Volume 19 Number 2
Hepatitis B vaccination in patients with stage 4/5 chronic kidney disease – a scoping review
Casey Light, Karen Heslop, Hemant Kulkarni
Keywords Chronic kidney disease, Hepatitis B virus, clinical practice guidelines, stage 4/5 CKD, Hepatitis B vaccination
For referencing Light C, Heslop K & Kulkarni H. Hepatitis B vaccination in patients with stage 4/5 chronic kidney disease – a scoping review. Renal Society of Australasia Journal 2023; 19(1):68-76.
DOI
https://doi.org/10.33235/rsaj.19.1.68-76
Submitted 17 February 2023
Accepted 3 May 2023
Abstract
Aim This scoping review aims to identify and map the extent of published literature on Hepatitis B vaccination in stage 4/5 chronic kidney disease (CKD) and dialysis patients, the availability of standardised practice guidelines, the optimal CKD stage to commence the vaccination for efficient response, and seroconversion rate.
Background Hepatitis B vaccination remains ‘standard-of-care’ in the haemodialysis (HD) population despite immunological challenges. CKD patients have decreasing immunity as the disease progresses, prompting further research to investigate the response of Hepatitis B vaccination in earlier stages of CKD for better response rate prior to requiring dialysis.
Method This scoping review was conducted using the Arksey and O’Malley (2005) five-stage approach: 1) identifying the research question; 2) identifying the relevant studies; 3) selecting the studies; 4) data charting; and 5) collating, summarising and reporting the results. Medline, PubMed, PubMed Central (PMC) and Cochrane databases were used to access research literature published between 2012–2022.
Results Of the 602 eligible articles,183 full text papers were identified. There were 41 studies retained for this review which were sorted out into domains relating to the vaccine immunogenicity, vaccine response and clinical practice guidelines. Although there were studies suggesting immunosuppression in declining renal function leads to low vaccine response, most of the studies were focused on HD patients. There have been no large, randomised control trials on optimal vaccination policy in CKD patients. This scoping review provided important knowledge for future studies to explore the efficiency of commencing vaccination at an earlier stage of CKD before reaching dialysis.
Background
Chronic kidney disease
Chronic kidney disease (CKD) is a leading global public health epidemic disease. The global prevalence, as reported in 2019, is estimated to be 13.4% (Lv & Zhang, 2019). In Australia, CKD is a major health concern. The Australian Institute of Health and Welfare (AIHW) report in 2020 showed an estimated 1.7 million Australians over 18 years old have CKD (AIHW, 2020), with a 9% prevalence among non-Indigenous adults and 18% among Aboriginal and Torres Strait Islander people. CKD is a major risk factor for cardiovascular disease, kidney failure and other complications (Usherwood & Lee, 2021). The most common causes of CKD are diabetes (38%), glomerular disease (16%), and hypertension (13%) as reported in July 2020 (AIHW, 2020).
CKD is defined as a decline in kidney function due to structural damage. The estimated glomerular filtration rate (eGFR) measures the level of kidney function and determines the stage of kidney disease. The eGFR can be calculated from the blood creatinine result, age, body size and gender using the Modification of Diet in Renal Disease (MDRD) method (Levey, Coresh & Greene, 2006) or the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) formula (Levey, Stevens & Schmiel, 2009). The eGFR is used globally to classify CKD into five stages according to the degree of kidney damage. Figure 1 summarises the five stages of CKD with corresponding eGFR (Kidney Health Australia, 2020).
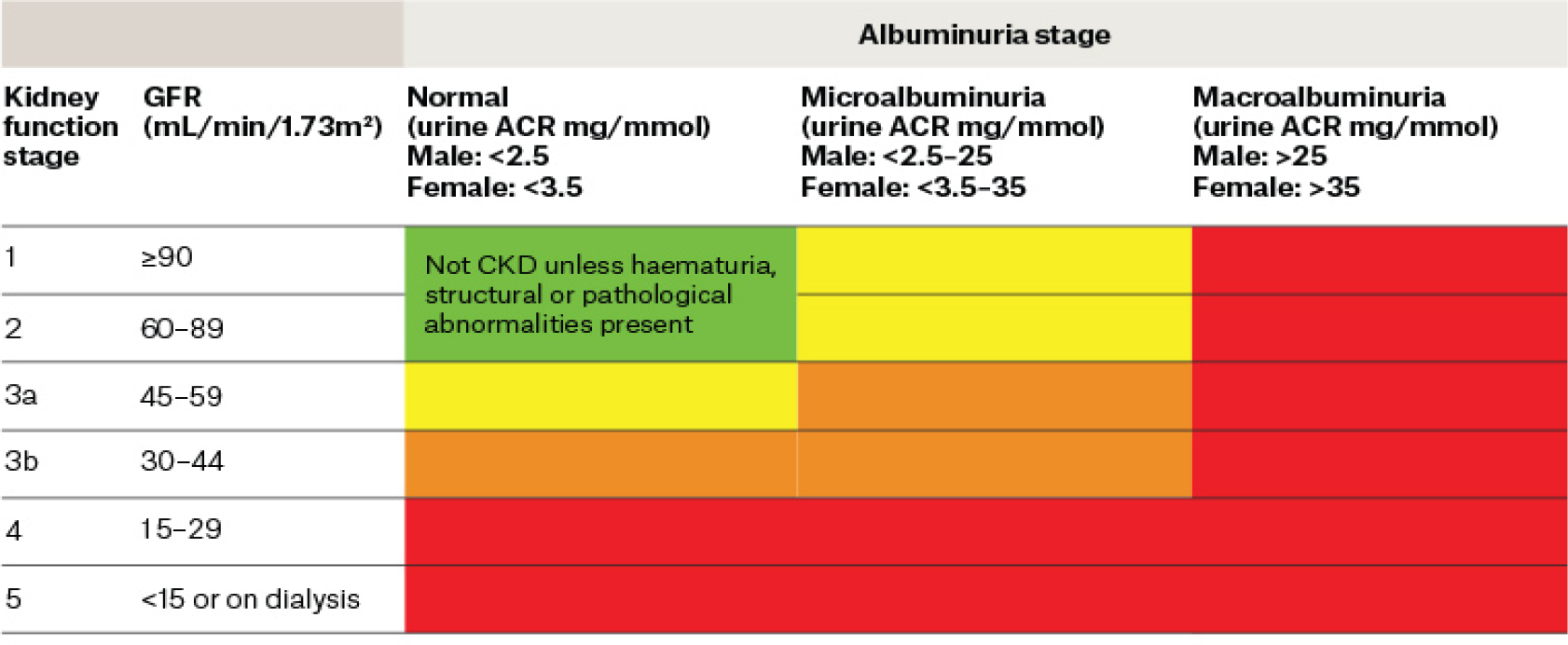
Figure 1. Classification of CKD according to corresponding eGFR. Reproduced with permission from Kidney Health Australia (2020)
ACR: albumin-to-creatinine ratio
Renal replacement therapy (RRT) or kidney transplantation is required when the progressive loss of kidney function develops from CKD to end stage kidney disease (ESKD) with uremic symptoms presented as cardiovascular, mental and gastrointestinal disturbances. The RRT includes various forms of dialysis, such as haemodialysis (HD) involving blood purification via a dialysis machine, peritoneal dialysis (PD) using special fluids and the peritoneal membrane, or pre-emptive kidney transplant (Phadke & Khanna, 2011). According to the 44th annual Australia New Zealand Dialysis and Transplant Registry (ANZDATA) report on data collected to 31 December 2020, there is a total of 14,554 patients on dialysis, with 82% on HD and 18% on PD in Australia (ANZDATA Registry, 2021).
