Volume 5 Issue 2
A new approach for early recognition of peripheral intravenous (PIV) infiltration: a pilot appraisal of a sensor technology in a neonatal population
Mr Matheus van Rens, Dr Kevin Hugill and Ms Airene Velez Francia
Keywords neonate, infiltration/extravasation, infusion therapy, vascular access technology
For referencing van Rens M et al. A new approach for early recognition of peripheral intravenous (PIV) infiltration: a pilot appraisal of a sensor technology in a neonatal population. Vascular Access 2019; 5(2):38-41.
DOI https://doi.org/10.33235/va.5.2.38-41
Abstract
Due to their unique developmental characteristics, preterm infants are more susceptible than most to iatrogenic harm arising from vascular cannulation and infusate infiltration or extravasation. It is therefore widely accepted that healthcare staff should take measures to prevent, detect, promptly treat, and mitigate these risks. Internationally, most neonatal units have implemented bundles of measures to reduce and manage risks associated with vascular access. One key element of these ‘care bundles’ is directed towards early detection of infiltration/extravasation events, often using a variety of visual assessment tools. The aim of this pilot product evaluation was to explore the feasibility of using a particular novel optical sensor-based infiltration detection technology (ivWatch®) in a neonatal unit.
The device was used on 15 preterm infants receiving short-term vesicant infusions while awaiting placement of central lines. Infiltration notifications were issued by the technology for 14 out of the 15 infiltrations confirmed by visual inspection. This corresponded to a sensitivity of 93.3% and, importantly, in all cases was detected earlier than was detected by staff using an hourly documented visual inspection tool. This pilot found that the technology was easy to use and apply, with only minor additions to existing intravenous (IV) access practice. Continuous IV site monitoring using optical sensor technology offers the potential to detect infiltration and extravasation events earlier than when using intermittent observational tools alone. Further study is required on larger neonatal study populations with a broader range of gestational ages, weights and IV site locations.
INTRODUCTION
It is widely known that in neonates short peripheral intravenous (PIV) catheters often develop complications before therapy is complete1,2. Many infusates have the potential to cause severe harm if they leak into the surrounding tissue. The inadvertent administration of a non-vesicant intravenous (IV) fluid is called infiltration; when it is a vesicant IV fluid it is called extravasation3. Both infiltration and extravasation can have serious consequences2,4. For the purpose of clarity in this article, we use the word infiltration for both situations.
The aetiology of PIV complication is complex, involving multiple patient and contextual factors5. Due to their unique anatomical and developmental state, preterm infants are at high risk of infiltration occurring and of harm developing afterwards2,4-6. Reported incidences of infiltration in neonatal patients range from 16–78%2,4,6. Infusion-related complications, such as pain, sloughing and blistering of the skin, permanent nerve damage, tissue necrosis and scarring, are widely reported in the literature1,2,4-6.
CURRENT PRACTICE
In 2016, in response to the challenges faced in reducing the incidence and potential harm related to PIV use, evidence-based care bundles and further preventative measures were developed and implemented in our unit by unit staff. This included new guidance on undertaking vascular assessment before cannulation utilising technologies such as the VeinViewer® [Christy Medical Corporation], appropriate site and catheter selection, use of a securement device, use of filter technologies, in-line pressure monitoring, infusate risk assessment, and vigilant hourly observation of the insertion site and surrounding tissue using the Infusion Nursing Society recommendation7 of “Touch, look and compare”. To reflect infiltration injury in a more objective way, our institution also transitioned from a grading score to a swelling measurement system as per the Cincinnati Children’s Hospital coding system8. In addition, a number of nurses – equating to four per 8-hour shift – received specialised training to develop expertise in vascular access. These nurses provide a 24-hour peripheral vascular access and support team across the unit; PIV insertions are performed only by the nurses from this team.
During the implementation of these PIV care bundles in our unit, an extensive database for PIV-related data was also established. This database substantiates a consistent overall rate of 40–45% of PIVs that infiltrate regardless of patient weight, gestation, cannula gauge, site, dwell time or infusate. Such a high infiltration rate puts a large number of patients at risk of injury, particularly if the PIV failure is not recognised in a timely manner.
In summary, in neonatal populations, short PIV failure rates are high, with relatively short dwell times and high incidences of infiltration. This situation poses significant challenges for healthcare practitioners in ensuring safe and effective vascular access. Despite our interventions and preliminary internal data suggesting decreases in the incidence and severity grading of PIV infiltrations, we felt more could be achieved. Consequently, we engaged with ivWatch, LLC to conduct a real-world appraisal of the ivWatch device’s ability to detect early-stage infiltrations in a preterm population. The pilot product evaluation described in this short report adds to our evolving knowledge about the effectiveness of technological interventions to reduce PIV infiltration-related harm by more timely recognition, and thus improve patient safety.
