephew, Watford, UK
Correspondence: Sudheer.karlakki@nhs.net
Conflicts of Interest: This study was sponsored and funded by Smith + Nephew.
Sarah Megginson and Brian Gilchrist were employees of Smith + Nephew at the time of this manuscript’s preparation and submission.
References
- Carroll K, Dowsey M, Choong P, Peel T. Risk factors for superficial wound complications in hip and knee arthroplasty. Clin Microbiol Infect 2014; 20(2):130–5.
- Llanos S, Danilla S, Barraza C, Armijo E, Piñeros JL, Quintas M et al. Effectiveness of negative pressure closure in the integration of split thickness skin grafts: A randomized, double-masked, controlled trial. Ann Surg 2006; 244(5):700–5.
- Reddy S, El-Haddawi F, Fancourt M, Farrant G, Gilkison W, Henderson N et al. The incidence and risk factors for lower limb skin graft failure. Dermatol Res Pract 2014; 2014:582080.
- Lin Y, He JF, Zhang X, Wang HM. Intraoperative factors associated with free flap failure in the head and neck region: A four-year retrospective study of 216 patients and review of the literature. Int J Oral Maxillofac Surg 2019; 48(4):447–51.
- Pugely AJ, Martin CT, Gao Y, Mendoza-Lattes S, Callaghan JJ. Differences in short-term complications between spinal and general anesthesia for primary total knee arthroplasty. J Bone Joint Surg Am 2013; 95(3):193–9.
- Nugent EK, Hoff JT, Gao F, Massad LS, Case A, Zighelboim I et al. Wound complications after gynecologic cancer surgery. Gynecol Oncol 2011; 121(2):347–52.
- Santema TB, Poyck PP, Ubbink DT. Skin grafting and tissue replacement for treating foot ulcers in people with diabetes. Cochrane Database Syst Rev 2016; 2:CD011255.
- Zeng R, Lin C, Lin Z, Chen H, Lu W, Lin C et al. Approaches to cutaneous wound healing: Basics and future directions. Cell Tissue Res 2018; 374(2):217–32.
- Braza ME, Fahrenkopf MP. Split-thickness skin grafts. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2020. Available from: https://www.ncbi.nlm.nih.gov/books/NBK551561/
- Novakovic D, Patel RS, Goldstein DP, Gullane PJ. Salvage of failed free flaps used in head and neck reconstruction. Head Neck Oncol 2009; 21:1-33.
- Fujioka M. Factors predicting total free flap loss after microsurgical reconstruction following the radical ablation of head and neck cancers. Int Sch Res Notices 2013; Article ID 952971.
- Yu P, Yu N, Yang X, Jin X, Lu H, Qi Z. Clinical efficacy and safety of negative-pressure wound therapy on flaps: A systematic review. J Reconstr Microsurg 2017; 33:358–66.
- Gore DC. Outcome and cost analysis for outpatient skin grafting. J Trauma 1997; 43(4):597–600; discussion 600-602.
- Lewis LS, Convery PA, Bolac CS, Valea FA, Lowery WJ, Havrilesky LJ. Cost of care using prophylactic negative pressure wound vacuum on closed laparotomy incisions. Gynecol Oncol 2014; 132(3):684–9.
- World Union of Wound Healing Societies (WUWHS) Consensus Document. Closed surgical incision management: Understanding the role of NPWT. Wounds International 2016.
- National Institute for Health and Care Excellence [NICE]. PICO negative pressure wound dressings for closed surgical incisions. Medical Technologies Guidance. 2019; May.
- Selvaggi F, Pellino G, Sciaudone G, Corte AD, Candilio G, Campitello, F. et al. New advances in negative pressure wound therapy (NPWT) for surgical wounds of patients affected with Crohn’s disease. Surg Technol Int 2014; 24:83–9.
- Canonico S, Campitiello F, Della Corte A, Padovano V, Giannini S, Luciani D, et al. Therapeutic possibilities of portable NPWT. Initial multidisciplinary observation with the negative pressure therapy device. Acta Vulnologica 2012; 10:57–66.