Immunodeficiency associated with ESKD has been identified as early as 1999 (Girndt et al., 1999). It is noted in contemporary literature that immune dysfunction in CKD predisposes patients to the increased risk of infection and diminished vaccine response (Syed-Ahmed & Narayanan, 2019; Ma et al., 2021; Fabrizi et al., 2021).
Cohen (2020) explained that kidney failure results in reduced renin, erythropoietin (EPO) and vitamin D production, causing adverse effects on the immune system. The retention of uremic toxins as a result of reduced glomerular filtration in kidney failure can compromise immune cells (Cohen, 2020). Thus, in CKD, especially patients with ESKD undergoing HD, there is increased risk for contracting viral infection due to recurrent blood exposure to the dialysis machine and skin breaches; the most common viral infection is Hepatitis B (Bernieh, 2015).
Hepatitis B virus
The Hepatitis B virus (HBV) is a double-stranded DNA blood-borne virus. This covalently closed circular (ccc) DNA virus has a long-lived minichromosome that is resistant to antiviral therapy and can persist on environmental surfaces for up to 7 days. The incubation of HBV is 45–100 days. The virus consists of three primary structural antigens – ‘surface’ (HBsAg), ‘core’ (HBcAg) and ‘e’ (HBeAg). Hepatitis B surface antigens (HBsAg) play a central role in the HBV infection diagnosis (McMahon, 2014; Holt, Locqrnini & Sasadeusz, 2021).
The World Health Organization (WHO) reported chronic HBV infection globally in 296 million people in 2019 (WHO, 2021), with high prevalence (8%) in Africa and South East Asia, intermediate prevalence (2–8%) in Eastern Europe, Japan and Russia, and low prevalence (<2%) in Western Europe, South America and Australia. According to the WHO classification of HBsAg, prevalence is determined as low (<2%), intermediate (2–8%) and high (>8%). Although Australia is classified as low prevalence, higher rates of HBsAg are reported in high-risk groups such as the Aboriginal population and migrants from high-risk countries. In 2022, 0.9% of the Australian population, i.e., an estimated 222,559 people, were living with chronic Hepatitis B infection (B-Positive, 2022).
The spread of HBV infection consists of horizontal and perinatal transmission modes. The horizontal mode is predominantly due to mucosal exposure to infected blood, saliva, seminal and vaginal fluids as well as the re-use of injections or tattooing equipment and body piercing (Bernieh, 2015; Nelson, Easterbrook & McMahon, 2016). The perinatal transmission is a vertical mode of mother to child at childbirth (Nelson et al., 2016; Veronese et al., 2021).
The consequences of Hepatitis B infection can be acute or chronic. An acute infection can be symptomatic or asymptomatic with the presence of jaundice. Chronic HBV infection is at high risk of cirrhosis and liver cancer (Cancer Council Victoria, 2016). A systemic review by Schweitzer showed 15–25% of people with chronic HBV die from cirrhosis or liver cancer (Schweitzer, 2015).
The most protective method against HBV infection is by vaccination against HBV. The first generation of HBV vaccines consisted of inactivated plasma-derived HBsAg from the asymptotic HBV carrier (Bernieh, 2015). Due to concerns over the transmission of HIV through plasma products, the first generation HBV vaccines were replaced by the second generation vaccines that used synthetic recombinant DNA technique (Jilg et al., 1986; Koumadakis et al., 2018). A third generation recombinant HBV vaccine containing pre-S1 and pre-S2 antigen expressed in mammalian cells has been developed and licensed in some countries only (Schouval, Roggendorf & Roggendorf, 2015; Fabrizi, Donato & Messa, 2017; El Hanan et al., 2018). Current vaccination is safe and effective.
A Hepatitis B surface antibody (HBsAb) titre of >10mIU/mL is defined as achieving seroprotection. In healthy individuals, seroprotection levels of 93% have been attained and long lived. The WHO and the Centers for Disease Control and Prevention (CDC) recommend a 6-month vaccination schedule of 20ug at 0, 1, 6 months (Holt et al., 2021).
In Australia, Hepatitis B vaccines are available as monovalent (Engerix-B or H-B-Vax II) or combined vaccines. The recommended dosing for healthy adults is a 6-month schedule of 20ug at 0, 1, 6 months (Department of Health and Aged Care, 2022).
Hepatitis B in CKD
Data from the Dialysis Outcomes and Practice Patterns Studies (DOPPS) reported HBV infection prevalence of 0–6.6% in Western Europe, Japan and USA. In the Asia Pacific regions (including Australia and New Zealand) prevalence ranged from 1.3% to 14.6% (Burdick et al., 2003).
In the CKD population, HBV infection is more prevalent in HD patients due to the exposure to blood handling, broken skin integrity from needling and sharing of dialysis equipment, as well as frequent hospitalisation for surgical procedures related to the HD vascular access. Holt et al. (2021) pointed out that HBsAg can also be found in PD fluids; however, transmission in PD populations seems rare (Holt et al., 2021).
In a systemic review of HBV outbreaks in dialysis units between 1992–2014, Fabrizi et al. (2015) reported 16 outbreaks in 12 papers involving 118 HD patients, demonstrating that despite rigorous measures, the risk of HBV infection still presents amongst HD patients in more recent years (Fabrizi et al., 2015; Victorian Renal Clinical Network, 2017). Rigorous practice of universal precaution is the single most important method of transmission prevention. Edey et al. (2010) pointed out the risk of infection is also increased when infected HD patients are likely to become carriers (Edey et al., 2010; Ghadini et al., 2012). Active vaccination remains vital against the virus (Garthwaite et al., 2019). As indicated in the literature, as the immune system’s abnormalities directly correspond to the degree of kidney failure, it would be beneficial to conduct a scoping review on the available knowledge relating to Hepatitis B vaccination in the high-risk HD group and stage 4/5 CKD before dialysis sets in as well as exploring for the most effective CKD stage to commence the vaccine.