PRODUCT PILOT EVALUATION: IV WATCH
As a first-of-its-kind technology approved by the US Food and Drug Administration (US FDA) to monitor for IV infiltration, the ivWatch Model 400 is manufactured by the company ivWatch, LLC. The intent of this technology is to provide continuous non-invasive surveillance of PIV sites for early evidence of infiltration, thus ensuring more timely remedy9.
The device uses a near-infrared (NIR) light sensor located near to the PIV catheter tip to monitor for changes in the optical properties of a patient’s subcutaneous tissues. The amount of light detected by the sensor depends primarily on the scattering and absorption properties of the monitored tissue. Scattering occurs when a light particle is redirected due to an interaction with some structure such as fluid in the tissue. Absorption occurs when the light particle is absorbed by a structure in the tissue such as haemoglobin9.
During an emerging infiltration event, fluid accumulates in subcutaneous tissue, causing a change in the light signal. The ivWatch Model 400 detects these changes and uses a software algorithm that is calibrated to issue a series of user alerts in response to changes in the optical properties of the monitored site. First, the device issues a ‘yellow check IV’ notification which indicates the possibility of an infiltration, advising nurses to check the site and confirm patency. If there is no resolution to the situation and the signal deteriorates further, then the device issues a ‘red check IV’ notification, indicating that infiltration is highly probable. The signal processing algorithm used to issue the alerts for early stage infiltration events has been developed and calibrated by the manufacturers using data from healthy adult volunteers and hospital in-patient children.
The sensitivity of the device to detect purposefully created infiltration – using slow or rapid infusions of 10mL of isotonic saline – in short PIVs placed in the upper limbs of healthy adult volunteers, and its performance across different people in terms of gender, age, body mass and skin pigmentation, were assessed in a small laboratory study (n=70)10. This study reported that 139 of the 140 visually confirmed infiltrations were accompanied by a ‘yellow check IV’ alert, suggesting a sensitivity of 99.3% (95% CI, 95.5–99.96%). A further 135 events also generated a ‘red check IV alert’, suggesting a sensitivity of 96.4% (95% CI, 91.4–98.7%)10.
A related clinical study reported by Doellmun and Rineair10,11 involving 156 paediatric patients receiving medically indicated continuous IV therapy sought to determine how many nurse observationally confirmed infiltrations were independently detected by the device, as well as the timing of these. The rationale was to compare the usual practice of visual confirmation of IV site integrity and device performance.
Patients enrolled in the study were aged between two weeks and 17 years, and all weighed more than 2.5kg. A wider range of PIV sites, including the leg and foot, were included in this study, as expected in this patient group. In this study, clinicians were blinded to the device’s alarm status until after they had determined the presence of infiltration (overall infiltration rate 14.7%). Interestingly, the device detected 16 of the 20 (80% sensitivity) infiltrations documented in the hand or forearm. Overall, the device was shown to have issued a yellow alert on average at 32.2 hours (5% CI, 17.3–47.3 hours) and a red alert averaged at 29.8 hours (95% CI, 14.8–44.8 hours) before staff documented a confirmed infiltration event.
A follow-up investigation11 (n= 57, 20 of whom were under 2 years of age) was carried out with the alarms enabled. In this group, the device detected 12 of the 15 clinician-confirmed events, that is it confirmed the 80% sensitivity. However, the ‘red check IV alarm’ was activated some 2.1 median hours before clinician confirmation, suggesting earlier identification of the problem. The authors of these studies10,11 therefore concluded that the device had the potential to provide clinicians with earlier notification of emerging infiltration in both paediatric and adult patients.
METHODS
For this pilot, the study site was provided with four ivWatch Model 400 monitoring systems and a quantity of consumables for the purposes of product appraisal in the site setting and patient population. An ivWatch clinical representative conducted short, ‘at-the-bedside’ training alongside previously trained hospital staff during the evaluation period which provided the staff with theory about the device operations and best use practices.
The purpose of this pilot product appraisal was to gain experience in using the device and to examine its utility in providing earlier notification of PIV infiltrations in a neonatal patient population when compared to existing methods that relied on staff observation alone. As this was a product appraisal it was deemed by local practice not to be research but was approved within the prevailing governance structures of a clinical audit. Device sensitivity to detect the presence of an infiltration was calculated using standard statistical sensitivity tests.
