Volume 42 Number 4
Risk assessment for pressure injuries
Elizabeth A Ayello and Barbara A Delmore
Keywords pressure injury, risk factors, risk assessment scales
For referencing Ayello EA & Delmore BA. Risk assessment for pressure injuries. WCET® Journal 2022;42(4):31-37
DOI
https://doi.org/10.33235/wcet.42.4.31-37
Submitted 18 November 2022
Accepted 9 December 2022
Abstract
This manuscript highlights commonly used pressure injury (PI) risk assessment instruments (scales) and other considerations that the clinician should contemplate for use in everyday practice to determine if their patient is at risk for a PI.
Introduction
Each year in November, many professional organisations participate in the Stop Pressure Injuries/Ulcers Day. It provides an opportunity to raise awareness about pressure injuries (PIs) to the general public as well as other healthcare professionals. Preventing PIs is an important part of a clinician’s everyday practice. The intent of this article is to provide a succinct summary of some of the commonly used PI risk assessment instruments (scales) as well as other patient characteristics to consider as part of a comprehensive risk assessment process.
Risk Assessment Overview
The purpose of risk assessment is to identify if a person is at risk for a PI and, if so, implement an individualised prevention plan especially considering modifiable and non-modifiable risk factors1. The 2019 International Guideline with implementation recommendations1 provides assistance for clinicians for best practices for individuals at risk for a PI regardless of the care setting. Risk assessment is one of the key components to consider when preventing PIs. It is a systematic process that at minimum includes examination of the person’s skin for any changes, awareness of any devices, including medical and other objects that can cause pressure, assessment of individual patient characteristics that are known to be risk factors, and assessment using a validated/reliable risk assessment instrument (scale) and the clinician’s clinical judgement.
Risk Assessment Instruments
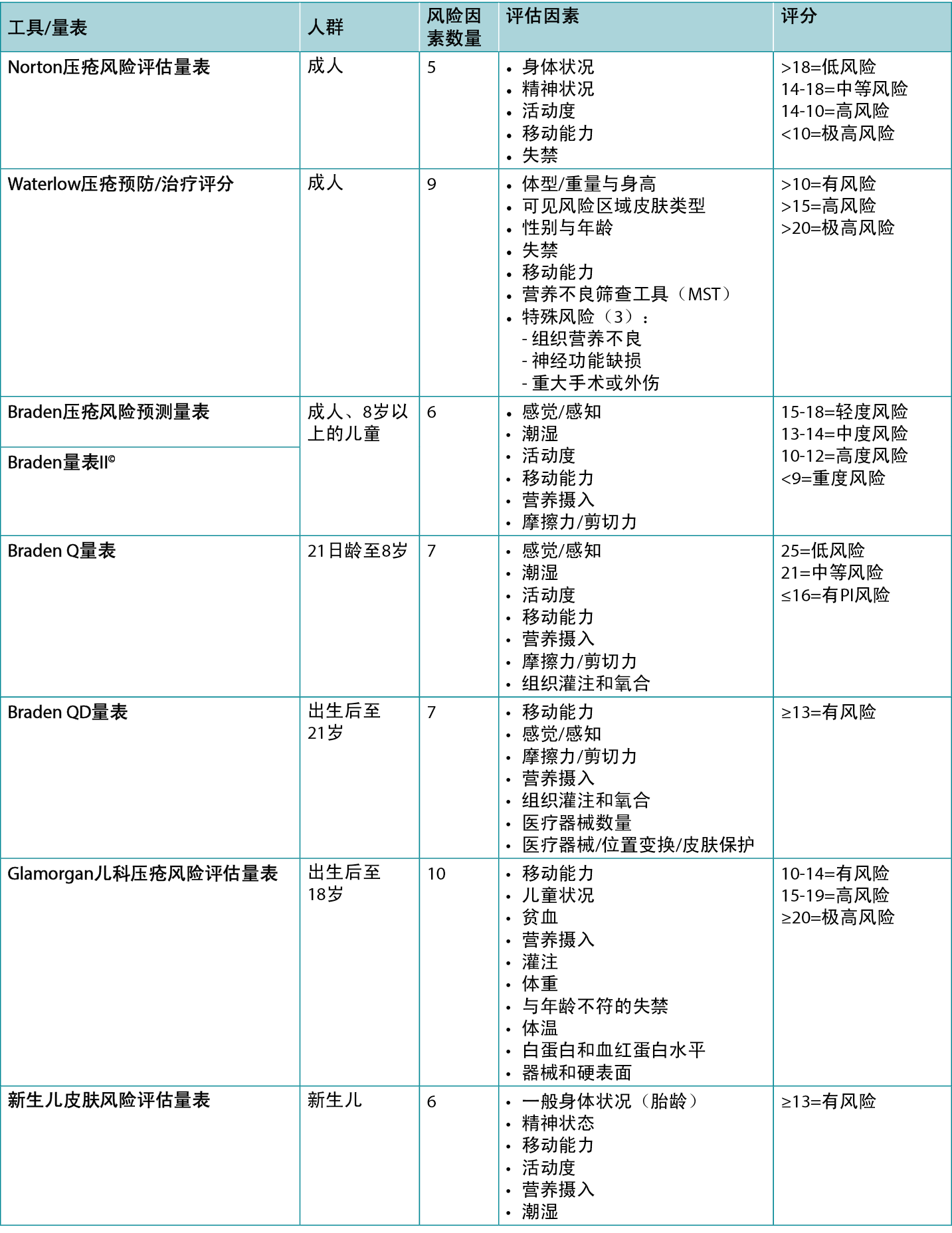
There are several valid and reliable risk assessment instruments (scales) available (Table 1), so it is important to use the one that reflects the age of your population and your practice setting. Some of the risk assessment instruments have a manual of instructions or glossary of terms for their use. The clinician should understand the definition of the terms used in the instrument so that they know how to accurately assess their patient for each of the risk factors outlined in the chosen instrument.