- Payne C, Edwards D. Application of the single use negative pressure wound therapy device (PICO) on a heterogeneous group of surgical and traumatic wounds. Eplasty 2014; 14:e20.
- Hudson DA, Adams KG, Van Huyssteen A, Martin R, Huddleston EM. Simplified negative pressure wound therapy: Clinical evaluation of an ultraportable, no-canister system. Int Wound J 2015; 12(2):195–201.
- Karlakki SL, Hamad AK, Whittall C, Graham NM, Banerjee RD, Kuiper JH, et al. Incisional negative pressure wound therapy dressings (iNPWTd) in routine primary hip and knee arthroplasties. A randomised controlled trial. Bone Joint Res 2016; 5:328–37.
- Strugala V, Martin R. Meta-analysis of comparative trials evaluating a prophylactic single-use negative pressure wound therapy system for the prevention of surgical site complications. Surg Infections 2017; 18:810–9.
- Mitchell SL, Ray E, Cordeiro PG. Miniaturized negative-pressure wound therapy for split-thickness skin graft donor sites. J Reconstr Microsurg Open 2018; 3(02):e46–9.
- Ray E, Mitchell SL, Cordeiro PG. Miniature negative pressure dressings on forearm donor sites after radial forearm flap harvest. Plast Reconstr Surg Glob Open 2018; 6(6):e1838.
- Malmsjö M, Huddleston E, Martin R. Biological effects of a disposable, canisterless negative pressure wound therapy system. ePlasty 2014; 14:e15.
- Hurd T. Evaluating the cost and benefits of innovations in chronic wound care products and practices. Ostomy Wound Manage 2013; Suppl:1-16.
- Data on file, reference DS/17/666/R2: Comparison of PICO 1.6 and 2.1 device air leak tolerance. January 2018.
- Karadsheh M, Nelson J, Rechner B, Wilcox R. Application of a skin adhesive to maintain seal in negative pressure wound therapy: Demonstration of a new technique. Wounds 2017; 29(11):E106–10.
- Moffatt CJ, Murray S, Aubeeluck A, Quere I. Communication with patients using negative wound pressure therapy and their adherence to treatment. J Wound Care 2019; 28(11):738–56.
- Hurd T, Kirsner RS, Sancho-Insenser J, Fumarola S, Garten A, Patel M, et al. International consensus panel recommendations for the optimization of traditional and single-use negative pressure wound therapy in the treatment of acute and chronic wounds. Wounds 2021; 33(suppl 2):S1–11.
- Galiano RD, Hudson D, Shin J, van der Hulst R, Tanaydin V, Djohan R, et al. Incisional negative pressure wound therapy for prevention of wounds healing complication following reduction mammaplasty. Plast Reconstr Surg Glob Open 2018; 6(1):e1560.
- Saunders C, Nherera LM, Horner A, Trueman P. Single-use negative-pressure wound therapy versus conventional dressing for closed surgical incisions: Systematic literature review and meta-analysis. BJS Open 2020; 00,1–8.
- Kirsner R, Dove C, Reyzelman A, Vayser D, Jaimes H. A prospective, randomised, controlled clinical trial on the efficacy of a single-use negative pressure wound therapy system, compared to traditional negative pressure wound therapy in the treatment of chronic ulcers of the lower extremities. Wound Repair Regen 2019;27(5):519–29
- Helito CP, Sobrado MF, Giglio PN, Bonadio MB, Pécora JR, Demange MR, et al. The use of negative-pressure wound therapy after total knee arthroplasty is effective for reducing complications and the need for reintervention. BMC Musculoskeletal Disorders 2020; 21(490):1–8.
- Smith+Nephew. Summary Report of in vitro wound model and negative pressure delivery (nominal -80mmHg) testing for PICO v2 (PICO 7 and PICO 14) system. Internal Report. 2019. RD/18/134 V2.
- Nherera LM, Trueman P, Karlakki SL. Cost-effectiveness analysis of single-use negative pressure wound therapy dressings (sNPWT) to reduce surgical site complications (SSC) in routine primary hip and knee replacements. Wound Repair Regen 2017; 25(3):474–82