Aim
This scoping review aims to identify and map the extent of published literature relating to Hepatitis B vaccination in stage 4 CKD, stage 5 CKD and dialysis patients. The broad nature of the scoping review focuses on summarising the breadth of evidence to ensure further research in this population group is beneficial. It adds to existing knowledge and informs future research study design, program and policy.
Methods
This scoping review was conducted according to the five-stage approach described by Arksey and O’Malley (2005): 1) identifying the research question; 2) identifying the relevant studies; 3) selecting the studies; 4) data charting; and 5) collating, summarising and reporting the results.
Stage 1: identifying the research questions
The broad research questions in the scoping process include:
- What is known about Hepatitis B vaccination in chronic kidney disease?
- What is the immunogenicity of the Hepatitis B vaccine in CKD?
- What is the Hepatitis B vaccination response in CKD?
- What is known about Hepatitis B vaccination in stage 4/5 CKD?
- What is the availability of established clinical practice guidelines for Hepatitis B vaccination in CKD?
Stage 2: identifying the relevant studies
Medline, PubMed, PubMed Central (PMC) and Cochrane databases were used to access research literature published between 2012–2022 on Hepatitis B vaccination for patients with CKD. Some historical literature that set milestones on Hepatitis B vaccine were also included (CDC, 1982; Jilg et al., 1986; Girndt et al., 1999). An example of a search strategy for the PubMed database included using search terms “Chronic Kidney Disease”, “Hepatitis B Virus”, “Immunisation”, and “Guidelines”.
Inclusion criteria:
- Population: adults with stage 4/5 CKD including dialysis.
- Study designs: full text published literature, systemic reviews, meta-analysis, case reports.
- Literature vintage: all peer reviewed studies that describe Hepatitis B vaccination in CKD published in English between 2012–2022 and grey literature such as fact sheets.
Exclusion criteria:
- Hepatitis B vaccination related to renal transplant recipients.
- Studies related to paediatric CKD patients.
- Studies older than 10 years.
- Studies not published in English.
Stage 3: selecting the studies
Studies and reviews that reported relevant data on Hepatitis B vaccination for CKD patients were included. Items such as immunogenicity of Hepatitis B, Hepatitis B vaccination response in stage 4/5 CKD patients, published clinical practice guidelines for Hepatitis B vaccination were included. Article selection progress is shown in Figure 2.
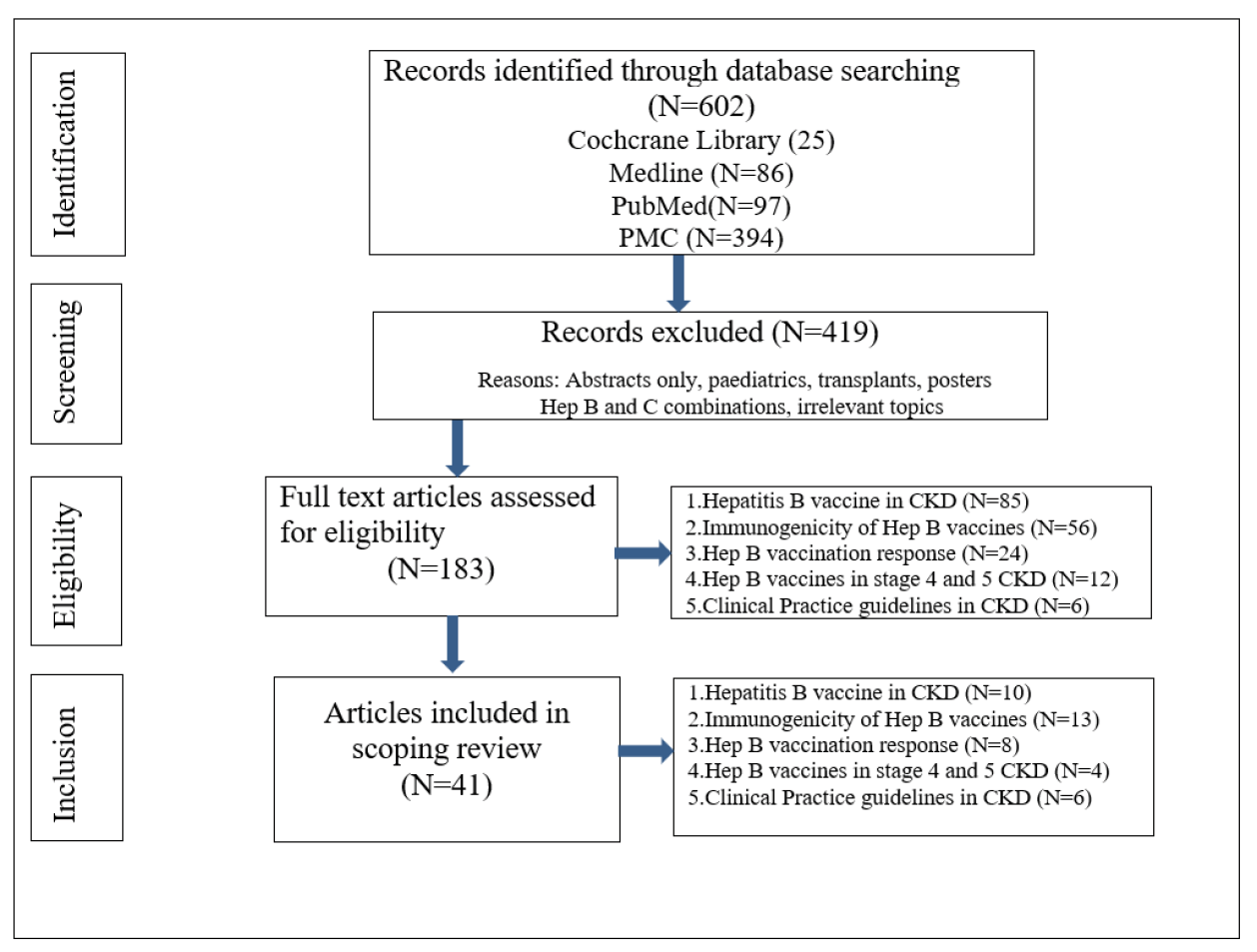
Figure 2. Article selection progress flow-chart
Stage 4: data charting
The following data from eligible articles was abstracted: the surname of the first author, year of publication, study design, study population, study setting, brief findings, outcomes and recommendations.
