Volume 7 Issue 1
A retrospective clinical audit investigating the prevalence of patients experiencing difficult peripheral intravenous cannulation afterhours in a tertiary referral hospital in Australia
Nathan Peters, Ranawaka Sandali De Alwis, Joanna Yu, Daniel Waters, and Christine Wood
Keywords catheterisation, peripheral, education, vascular access devices, shift work schedule, difficult intravenous access
For referencing Peters N et al. A retrospective clinical audit investigating the prevalence of patients experiencing difficult peripheral intravenous cannulation afterhours in a tertiary referral hospital in Australia. Vascular Access 2021; 7(1):6-11.
DOI https://doi.org/10.33235/va.7.1.6-11
Abstract
Background Difficult peripheral intravenous cannulation (DPIVC) outside of normal working hours could be particularly problematic due the relatively limited resources available to support afterhours staff. Our objective was to determine the prevalence and impact DPIVC had on patients afterhours.
Methods A retrospective clinical audit of 561 afterhours requests to junior medical staff between 1 September to 30 November 2019 at the Royal Brisbane and Women’s Hospital, Australia. Junior medical staff relied solely on landmark/palpation insertion technique when tending to adult medical and surgical wards afterhours. DPIVC encounters were determined by analysing patients’ electronic medical records and applying predetermined criteria reflecting difficulty with insertion.
Results Twenty-two percent (n=121) of insertions were identified as difficult. This resulted in a median (IQR) delay in medical therapy of 225 (119–473) minutes. Forty-eight percent (n=58) of DPIVC cases required escalation to another healthcare professional for assistance. Where a number of attempts were documented in the patient’s medical record, 50% (n=26) required three or more attempts at peripheral intravenous cannulation.
Conclusion DPIVC is a problem commonly encountered by junior medical staff afterhours. The current system for managing these patients, including escalation to staff who utilise the same insertion technique, results in a high likelihood of failed insertion attempts and clinical inefficiencies such as multiple staff attending the bedside and delays in intravenous medical therapies. These results support the need for additional training and resources being provided to all afterhours staff with the aim of reducing the burden DPIVC creates for patients, staff and the institution.
Background
Intravenous (IV) access is a necessity to facilitate the delivery of IV medications and fluids for up to 70% of patients who are admitted to hospital in Australia.1 Establishment of IV access is a procedure that is required both day and night as new therapy is commenced, or when an existing catheter becomes non-functional and needs replacement.
Difficult peripheral intravenous cannulation (DPIVC) is a well-recognised problem and is commonly identified after multiple attempts have been made at insertion.2 First-attempt failure at peripheral intravenous catheter (PIVC) insertion has been reported as 12–39% in adults.3–5 However, in highly complex hospitalised patients, the reported prevalence of failed first attempts at PIVC insertion was as high as 59%.6 Difficult peripheral intravenous cannulation is a significant problem for patients who can be subjected to multiple failed cannulation attempts, causing distress and delaying their medical treatment.2 Multiple unsuccessful attempts have been associated with complications such as haematoma, arterial puncture, and nerve damage.7 When difficulty was encountered in PIVC insertion in an urban Australian emergency department, delays of 3 or more hours in patients receiving prescribed medical therapy were noted.8 Delays in medical therapy are not only problematic for patients but also for healthcare workers and institutions as delays in the delivery of required medical therapy inevitably consume more costly resources, including staff time.
Difficult peripheral intravenous cannulation could be expected to be particularly problematic afterhours due to the reduced availability of staff possessing the skill, expertise, and equipment required to manage these scenarios. Junior medical staff are primarily tasked with performing PIVC insertion afterhours in our institution and they are therefore more likely to encounter difficulty. However, the scale and significance of this problem is currently unknown or unreported.
Objectives
The primary objective of this clinical audit was to determine the prevalence of hospitalised patients experiencing DPIVC when managed by junior medical staff afterhours.
The secondary objectives were to quantify the impacts an episode of DPIVC had on patients afterhours. These included the following:
- the number of attempts made on patients experiencing an episode of DPIVC;
- time delays in administration of IV medical therapy when DPIVC was encountered; and,
- patient sentiments as recorded in medical notes related to the DPIVC episode.