The study site was a large 112-bed tertiary level neonatal intensive care unit located in the Women’s Wellness and Research Center of Hamad Medical Corporation (Doha, Qatar). This unit is the largest in the country and has an average of approximately 300 admissions and 800 PIVs placed per month.
The study population consisted of preterm infants who were receiving at least one vesicant infusion though a PIV, mostly while awaiting the placement of a more suitable central line. All IVs monitored during the appraisal had continuous parenteral nutrition (PN) infusions and at least one additional medication such as antibiotics and/or caffeine. We chose to select our highest risk group for PIV infusion-related harm to assess the device’s performance as it was felt that this group of patients had the most to gain from earlier detection of infiltration.
RESULTS
The evaluation was conducted from 9–12 December 2018 and from 7–16 February 2019 as determined by the availability of sensor consumables. The total monitoring time was 673.01 hours (equivalent to 28.04 days), with an average monitoring time of 29.26 hours per PIV (Figure 1).
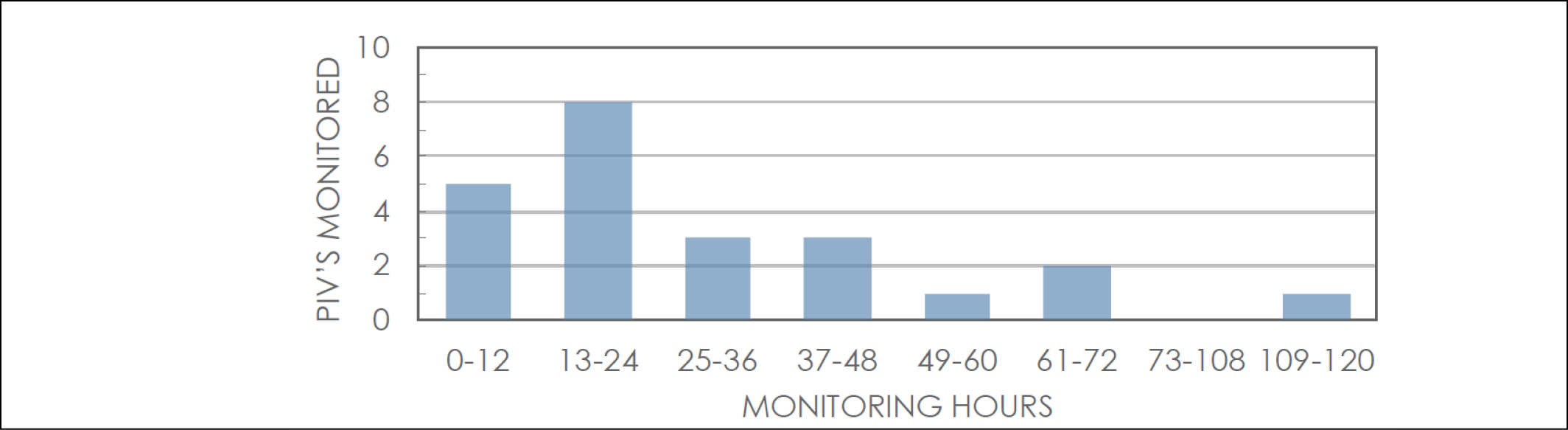
Figure 1. Duration of monitoring hours and number of PIVs monitored in that timeframe.
During this period, a total of 15 preterm infants (11 male and four female) and 23 PIVs were continuously monitored by four ivWatch Model 400 monitoring systems. Gestational age at birth ranged from 26 weeks plus 4 days to 36 weeks plus 6 days. Infants’ ages at the time of PIV insertion ranged from 1–42 days, and the current weight was recorded as between 920–2940g. All the 23 monitored PIVs used a 26-gauge BD Neoflon™ [Becton, Dickinson and Company] cannula; 17 were inserted in the hand, five in the foot and one in the lower arm.
During the monitoring timeframe, 15 infiltrations were detected among the PIVs monitored, suggesting an infiltration rate of 65.2%. This is higher than our unit baseline average figure of 40–45% for all PIVs. However, despite all the infusates being vesicant, all infiltrations were graded as minor. Infiltration notifications were issued for 14 out of the 15 infiltrations, corresponding to 93.3% sensitivity and, importantly, all before the clinician identified the event.
Yellow notifications that were considered on inspection to be related to an infiltration event occurred once every 4.67 days. Red notifications that were related to an infiltration event occurred once every 4.01 days, a figure similar to that reported in the literature10,11.