Stage 5: collating, summarising and reporting the results
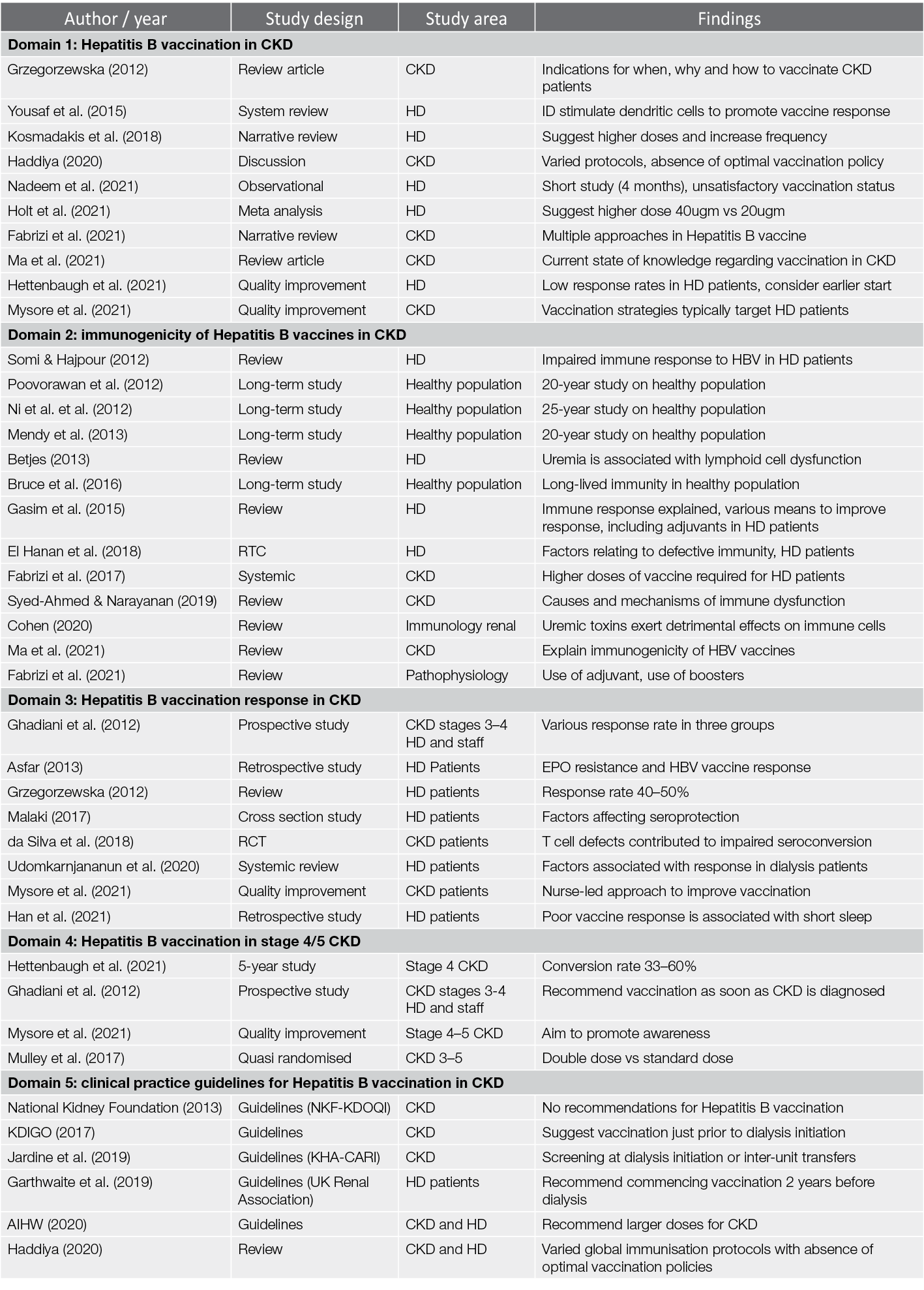
The database search identified 602 articles; after screening titles and abstracts, 183 full-text papers were assessed for eligibility and 41 studies were retained for this review. The papers were further sorted out into the five content domains as shown in Table 1:
- Domain 1: Hepatitis B vaccination in CKD
- Domain 2: immunogenicity of Hepatitis B vaccines in CKD
- Domain 3: Hepatitis B vaccination response in CKD
- Domain 4: Hepatitis B vaccination in stage 4/5 CKD
- Domain 5: clinical practice guidelines for Hepatitis B vaccination in CKD.
Table 1. Collating, summarising and reporting the results in five domains
Discussion
Hepatitis B vaccination in CKD
Since its inception in 1982, the Hepatitis B vaccine remains the best protection against HBV (CDC, 1982). Grzegorzewska reported a 99% response rate in the general population with long lived immunity, while in patients with CKD it is less effective and does not provide sustained immunity (Grzegorzewska, 2012). Fabrizi et al., pointed out CKD patients have suboptimal response. Studies have demonstrated multiple approaches and effects to improving Hepatitis B vaccination in HD patients – Holt suggested increasing vaccine dose from 20ugm to 40ugm (Holt et al., 2021) while others proposed to increase the frequency from 0, 1, 6 months to 0, 1, 2, 6 months (Fabrizi et al., 2015; da Silva et al., 2018; Komadakis et al., 2018). A systemic review by Yousef et al. (2015) on intradermal (ID) versus intramuscular (IM) technique demonstrated the ID technique enhanced vaccine response by direct dendritic cell stimulation; however, this study only included those who did not respond to the primary dose given using the IM technique, so it is not clear whether the ID technique was effective if given as the primary dose (Yousef et al., 2015). The use of an adjuvant (e.g. Levamisole) to increase seroprotection rate was also explored by Fabrizi et al. (2021) and Ma et al., (2021). Fabrizi et al. (2021) also emphasised the importance of vaccination in HBV infection management, but only focused on dialysis patients, providing little data on stage 4/5 CKD patients (Fabrizi et al., 2021; Mysore et al.,2021).
Immunogenicity of Hepatitis B vaccine in CKD
CKD and ESKD patients have suboptimal response and shorter duration of seroprotection due to immune deficiency (Somi & Hajpour, 2012; Betjes, 2013; Grzegorzewska, 2014). Fabrizi et al. (2021) pointed out that it remains unclear how the immune response of CKD patients to HBV can be boosted (Fabrizi et al., 2021). The importance of exploring the immunogenicity of the Hepatitis B vaccine in CKD patients was highlighted by Ma et al. (2021) who explained that for an effective immunisation response, both innate (genetic or natural) immunity and adaptive (acquired) immunity are required. The reduced vaccination response is due to various immunological dysfunctions in CKD such as impaired monocytes endocytosis and maturation, defective antigen, impaired T cell activation and proliferation leading to decreased T cell cytokines and reduced B cell memory (Ma et al., 2021).