Methods
Study design and setting
This study was a retrospective clinical audit analysing all new PIVC insertion requests to afterhours junior medical staff between 1 September to 30 November in 2019. This study was conducted at the Royal Brisbane and Women’s Hospital (RBWH), the largest tertiary referral hospital in Queensland, Australia, with 969 beds. Data was collected and analysed in April 2020. This study was prospectively given a waiver of full ethical review by the RBWH Human Research Ethics Committee (HREC) (Ref No: LNR/2020/QRBW/63558) and follows the STROBE guidelines for reporting of observational studies in healthcare.9
Population
Adult (>18 years of age) inpatients from medical and surgical wards who had an electronically recorded request submitted for the placement of a new PIVC afterhours were included in this study. Requests from these general and subspecialty medical and surgical wards are managed by junior medical staff afterhours. Afterhours was defined as Monday to Friday 2130–0800 and Saturday and Sunday 2030–0800 as this corresponded to junior medical staff shift times.
The following requests were excluded:
- multiple requests for PIVC insertion for the same patient were classified as one request only, with the initial request being considered the primary request;
- afterhours requests for PIVC insertion where the patient already had a functional PIVC and the request was for routine replacement, ie, PIVC had been in place >72 hours (these are not actioned by junior medical staff overnight as they are a lower priority afterhours and are deferred to normal working hours for management);
- requests for new PIVC insertion that were subsequently assessed by the junior medical staff member who decided that a PIVC was not required and no attempts were made, ie, oral therapy was appropriate;
- requests from areas that junior medical staff were not responsible for managing afterhours that included obstetric and gynaecology wards and critical care areas – anaesthesia, intensive care, emergency medicine; and
- where no electronic medical record was available for the time of request.
Junior medical staff were defined as prevocational doctors usually in the first 1–3 years following completion of their medical degree. All of these junior medical staff rely on the traditional landmark/palpation technique for PIVC insertion. No additional vein locating technologies (ie, ultrasounds) were available to them afterhours.
Variables
The research team prospectively devised a screening tool to identify patients who had a likely episode of DPIVC afterhours through retrospective review of written documentation. This DPIVC identification tool was comprised of four criteria, with DPIVC defined as meeting at least one of these specific criteria. The components that formed the DPIVC criteria have similarly been used by other researchers to reflect episodes of cannulation where the inserter had difficulty, such as two or more failed attempts, the need for escalation for assistance, as well as history of, or anticipated, difficulty identified by nursing staff.4,10–12 The DPIVC criteria can be seen in Table 1.
Table 1. Criteria used in determining an episode of DPIVC insertion
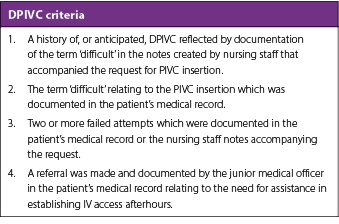
The number of attempts, if documented, was calculated by summation of all documented attempts related to that episode of cannulation by the healthcare staff managing the PIVC insertion request.
The delay in administration of IV medical therapy was calculated by measuring the difference between the date and time the IV therapy was prescribed to commence, and the date and time the IV therapy was documented actually to have been administered. These data were collected from medication and fluid therapy charts from the patients’ electronic medical record.
Data source
Outside of clinical emergencies, at the RBWH the accepted referral pathway to afterhours junior medical staff, across medical and surgical wards, for new PIVC insertions is through the electronic task management system Patient Flow Manager® (PFM). When ward nursing staff logged an electronic request using this system, it included the patient’s medical record number, location (ward), time of request, indication and general notes relating to the request. All electronically recorded requests for the insertion of a new PIVC were included and analysed in addition to patients’ electronic medical records 6 hours prior to, and 6 hours after the PFM request time.
Data analysis
All electronic requests that fell within the audit period were downloaded into a Microsoft Excel spreadsheet and screened against the inclusion and exclusion criteria. Patients who met one or more DPIVC criteria then underwent a second, more in-depth analysis of their electronic medical records looking for additional data for assessment of secondary objectives. These data were collated and analysed using the REDCap® electronic research database analysis system. During this phase, data collected included age, gender, ward, IV therapy, time elapsed between prescribed and administered, number of attempts made at PIVC, need for assistance from another healthcare worker, time of day that IV access was established, and documentation relating to patient experiences.