Most practice settings have a specific policy or guidance as to when risk assessments should be performed. The first general practice for performing a risk assessment is upon the person’s admission to a facility, e.g., hospital, long-term care/nursing home, rehabilitation, outpatient setting (e.g., clinics) or homecare assignment. Subsequent risk assessments are based on the clinical setting. For example, in acute care facilities, clinicians perform a risk assessment daily upon transfer to another nursing unit, when the patient’s condition changes, and upon discharge from the facility. In nursing homes or long-term care facilities, clinicians tend to perform risk assessments weekly and upon discharge. In homecare organisations, clinicians tend to perform a risk assessment on every visit, much like outpatient settings. It goes without saying that the clinician should follow the policy of their work setting and accurately evaluate the person according to each risk factor on the instrument. It is important to note that a clinician should also employ their judgment to a person’s PI risk outside of using a risk assessment instrument.
The following section provides a short description of the more commonly used risk assessment instruments.
Adult risk assessment instruments
Norton Pressure Sore Risk-Assessment Scale
Widely acknowledged as the first known scale is the Norton Pressure Sore Risk-Assessment Scale2. It was created in England in 1962 by Doreen Norton. It has five categories (Table 1) to which a number score is assigned based on the descriptor terms. When the numbers are totalled, low risk is determined to be >18, medium risk from 14–18, and lower numbers indicate higher risk, with <10 considered very high risk3.
Waterlow Pressure Ulcer Prevention/Treatment Score
The Waterlow Score was created by Judy Waterlow of the UK in 1985 and was revised in 2005 by Queensland Health4. As seen in Table 1, it has six categories. Additionally, the Malnutrition Screening Tool (MST) is used to assess the person’s nutritional status on this scale. There is also a section entitled ‘Special risks’. The scores are added, with a person being considered at risk when the score is >10, high risk at >15 and very high risk at >20. The back of the scale card has a short summary of prevention strategies as well as the European Pressure Ulcer Advisory Panel (EPUAP) classification definitions; further details can be found at the judy-waterlow.co.uk website5.
Braden Scale for Predicting Pressure Sore Risk
Known by many as the Braden Scale, it was created in the USA by Drs Barbara Braden and Nancy Bergstrom based on a conceptual schema which they published in 19876–8. The Scale has six assessment risk factors – sensory/perception, moisture, activity, mobility, nutrition and friction/shear (Table 1). Several early publications on the validation of the scale were subsequently published8–11. Over the years it has been used around the world and has had much research to validate its use in a variety of skin tones12. Its intended use is for ages 8–100+ years old. A score of 15–18 is considered to be mild risk, 13–14 moderate risk, 10–12 high risk and <9 severe risk.
Braden Scale II©
The Braden Scale for Predicting Pressure Sore Risk was originally published in the late 1980s6–8. Since April 2021, the Braden Scale copyright is now owned by Health Sense Ai and termed the Braden II©13. It has been updated in collaboration with original scale developers, Drs Barbara Braden and Nancy Bergstrom, to the Braden Scale II©. You can apply for copyright permission to use the Braden Scale II© by going to their website (www.bradenscale.com13), completing the licence use forms and paying the fee.