Long-term studies in healthy adults have demonstrated sustained immunity for up to 30 years in an Alaskan study (Bruce et al., 2016); 25 years in a Taiwanese study (Ni et al., 2012), 20 years in a Thai study (Poovorawan et al., 2012), and 24 years in a Gambian study (Mendy et al., 2013). However, there was limited long-term immunity study in CKD patients. One article was identified describing a 13-year follow-up study by Pin et al. (2009) on 136 HD patients; the study reported that 32% of responders lost their immunity, with only 18% of the responders remaining seroconverted for 6 years (Pin et al., 2009).
Hepatitis B vaccination response in CKD
Ghadiani et al. classified response rates using anti-HBs titres, with <10mIU/mL as non-seroconversion, >10mIU/mL as seroconversion and >100mIU/mL as seroprotective (Ghadiani et al., 2012). da Silva et al. conducted a RCT describing the serological and cellular response to hepatitis vaccination in CKD, and concluded that T cell defects contributed to impaired seroconversion (da Silva et al., 2018). Mysore et al. noted that Hepatitis B vaccination strategies for CKD patients typically target patients on dialysis (Mysore et al., 2021). Response rate in dialysis had been shown to be 40–50% (Grzegorzewska, 2012; Mysore et al., 2021) compared to a 90% response rate in the general population, with 50% of the dialysis responders losing their immunity quicker than the healthy population (Grzegorzewska, 2012).
Elevated serum urea due to a decline in kidney functions has an adverse effect on vaccine response rate (Malaki, 2017). Other factors affecting vaccine response were also investigated; however, the studies were limited to dialysis patients only, such as the systemic review and meta-analysis conducted by Udomkarnjananun et al. (2020) that identified 61 studies that explored the factors associated with immune response in the dialysis patients. This review identified multiple factors affecting the immune response – older age, diabetes, HLA-DR3 status, shorter dialysis time leading to lower dialysis adequacy, poor nutritional status, lower parathyroid hormone (PTH) and lower haemoglobin levels (Udomkarnjananun et al., 2020). EPO use for treating low haemoglobin was also studied by Afsar (2013) in the Turkey Konya State Hospital on 97 HD patients to analyse the relationship between anti-HBs response and EPO resistance. It was concluded there was no association between EPO resistance and Hepatitis B vaccine response (Afsar, 2013). In a retrospective study, Han et al. studied the effect of sleep in adaptive Hepatitis B vaccination response and demonstrated that short sleep duration was associated with poor antibody response to the vaccine (Han et al., 2021).
Hepatitis B vaccination in stage 4/5 CKD
Amongst various strategies to improve response rates, commencing the vaccination at earlier CKD stage has been suggested. For example, commencing vaccination earlier at stage 4 CKD (16–29ml/min/1.73m2) and stage 5 CKD (<15ml/min/1.73m2) before dialysis commences, as about 40% of dialysis patients do not develop protective titre of antibodies due to compromised immune system (Grzegorzewska, 2012).
However, there was limited literature on commencing vaccination in stage 4/5 CKD. Ghadiani et al. (2012), one of the early proponents of vaccination at an early CKD stage, conducted a study on three groups of participants – Group A HD patients; Group B: stage 3–4 CKD patients; Group C: healthy medical staff (as control group). The vaccination consisted of a regime of four doses of 40ug on a 6-month schedule (0, 1, 2 and 6 months), IMI in the Deltoid muscle. Results demonstrated seroconversion rates of 44% (Group A), 89.7% (Group B) and 96.2% (Group C) (Ghadiani et al., 2012). Although early CKD stage was studied, Group B consisted of stage 3–4 CKD patients who have a wide range of eGFR spanning from 16–60ml/min/1.73m2. From this data it is difficult to judge if commencing at stage 3 or at stage 4 is more beneficial or effective. Mulley et al. conducted a systemic review on stage 3–5 CKD comparing standard dose vaccination to double dosing, but found no increase in seroconversion in the double dose vaccination (Mulley, Le & Ives, 2017).
A more current study by Hettenbaugh et al. investigated the vaccination at stage 4/5 CKD over a 5-year period in Omaha, USA. A total of 198 patients were screened, with 56 eligible and 37 completing the course. The seroconversion rate ranged from 33–60%, with one of two US FDA approved vaccines (ReCombivexTM or EngerixTM). The study concluded the importance of awareness by nephrologists and nursing staff to vaccinate patients at earlier stage of CKD, at stage 4/5, prior to the onset of dialysis (Hettenbaugh et al., 2021).
Clinical practice guidelines in Hepatitis B vaccination in CKD
HBV causes serious disease such as acute hepatitis, liver fibrosis and liver cancer. Hepatitis B infection is difficult to treat, so prevention by vaccination is the most effective way.
Despite widespread HBV vaccine use since 1982 with a clear benefit to Hepatitis B vaccination in CKD patients – in particular the immunocompromised HD patients who are at higher risk of the infection due to frequent blood exposure and skin breaches – there have been limited published studies on vaccination implementation strategies and clinical practice guidelines (Mysore, 2021). Vaccination regime variations and a lack of optimal policy between countries continues to be recognised in recent literature (Haddiya, 2020; Nadeem et al., 2021). Although the CDC guidelines strongly recommend all CKD patients should be vaccinated against HBV, studies have shown the range of CKD patients vaccinated to vary greatly, between 20–73.1%: 20% in Pakistan (Nadeem et al., 2021); 31% in Belgium (Boey et al., 2020); 46% in the UK (Ray et al., 2002); and 73.1% in the US (Ayoola et al., 2019).
Global organisations such as the National Kidney Foundation Kidney Dialysis Outcome Quality Initiative Guidelines (NKF-KDOQI) (National Kidney Foundation, 2013) and Kidney Disease Improving Global Outcomes (KDIGO, 2017) are involved in developing and implementing evidence-based clinical practice guidelines in kidney disease. The NKF-KDOQI showed no recommendations for Hepatitis B vaccination amongst other 13 published renal guidelines (National Kidney Foundation, 2013). The KDIGO guidelines have developed 11 clinical practice guidelines on renal topics since 2008 to 2017; however, there is no specific practice guidelines on Hepatitis B vaccination except to suggest vaccination just prior to initiation of dialysis. The UK Renal Association recommends screening all patients starting HD, preferably to commence vaccination 2 years before dialysis as well as vaccinating patients returning to HD from failed PD (Garthwaite et al., 2019). No recommendations were noted in the Canadian Society of Nephrology or the European Best Practice Guidelines.