Bias
Junior medical staff are not vascular access experts but all were deemed competent in landmark/palpation PIVC insertion. All junior medical staff at the RBWH undergo supervised training and insertion by a vascular access expert upon introduction to the hospital and must be recognised as competent before commencing unsupervised practice. The majority of junior medical staff rostered afterhours are predominantly 2 years postgraduate or greater. This means that the majority of the afterhours junior medical staff are experienced in landmark/palpation cannulation technique and are familiar with local hospital equipment and procedures.
The period of September, October, and November was selected to minimise any bias created by the presence of junior medical staff who were new to the hospital. The intake of new junior medical staff is based on the calendar year and occurs predominantly in January. This means that there were few, if any, junior medical staff included in this study who were new to PIVC insertion or the institution.
Study size and statistical analysis
A study period of 3 months was chosen to create a sample size of 500 or greater PIVC requests. As this was a retrospective audit, no sample size calculation was performed and no inferential statistical analysis was planned.
Results
There were 691 requests identified that fell within the afterhours timeframe during the 3-month study period, equating to 561 eligible requests for new IV access after the exclusion criteria were applied (Table 2). Twenty-two percent (n=121) of patients met at least one of the DPIVC criteria indicating an episode of difficult PIVC insertion (Table 3). It was common for patients to meet two or more of these criteria and this occurred in 60% (n=73) of patients. The number of DPIVC criteria met per patient can be seen in Figure 1. The most frequently noted criteria indicating difficulty was “documentation of difficulty at time of insertion” occurring in 69% (n=83) of patients experiencing difficult insertion (Table 4). Table 5 describes the patient characteristics, indication, and type of IV therapy required for all patients who experienced DPIVC.
Table 2. Excluded requests
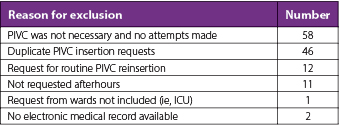
Table 3. Proportion of eligible patients with one or more documented criteria suggestive of DPIVC detected during each 1-month period
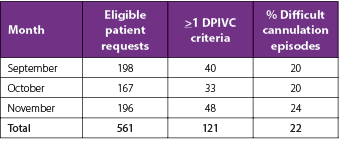
Table 4. Number of patients meeting each DPIVC criterion per month
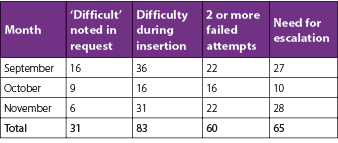
Table 5. Characteristics of patients experiencing DPIVC insertion
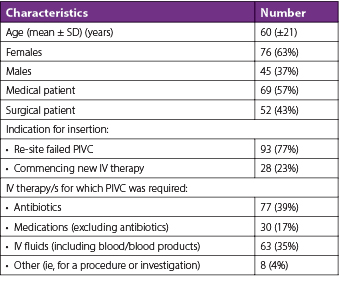
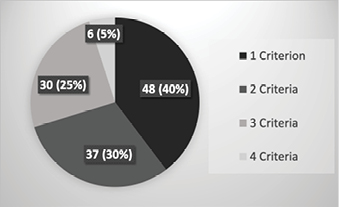
Figure 1. Number of DPIVC criteria met per patient
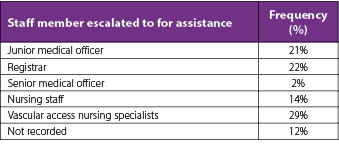
Of the 115 DPIVC patients where the data available allowed for calculation of difference between the time IV therapy was prescribed and administered, the median (IQR) delay in therapy was 225 (119–473) minutes. Escalation to another staff member for assistance was documented to occur in 48% (n=58) of patients with DPIVC. The health professional called upon to assist with PIVC placement in these difficult cases afterhours could be another more experienced medical officer, a senior afterhours nurse, or a senior medical officer. Vascular access specialist nurses were only available during normal working hours and were called upon to manage some requests that could not be successfully managed afterhours. Table 6 outlines which staff member was contacted for assistance and was able to successfully insert the PIVC. Notably, only 57% (n=68) of DPIVC patients had a PIVC successfully inserted within the afterhours timeframe. The remainder of these requests were handed over to be managed by daytime health professionals.