DISCUSSION
In this pilot study, the device detected 14 of the 15 infiltrations with 93.3% sensitivity which is higher than that reported in other paediatric populations11 but which broadly aligned with figures from adult studies9,10. It is interesting to note that the one infiltration confirmed by clinical staff on hourly inspection did not trigger an alert, suggesting that the software algorithm might need further refinement for use in neonatal populations. False positive alerts – for example alarm but no evidence of infiltration – were seldom reported; it is unclear what factors precipitated these alerts and further study with a larger patient cohort is required to elucidate these causes. In our opinion, notifications not associated with an infiltration event were minimal and did not contribute to alarm fatigue.
This pilot appraisal has a number of caveats and limitations. With a nursing staff in excess of 400 full-time equivalents, it was not feasible during the equipment loan period to introduce the device to every nurse. Instead, we focused on a small cohort of staff, largely determined by previous training or patient allocation – for example to infants receiving high-risk infusions. Because this is a relatively novel device in neonatal care, for more optimal implementation additional advanced staff training would be required.
A limitation on the data collected during the evaluation timeframe was also a feature of this pilot. The sample was, by necessity, small, given the limited number of sensors available for patient use at the time. Furthermore, while we purposefully chose to include only the most high risk PIV infusions to determine the product’s utility, this might have affected our findings. In general, in our unit, PIV vascular access is more commonly used for non-vesicant clear fluids or antibiotics only, and further evaluation in this larger but lower risk group of patients is required.
CONCLUSION
Detecting PIV infiltration events in a timely manner enables healthcare staff to take appropriate actions, ensuring patent safety and reducing the potential for harm. The ivWatch Model 400 is a device that is simple to use and offers an effective adjunct to existing intermittent observational visual tools such as “Touch, look and compare”7, and offers a potential solution to some of the challenges faced with early detection of infiltration in a neonatal critical care setting. Larger multicentre studies are planned.
DISCLOSURE
The authors have not received an honorarium nor benefits for this work. The manufacturer, ivWatch, LLC, loaned the patient monitors and provided training and the single-use sensors at no cost.
Author(s)
*Mr Matheus van Rens1; Dr Kevin Hugill2; Ms Airene Velez Francia1
1NICU, Women’s Wellness and Research Center, Hamad Medical Corporation, PO Box 3050, Doha, Qatar
2Nursing and Midwifery, Education and Research, Hamad Medical Corporation, Qatar
*Corresponding author
Mr Matheus van Rens, RN, MaANP, Director of Nursing and Vascular Access, NICU, Women’s Wellness and Research Center,
Hamad Medical Corporation, PO Box 3050, Doha, Qatar
Email mrens@hamad.qa
References
- Helm RE, Klausner JD, Klemperer JD, Flint M & Huang E. Accepted but unacceptable: peripheral IV catheter failure. J Inf Nurs 2015;38(3):189–203.
- Unbeck M, Förberg U, Ygge B-M, Ehrenberg A, Petzold M, Johansson E. Peripheral venous catheter related complications are common among paediatric and neonatal patients. Acta Paediatr 2015;104(6):566–74
- Hadaway L. Infiltration and extravasation. Am J Nurs 2007;107(8):64–72.
- Legemaat, M, Carr PJ, van Rens RM, VanDijk M, Poslawsky IE, van den Hoogen A. Peripheral intravenous cannulation: complication rates in the neonatal population: a multicenter observational study. J Vasc Access 2016;17(4):360–65.
- Hugill K. Vascular access in neonatal care settings: selecting the appropriate device. Brit J Nurs 2016;25(3):171–76.
- Atay S, Sen S, Cukurlu D. Incidence of infiltration/extravasation in newborns using peripheral venous catheter and affecting factors. Rev Esc Enferm USP [Internet] 2018;52:e03360. doi:10.1590/S1980–220X2017040103360
- Gorski LA, Hallock D, Kuehn SC, Morris P, Russell JM, Skala LC. Recommendations for frequency of assessment of the short peripheral catheter site. J Infus Nurs 2012;35(5):290–92.
- Tofani B, Rineair S, Gosdin CH, Pilcher PM, McGee S, Varadarajan KR, Schoettker PJ. Quality improvement project to reduce infiltration and extravasation events in a pediatric hospital. J Pediatri Nurs 2012;27(6):682–89.
- ivWatch®. ivWatch Model 400 overview, how it works; 2019 [cited 2019 June 19]. Available from: www.ivWatch.com/how-it-works/
- ivWatch®. Breakthrough in IV safety. Whitepaper; 2019 [cited 2019 June 19]. Available from: https://www.ivwatch.com/wp-content/uploads/2018/05/Model-400-Whitepaper-Rev-4-718.pdf
- Doellmun D, Rineair S. The use of optical detection for continuous monitoring of pediatric IV sites. JAVA 2019;24(2):44–47.