In Australia, the Australian Immunisation Handbook (Department of Health and Aged Care, 2018) recommend HD patients and CKD patients nearing dialysis to receive larger than usual doses of Hepatitis B vaccine (40ugm in CKD patients compared to 20ugm in the general population); however, there is no further clarity to what CKD stage to commence vaccination, nor the booster regime for non-responders <10IU/mL (Department of Health and Aged Care, 2018). The Australian renal guideline (the Kidney Health Australia: Caring for Australian with Renal Impairments (KHA-CARI) guidelines) for infection control for HD units recommended standard precautions, screening of HBV at the start of HD or transferring between units (Jardine et al., 2019).
Conclusion
From the literature presented, the immune system abnormalities correlate with the degree of kidney failure – CKD patients not yet on dialysis may have a stronger immune system and higher antibody response to the HBV vaccine. This review provided awareness about why it is so important to continually vaccinate against HBV; although there has been a substantial decline in HBV infection in HD patients – likely due to blood donor screening and reduced use of blood transfusions requirement related to correction of renal anaemia with EPO use (Somi & Hajpour, 2012) – outbreaks of Hepatitis B still occur despite rigorous precaution measures (Victoria Renal Clinical Network, 2017). There have also been studies showing that Hepatitis B vaccination was not routinely implemented. In the UK, a published study showed only 46% of dialysis units were routinely immunising patients according to the Renal Association’s recommendation, while only 20% patients were vaccinated in a study conducted in Pakistan (Nadeem et al., 2021). Besides preventing transmission in the dialysis unit, successful Hepatitis B vaccination also allows for renal transplantation recipient from an anti-HBc positive donor (Holt et al., 2021). Moreover, it is important to note that not only HD patients are at high risk of the infection, they are also at increased risk for becoming HBV carriers (Edey et al., 2010; Ghadini et al., 2012).
This scoping review provided broad knowledge to fulfil the research questions as set out in the aims of the review as well as identifying the parameters and gaps in the literature. There was ample literature describing Hepatitis B vaccination in CKD, the immunogenicity of the vaccine, and the diminishing response as the kidney function declines with escalated inflammatory and immunological changes. In order to improve the vaccine response, many strategies such as increasing the dosing, increasing the frequencies, variations of schedules, and the use of adjuvants were found in the literature. There were also few recommendations in commencing the vaccine in earlier stages of CKD; however, the optimal strategy for vaccination remains unclear, with limited standardised clinical practice guidelines available to guide clinicians. The results of the review indicated the need for further studies to define a stage of CKD and standardise clinical practice guidelines for optimal Hepatitis B vaccination management.
Author(s)
Casey Light PhD student, MN(NP), PGD, Grad Cert (Neph), BN, RN
Curtin School of Nursing, Curtin University, Bentley, WA, Australia
Armadale Renal Service, Armadale Hospital, Armadale, WA, Australia
Karen Heslop PhD, MEd, Grad Dip Soc Sci, BN, RN
Curtin School of Nursing, Curtin University, Bentley, WA, Australia
Hemant Kulkarni MBBS, MD, DCH, DM(Neph)MRCPH(UK), FRACP
Armadale Renal Service, Armadale Hospital, Armadale, WA, Australia
Correspondence to Casey Light, Renal Nurse Practitioner, Armadale Renal Service, Armadale Hospital,
3056, Albany Highway, Armadale, WA 6112, Australia
Email casey.light@health.wa.gov.au
References
Afsar, B. (2013). The relationship between erythropoietin resistance and antibody response to hepatitis B vaccine in hemodialysis patients. Nephro-urology Monthly, 5(3), 806–812. https://doi.org/10.5812/numonthly.8919
ANZDATA Registry. (2021). ANZDATA Registry 44th report, Australian Data Infographic. Retrieved from: http://www.anzdata.org.au/wp-content/uploads/2021/11/2020-ANZDATA-Summary-Consumer-Infographic-v1.1.pdf
Arksey, H., & O’Malley, L. (2005). Scoping studies: Towards a methodological Framework. International Journal of Social Research Methodology 8, 19–32.
Australian Institute of Health and Welfare (AIHW). (2020). AIHW web report on chronic kidney disease 15 July 2020. Retrieved from: https://www.aihw.gov.au/reports-data/health-conditions-disability-deaths/chronic-kidney-disease/overview
Ayoola, R., Larion, S., Poppers, D. M., & Williams, R. (2019). Clinical factors associated with hepatitis B screening and vaccination in high-risk adults. World Journal of Hepatology, 11(1), 86–98. https://doi.org/10.4254/wjh.v11.i1.86
Bernieh, B. (2015). Viral hepatitis in hemodialysis: An update. Journal of Translational Internal Medicine, 3(3), 93–105. https://doi.org/10.1515/jtim-2015-0018
Betjes, M. (2013). Immune cell dysfunction and inflammation in end-stage renal disease. Nature Reviews: Nephrology, 9(5), 255–265. https://doi.org/10.1038/nrneph.2013.44
Boey, L., Bosmans, E., Ferreira, L. B., Heyvaert, N., Nelen, M., Smans, L., ... Vandermeulen, C. (2020). Vaccination coverage of recommended vaccines and determinants of vaccination in at-risk groups. Human Vaccines & Immunotherapeutics, 16(9), 2136–2143. https://doi.org/10.1080/21645515.2020.1763739
B-Positive. (2022). Prevalence and epidemiology of hepatitis B. Retrieved from: https://www.hepatitisb.org.au/prevalence-and-epidemiology-of-hepatitis-b/
Bruce, M. G., Bruden, D., Hurlburt, D., Zanis, C., Thompson, G., Rea, L., ... McMahon, B. J. (2016). Antibody levels and protection after hepatitis B vaccine: Results of a 30-year follow-up study and response to a booster dose. The Journal of Infectious Diseases, 214(1), 16–22. https://doi.org/10.1093/infdis/jiv748
Burdick, R., Bragg-Gresham, J., Woods, J. D., Hedderwick, S. A., Kurokawa, K., Combe, C., ... Young, E. W. (2003). Patterns of hepatitis B prevalence and seroconversion in hemodialysis units from three continents: The DOPPS. Kidney International, 63(6), 2222–2229. https://doi.org/10.1046/j.1523-1755.2003.00017.x.
Cancer Council Victoria. (2016). Hepatitis B and liver cancer fact sheet. Retrieved from: https://www.cancervic.org.au/downloads/cpc/hepatitis-b-and-liver-cancer-fac-sheet-pdf
CDC. (1982). Recommendation of the Immunization Practices Advisory Committee (ACIP) inactivated Hepatitis B virus vaccine. MMWR Weekly, 31, 317–22.