Table 6. Health professional attending to assist the junior medical officer with difficult PIVC insertion (n=58)
In 52 DPIVC cases, a number of attempts made to secure IV access was documented in the patients’ medical records (Figure 2). Notably, 50% (n=26) required three or more attempts to establish a PIVC, while 15% (n=8) required five or more attempts.
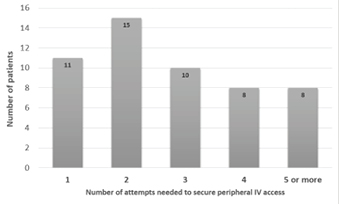
Figure 2. Number of documented attempts in patients experiencing DPIVC (n=52)
Of the 121 patients identified as having DPIVC, 18% (n=22) had their comments documented in their medical record reflecting their experience. Nine patients expressed physical discomfort and ten patients refused any further attempts. Two patients requested more experienced staff to attend to their request, and one requested the use of ultrasound-guidance or a peripherally inserted central catheter.
Discussion
Key results
We identified that 22% of PIVC insertion episodes performed by junior medical staff afterhours were considered difficult, indicating that DPIVC is a commonly encountered problem. At the RBWH the task of establishing IV access for patients on the medical and surgical wards is allocated to medical staff in more than 80% of cases.13 This system of medical staff-performed PIVC is commonly adopted throughout Australian hospital medical practice.
When difficulty was encountered by junior medical staff afterhours, 75% of patients had their medical therapy delayed by 2 or more hours and 79% of patients required two or more attempts to secure IV access. Within healthcare systems, DPIVC has previously been shown to result in delayed medical treatment, multiple failed attempts, and increased patient discomfort.4,14 Witting et al.4 reported that 22% of patients presenting to an urban emergency department had two failed attempts at PIVC insertion. Using our DPIVC identification tool, we were able to identify a similar prevalence to these other published rates. Interestingly, 76% of requests for IV access afterhours were in patients where a pre-existing IV access device had failed. This may have been a reflection of a higher failure rate of PIVCs in patients with DPIVC.
Documentation of the patients’ experience was uncommon in those identified as having an episode of DPIVC. This, however, is likely a reflection of inconsistencies and gaps in documentation rather than the absence of expressed negative emotions by patients when faced with multiple failed peripheral IV cannulation attempts. When patients’ experiences were documented in their medical record, it reflected fear and frustration expressed through their refusal of further attempts and complaints of physical discomfort.
Limitations
The main limitations of our study arose from the retrospective nature of our data collection and the highly variable nature of medical documentation by individual healthcare workers. Other researchers have noted that documentation relating to PIVC insertion is generally poor.15,16 Despite the limitation created by relying on existing documentation, we still were able to identify a significant number of patients experiencing DPIVC afterhours based on what was available.
The DPIVC identification tool used in this study is not a diagnostic tool, rather a method of identifying patients who experience difficulty at the time of insertion. Also, PIVCs inserted afterhours where an electronic request was not logged could not be identified and therefore would not have been included in this study. However, due to ease of use and the hospital-wide adoption of the electronic task management system, the number of these would be small in comparison to the electronically logged requests.
The definition of attempt was according to the healthcare staff attending the request and may not have been the same across all staff. The number of attempts made was defined as the documented number in the medical record and, given the retrospective nature of the study, the research team had no method of standardising or verifying this number.
Interpretation
A stepwise escalation policy following two failed attempts is adopted by our institution and has also been used by other researchers as an indicator of difficulty.4,11 Our results highlight the problematic nature of this system of escalation afterhours. If a patient with DPIVC is encountered by junior medical staff who is unsuccessful in securing IV access, then our hospital protocol requires them to escalate the management to a more senior or experienced clinician. Delays in IV therapy would be inevitable when waiting for other busy afterhours staff to attend to a DPIVC request at a time when the overall number of staff is reduced. This delay in accessing another staff member to assist with cases of DPIVC was also evidenced in our audit by the frequent need to delay the task until normal working hours when more resources and expertise were available.
Also problematic at our institution was that the healthcare worker to whom the task was escalated afterhours relied on the same landmark/palpation insertion technique that the junior medical staff had already encountered difficulty with. Without additional training or resources, escalation within the hospital is only effective if the problem stemmed from the lack of proficiency of the initial inserter using the traditional insertion technique. This escalation system is unable to overcome the problem where patient specific factors (ie, small or not palpable/visible veins) contribute to difficulty significantly more than inserter proficiency. It has been recommended that when no visible or palpable veins are present, or when PIVC insertion proves to be difficult, an ultrasound assisted technique should be considered early.17 The recommendation to use ultrasound in cases of DPIVC is supported by evidence demonstrating a reduction in the number of attempts needed as well as a reduction in the time to successful cannulation.18 Specifically, utilising ultrasound afterhours for patients with DPIVC has been reported to result in high first pass success rates and lower patient pain scores in a study by Sou et al.14
Our results support the need for development of a hospital-wide system where DPIVC patients afterhours are recognised early and more promptly attended to by staff who have more advanced skills and the resources needed to better manage them. The healthcare staff who are provided with this additional training in management of DPIVC could be medical, nursing, or a combination of both. When Nye et al. introduced a team of nursing staff trained in ultrasound-guided PIVC placement for difficult patients, they noted that most calls for assistance were afterhours and also that most patients underwent three or more attempts at cannulation prior to calling for assistance.8 Staff members trained in alternative approaches, such as ultrasound-guided insertion, should be a component of this afterhours DPIVC management system and there should be clear referral processes to these more skilled staff in place afterhours.
Generalisability
This study was undertaken in a large metropolitan tertiary referral hospital. The results reflect the afterhours staffing system in combination with hospital policies relating to the insertion of PIVCs. Our institution tasks competent junior medical staff to perform PIVC insertion afterhours across the medical and surgical wards and requires them to escalate to a more experienced health professional if they are unsuccessful. Ultrasound or other vein finding technologies are not available to assist medical or nursing staff for patients with DPIVC on medical and surgical wards afterhours.
Conclusion
Our retrospective clinical audit demonstrated that DPIVC is a commonly encountered problem by junior medical officers afterhours at our institution. The current system for management of these patients, including escalation to staff who utilise the same insertion technique, results in a high likelihood of failed insertion attempts and system-wide inefficiencies such as multiple staff attending the bedside leading to delays in patients receiving prescribed medical therapies.
Given the frequency and significance of DPIVC as a problem afterhours, better support should be provided to all afterhours staff who are likely to encounter patients with DPIVC. This may include providing additional training and resources to selected afterhours healthcare staff who are more accessible to junior medical staff when they encounter DPIVC. This intervention would be specially aimed at reducing the burden DPIVC creates for patients, staff and the institution, and would also warrant further investigation.
About the authors
Nathan Peters, BPharm MBBS FANZCA, is Staff Specialist Anaesthetist, Department of Anaesthesia and Perioperative Medicine at the Royal Brisbane and Women’s Hospital, in Brisbane QLD, Australia. Ranawaka Sandali De Alwis, Bmed/MD, Joannu Yu, MBBS, and Daniel Waters, MBBS, are Resident Medical Officers at the Royal Brisbane and Women’s Hospital in Brisbane QLD, Australia. Christine Woods, BNurs, is Research Manager in the Department of Anaesthesia and Perioperative Medicine at the Royal Brisbane and Women’s Hospital in Brisbane QLD, Australia.
Conflict of interest
The authorship team received no funding for this research.
Acknowledgments
The authors would like to acknowledge J Gonzel and H Reynolds for their assistance in data collection.
Author(s)
Nathan Peters*,1 Ranawaka Sandali De Alwis,2 Joanna Yu,2 Daniel Waters,2 and Christine Woods1
1 Department of Anaesthesia and Perioperative Medicine, Royal Brisbane and Women’s Hospital, Herston, QLD 4029, Australia
2 Royal Brisbane and Women’s Hospital, Brisbane QLD, Australia
*Corresponding author
Nathan Peters, Staff Specialist Anaethetist, Department of Anaesthesia and Perioperative Medicine, Level 4, Ned Hanlon
Building, Royal Brisbane and Women’s Hospital, Corner of Butterfield Street and Bowen Bridge Road, Herston, QLD 4029, Australia.