Cohen, G. (2020). Immune dysfunction in uraemia. Toxins, 12(7), 439. https://doi.org/10.3390/toxins12070439
da Silva, E. N., Baker, A., Alshekaili, J., Karpe, K., & Cook, M. C. (2018). A randomized trial of serological and cellular responses to hepatitis B vaccination in chronic kidney disease. PloS One, 13(10), e0204477. https://doi.org/10.1371/journal.pone.0204477
Department of Health and Aged Care. (2018). Australian Immunisation Handbook. Hepatitis B: Recommendations. Retrieved from: https://immunisationhandbook.health.gov.au/contents/vaccine-preventable-diseases/hepatitis-b#recommendations
Department of Health and Aged Care. (2022). Australian Technical Advisory Group on Immunisation (ATAGI). Retrieved from: https://www.health.gov.au/committees-and-groups/australian-technical-advisory-group-on-immunisation-atagi?language=und
Edey, M., Barraclough, K., & Johnson, D. W. (2010). Review article: Hepatitis B and dialysis. Nephrology, 15(2), 137–145.
El Hanan, E., Boaz, M., Schwartz, I., Schwartz, D., Chernin, G., Soetendorp, H., & Weinstein, T. (2018). A randomized, controlled clinical trial to evaluate the immunogenicity of a PreS/S hepatitis B vaccine Sci-B-Vac™, as compared to Engerix B®, among vaccine naïve and vaccine non-responder dialysis patients. Clinical and Experimental Nephrology, 22(1), 151–158.
Fabrizi, F., Dixit, V., Messa, P., & Martin, P. (2015). Transmission of hepatitis B virus in dialysis units: A systematic review of reports on outbreaks. The International Journal of Artificial Organs, 38(1), 1–7. https://doi.org/10.5301/ijao.5000376
Fabrizi, F., Donato, F. M., & Messa, P. (2017). Efficacy and safety of reinforced versus standard vaccine scheduled towards Hepatitis B in chronic kidney disease: A systematic review and meta-analysis. Hepatitis Monthly, 17(7), e44179. https://doi.org/10.5812/hepatmon.44179.
Fabrizi, F., Cerutti, R., Dixit, V., & Ridruejo, E. (2021). Hepatitis B virus vaccine and chronic kidney disease. The Advances, Nefrologia, 41(2), 115–122. https://doi.org/10.1016/j.nefro.2020.08.016
Garthwaite, E., Reddy, V., Douthwaite, S., Lines, S., Tyerman, K., & Eccles, J. (2019). Clinical practice guideline management of blood borne viruses within the haemodialysis unit. BMC Nephrology, 20(1), 388. https://doi.org/10.1186/s12882-019-1529-1
Gasim, G. I., Bella, A., & Adam, I. (2015). Immune response to hepatitis B vaccine among patients on hemodialysis. World Journal of Hepatology, 7(2), 270–275. https://doi.org/10.4254/wjh.v7.i2.270
Ghadiani, M. H., Besharati, S., Mousavinasab, N., & Jalalzadeh, M. (2012). Response rates to HB vaccine in CKD stages 3–4 and hemodialysis patients. Journal of Research in Medical Sciences, 17(6), 527–533.
Girndt, M., Sester, U., Sester, M., Kaul, H., & Köhler, H. (1999). Impaired cellular immune function in patients with end-stage renal failure. Nephrology Dialysis Transplantation, 14(12), 2807–2810. https://doi.org/10.1093/ndt/14.12.2807
Grzegorzewska, A. (2012). Hepatitis B vaccination in chronic kidney disease: Review of evidence in non-dialyzed patients. Hepatitis Monthly, 12(11), 7359. https://dx.doi.org/10.5812/hepatmon.7359
Grzegorzewska, A. (2014). Hepatitis B vaccination in chronic kidney disease patients: A call for novel vaccines. Expert Review of Vaccines, 13(11), 1317–1326. https://doi.org/10.1586/14760584.2014.944508
Haddiya, I. (2020). Current knowledge of vaccination in chronic kidney disease patients. International Journal of Nephrology and Renovascular Disease, 13, 179–185. Retrieved from: https://www.dovepress.com/current-knowledge-of-vaccinations-in-chronic-kidney-disease-patients-peer-reviewed-fulltext-article-IJNRD6
Han, M., Ye, X., Rao, S., Williams, S., Thijssen, S., Hymes, J., Maddux, F. W., & Kotanko, P. (2021). Hepatitis B vaccination response in hemodialysis patients: The impact of dialysis shift. Blood Purification, 50(4–5), 628–635. https://doi.org/10.1159/000513154
Hettenbaugh, J., Mullane, R., Gillispie, G., Shostrom, V., Flores, L., Fillaus, J. A., Florescu, M. C., Murcek, D., & Tendulkar, K. K. (2021). Hepatitis B vaccination in advanced chronic kidney disease: A quality improvement project at a veteran affairs chronic kidney disease clinic. Infectious Disease Reports, 13(4), 1036–1042. https://doi.org/10.3390/idr13040094
Holt, S., Locqrnini, S., & Sasadeusz, J. (2021). Hepatitis B related dilemmas in the renal unit. Nephrology, 26(4), 287–293.
Jardine, M., Commons, R. J., de Zoysa, J. R., Wong, M. G., Gilroy, N., Green, J., ... Athan, E. (2019). Kidney Health Australia: Caring for Australians with renal impairment guideline recommendations for infection control for haemodialysis units. Nephrology, 24(9), 951–957.
Jilg, W., Schmidt, M., Weinel, B., Küttler, T. H., Brass, H., Bommer, J., & Deinhardt, F. (1986). Immunogenicity of recombinant hepatitis B vaccine in dialysis patients. Journal of Hepatology, 3(2), 190–195.
Kidney Disease Improving Global Outcomes (KDIGO). (2017). KDIGO guidelines. Retrieved from: http://kdigo.org/guidelines
Kidney Health Australia. (2020). Chronic kidney disease (CKD) management in primary care. 4th edn. Melbourne, Vic: Kidney Health Australia.
Kosmadakis, G., Albaret, J., Correia, E. D. C., Somda, F., & Aguilera, D. (2018). Vaccination practices in dialysis patients: A narrative review. Seminars in Dialysis, 31(5), 507–518. https://doi.org/10.1111/sdi.12709
Levey, A., Coresh, J., & Greene, Y. (2006). Using standardised serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Annals of Internal Medicine, 145(4), 247–54.
Levey, A., Stevens, L., & Schmiel, C. (2009). A new equation to estimate glomerular filtration rate. Annals of Internal Medicine, 150(9), 604–612.