Email Nathan.Peters@health.qld.gov.au
References
- Hawthorn A, Kleidon T, Larsen E, Marsh N, Marshall A, Ray-Barruel G, et al. Peripheral intravenous catheters: a review of guidelines and research. Australian Commission on Safety and Quality in Health Care. 2019 [cited 2020 Sept 1]. Available from: https://wwwsafetyandquaity.gov.au/upblication-and-resources/resource-library/peripheral-intravenous-catheters-review-guidelines-and-research
- van Loon FH, van Hooff LW, de Boer HD, Koopman S, Buise MP, Korsten HH, et al. The Modified A-DIVA Scale as a predictive tool for prospective identification of adult patients at risk of a difficult intravenous access: a multicenter validation study. J Clin Med. 2019;8(2):144.
- Sabri A, Szalas J, Holmes KS, Labib L, Mussivand T. Failed attempts and improvement strategies in peripheral intravenous catheterization. Bio-Med Mater Eng. 2013;23:93–108.
- Witting MD. IV access difficulty: incidence and delays in an urban emergency department. J Emerg Med. 2012;42(4):483–7.
- Piredda M, Biagioli V, Barrella B, Carpisassi I, Ghinelli R, Giannarelli D, et al. Factors affecting difficult peripheral intravenous cannulation in adults: a prospective observational study. J Clin Nurs. 2017;26(7–8):1074–84.
- Armenteros-Yeguas V, Gárate-Echenique L, Tomás-López MA, Cristóbal-Domínguez E, Moreno-de Gusmão B, Miranda-Serrano E, et al. Prevalence of difficult venous access and associated risk factors in highly complex hospitalised patients. J Clin Nurs. 2017;26(23–24):4267–75.
- Aponte H, Acosta S, Rigamonti D, Sylvia B, Austin P, Samolitis T. The use of ultrasound for placement of intravenous catheters. AANA J. 2007;75(3):212–6.
- Nye M, Sweeny A, Watkins S, Ingold J, Sharwood P. Difficult vascular access in hospitalised patients: delays to treatment, cannulation attempts, and use of ultrasound. Vascular Access. 2020;6(1):5–9.
- Vandenbroucke JP, von Elm E, Altman DG, Gøtzsche PC, Mulrow CD, Pocock SJ, et al. Strengthening the reporting of observational studies in epidemiology (STROBE): explanation and elaboration. PLoS Med. 2007;4(10):e297.
- Piredda M, Fiorini J, Facchinetti G, Biagioli V, Marchetti A, Conti F, et al. Risk factors for a difficult intravenous access: a multicentre study comparing nurses’ beliefs to evidence. J Clin Nurs. 2019;28(19–20):3492–504.
- Bauman M, Braude D, Crandall C. Ultrasound-guidance vs standard technique in difficult vascular access patients by ED technicians. Am J Emerg Med. 2009;27(2):135–40.
- Ehrhardt BS, Givens KE, Lee RC. Making it stick: developing and testing the difficult intravenous access (DIVA) tool. AJN. 2018;118(7):56–62.
- Marsh N, Webster J, Larson E, Cooke M, Mihala G, Rickard CM. Observational study of peripheral intravenous catheter outcomes in adult hospitalized patients: a multivariable analysis of peripheral intravenous catheter failure. J Hosp Med. 2018;13(2):83–9.
- Sou V, McManus C, Mifflin N, Frost SA, Ale J, Alexandrou E. A clinical pathway for the management of difficult venous access. BMC Nurs. 2017;16:64.
- McGuire R. Assessing standards of vascular access device care. Br J Nurs. 2015;24(Sup8):S29-S35.
- Ray-Barruel G, Cooke M, Mitchell M, Chopra V, Rickard CM. Implementing the I-DECIDED clinical decision-making tool for peripheral intravenous catheter assessment and safe removal: protocol for an interrupted time-series study. BMJ Open. 2018;8(6):e021290. doi:10.1136/bmjopen-2017-021290
- Bodenham A, Babu S, Bennett J, Binks R, Fee P, Fox B, et al. Association of Anaesthetists of Great Britain and Ireland: Safe vascular access 2016. Anaesthesia. 2016;71(5):573–85.
- van Loon FHJ, Buise MP, Claassen JJF, Dierick-van Daele ATM, Bouwman ARA. Comparison of ultrasound guidance with palpation and direct visualisation for peripheral vein cannulation in adult patients: a systematic review and meta-analysis. Br J Anaesth. 2018;121(2):358–66.