Lv, J. C., & Zhang, L.X. (2019). Prevalence and disease burden of chronic kidney disease. Advances in Experimental Medicine and Biology, 1165, 3–15.
Ma, B. M., Yap, D., Yip, T., Hung, I., Tang, S., & Chan, T. M. (2021). Vaccination in patients with chronic kidney disease: Review of current recommendations and recent advances. Nephrology, 26(1), 5–11. https://doi.org/10.1111/nep.13741
Malaki, M. (2017). Factors affecting on hepatitis B seroprotection in hemodialysis patients. Saudi Journal of Kidney Diseases and Transplantation, 28, 672–4. https://doi.org/10.4103/1319-2442.206472.
McMahon, B. (2014). Chronic hepatitis B virus infection. The Medical Clinics of North America, 98(1), 39–54.
Mendy, M., Peterson, I., Hossin, S., Peto, T., Jobarteh, M. L., Jeng-Barry, A., ... Whittle, H. (2013). Observational study of vaccine efficacy 24 years after the start of hepatitis B vaccination in two Gambian villages: No need for a booster dose. PloS One, 8(3), e58029. https://doi.org/10.1371/journal.pone.0058029
Mulley, W., Le, S., & Ives, K. (2017). Primary seroresponses to double-dose compared with standard-dose hepatitis B vaccination in patients with chronic kidney disease: A systematic review and meta-analysis, Nephrology Dialysis Transplantation, 32 (1); 136–143, https://doi.org/10.1093/ndt/gfv443
Mysore, P., Khinkar, R. M., McLaughlin, D., Desai, S., McMahon, G. M., Ulbricht, C., & Mendu, M. L. (2021). Improving hepatitis B vaccination rates for advanced chronic kidney disease patients: A quality improvement initiative. Clinical and Experimental Nephrology, 25(5), 501–508. https://doi.org/10.1007/s10157-020-02013-4
Nadeem, M., Shah, S. A. A., Arshad, N., Arshad, N., Riaz, F., Kian, R. S., Quddus, M. A. (2021). Vaccination against hepatitis B virus in hemodialysis patients: Trends in dialysis centres of Northern Pakistan. F1000Research, 10, 55. https://doi.org/10.1268/f1000research.28045.2
National Kidney Foundation. (2013). NKF KDOQI clinical practice guidelines. Retrieved from: http://www.kidney.org/professionals/guidelines
Nelson, N. P., Easterbrook, P. J., & McMahon, B. J. (2016). Epidemiology of Hepatitis B virus infection and impact of vaccination on disease. Clinics in Liver Disease, 20(4), 607–628. https://doi.org/10.1016/j.cld.2016.06.006
Ni, Y. H., Chang, M. H., Wu, J. F., Hsu, H. Y., Chen, H. L., & Chen, D. S. (2012). Minimization of hepatitis B infection by a 25-year universal vaccination program. Journal of Hepatology, 57(4), 730–735. https://doi.org/10.1016/j.jhep.2012.05.021
Phadke, G., & Khanna, R. (2011). Renal replacement therapies. Missouri Medicine, 108(1), 45–49.
Pin, M., Compte, M. T., Angelet, P., Gállego, C., Gutiérrez, C., & Martinez Vea, A. (2009). Long-term evaluation of immune response to hepatitis B vaccine in 136 patients undergoing hemodialysis. Nefrologia, 29(5), 415–420.
Poovorawan, Y., Chongsrisawat, V., Theamboonlers, A., Leroux-Roels, G., Crasta, P. D., & Hardt, K. (2012). Persistence and immune memory to hepatitis B vaccine 20 years after primary vaccination of Thai infants, born to HBsAg and HBeAg positive mothers. Human Vaccines & Immunotherapeutics, 8(7), 896–904. https://doi.org/10.4161/hv.19989
Ray, S., Samuel, T., Hawker, J., & Smith, S. (2002). Hepatitis B immunisation in renal units in the United Kingdom: Questionnaire study. BMJ, 324(7342), 877–8. https://doi.org/10.1136/bmj.324.7342.877.
Schweitzer, A., Horn, J., Mikolajczyk, R., Krause, G., & Ott, J. (2015). Estimations of worldwide prevalence of chronic hepatitis B virus infection: A systemic review of data published between 1965 and 2013. Lancet, 386(10003), 1546–1555. https://doi.org/10.1016/S0140-6736(15)61412-X
Somi, M., & Hajipour, B. (2012). Improving Hepatitis B vaccine efficacy in end-stage renal diseases patients and role of adjuvants. International Scholarly Research Notices, 2012, 960413. https://doi.org/10.5402/2012/960413
Shouval, D., Roggendorf, H., & Roggendorf, M. (2015). Enhanced immune response to hepatitis B vaccination through immunization with a Pre-S1/Pre-S2/S vaccine. Medical Microbiology and Immunology, 204(1), 57–68.
Syed-Ahmed, M., & Narayanan, M. (2019). Immune dysfunction and risk of infection in chronic kidney disease. Advances in Chronic Kidney Disease, 26(1), 8–15. https://doi.org/10.1053/j.ackd.2019.01.004
Udomkarnjananun, S., Takkavatakarn, K., Praditpornsilpa, K., Nader, C., Eiam-Ong, S., Jaber, B. L., & Susantitaphong, P. (2020). Hepatitis B virus vaccine immune response and mortality in dialysis patients: A meta-analysis. Journal of Nephrology, 33(2), 343–354. https://doi.org/10.1007/s40620-019-00668-1
Usherwood, T., & Lee., V (2021). Advances in chronic kidney disease pathophysiology and management. Australian Journal of GP Practice, 50(4).
Veronese, P., Dodi, I., Esposito, S., & Indolfi, G. (2021). Prevention of vertical transmission of hepatitis B virus infection. World Journal of Gastroenterology, 27(26), 4182–4193. https://doi.org/10.3748/wjg.v27.i26.4182
Victorian Renal Clinical Network (2017). Hepatitis B infection control in haemodialysis centres. Retrieved from: https://www2.health.vic.gov.au/about/publications/policiesandguidelines/hepatitis-b-infection-control-in-haemodialysis-centres
World Health Organization (WHO). (2021). Hepatitis B. Retrieved from: http://www.who.int/news-room/fact-sheets/detail/hepatitis-b
Yousaf, F., Gandham, S., Galler, M., Spinowitz, B., Charytan, C. (2015). Systematic review of the efficiency and safety of intradermal versus intramuscular hepatitis B vaccination in end-stage renal disease population unresponsive to primary vaccination series. Renal Failure, 37(7), 1080–8. Retrieved from: https://pubmed.ncbi.nim.nih.gov/26258528