The Braden Scale II© has the same six risk assessment factors as the original Braden Scale – sensory/perception, moisture, activity, mobility, nutrition and friction/shear. Updates to the Braden Scale II© include language to bring the Scale into compliance with currently used taxonomy, like changing pressure sore to pressure injury. In addition, there are updates to the subsection descriptions to facilitate accurate scoring of the instrument among users. There are no changes to the cut scores at which a patient is considered to be at risk, but plan to address in the patient’s plan of care any subscales with higher scores even if the total overall scale score does not indicate the patient is at risk. The Braden Scale II© is available in English, French and Spanish.
To help clinicians score the scale, a glossary of terms has been created and available to use when you obtain copyright use permission. Health Sense AI/HD Nursing also has available several resource materials to help educate clinicians about the Braden Scale II©, including case examples that illustrate how to correctly use the scale13. The Braden Scale II© glossary and training module now make up the Braden Scale II Toolkit© which comes as a package when you licence the Braden Scale II©. This helps ensure staff are trained correctly to use the scale in direct patient care.
Paediatric risk assessment instruments
Braden Q Scale
The Braden Q risk assessment instrument was adapted from the Braden Scale by Curley and colleagues14 and since then has been frequently tested for its reliability and validity15. Its intended use in practice is for paediatric patients aged from 21 days (including corrected to gestational age of 21 days) up to age 8. The instrument includes the same six subscales of the Braden Scale with the addition of a seventh item – tissue perfusion and oxygenation. A score of 25 is considered low risk, 21 is medium risk and 16 or below is considered at risk for a PI (Table 1).
Table 1. Commonly used PI risk assessment instruments (scales) [©Delmore & Ayello 2022]
Braden QD Scale
The Braden QD is one of the newer risk assessment instruments created by Curley and colleagues16 and is based on the Braden Q Scale. Its intended use is for paediatric patients from pre-term ages to 21 years old. It contains five items from the Braden Q (mobility, sensory perception, friction/shear, nutrition, tissue perfusion and oxygenation) plus the addition of number of medical devices and repositionability/skin protection, the latter item specifically addressing medical devices (Table 1). A score of >13 is considered at risk for a PI17.
Glamorgan Paediatric Pressure Ulcer Risk Assessment Scale
This scale was created in the late 2000s as the Glamorgan Paediatric Pressure Ulcer Risk Assessment Scale (Glamorgan Scale) and noted to be the first paediatric risk assessment scale to include devices as one of the risk assessment factors18. Other Scale points address mobility, the child’s condition, anaemia, nutrition, perfusion, weight, incontinence inappropriate for the age, body temperature, albumin and haemoglobin levels, and devices. Any score of 10–14 is considered to be at risk, 15–19 is at high risk, and a score of ≥20 is considered very high risk for a PI.
Neonatal Skin Risk Assessment Scale
This scale was created by Huffines and Logsdon in the late 1990s and was based on the Braden Scale19. It was the first scale tested for reliability and validity for the neonatal population. The neonate is scored based on general physical condition (gestational age), mental state, mobility, activity, nutrition and moisture. A score of ≥13 is considered to be at risk.
Populations at Risk
Older adults
Advanced age (>65 years) is a PI intrinsic risk factor. Much of the risk is from skin changes that occur due to the ageing process such as epidermal thinning and loss of adipose tissue as a protective function. Additionally, disease burden and presence of co-morbidities create PI risk in this population20,21. Assessing risk using a valid and reliable scale is only one component of assessing an older adult’s PI risk. In this case, risk factors should be considered that are not included (e.g., age, disease burden) or reflect the degree of a condition’s severity (e.g., malnutrition)20–22.
Patients with obesity
According to the 2019 International Guideline, patients with obesity are considered a population that requires diligent PI risk assessments23. Obesity is an under recognised complex condition22. The Centers for Disease Control and Prevention (CDC) defines obesity by body mass index (BMI) categories: Class 1, BMI of 30–35kg/m2; Class 2, BMI of 35–40kg/m2; and Class 3, BMI of 40kg/m2 or higher and considered severe24. In this population, PIs occur due to a variety of factors such as malnutrition, diseases and conditions associated with obesity and device-related PIs due to ill-fitting equipment22,23.
Surgical patients
Assessment of the research literature in the 2019 International Guideline supports that the duration of time from when a person is admitted to when they have surgery as well as the length of time they are in surgery may be markers of a patient’s immobility1. Additionally, a person’s American Society of Anesthesiologists (ASA) Physical Status Classification may be a marker of the patient’s clinical status22. All three of these should be considered as risk factors for a person undergoing surgery.
Critical care
Critically ill patients are another special population that should be considered high risk for PI formation and therefore require diligent PI risk assessments23. The reason for this high risk is due to the critical illness of this population, the setting itself, and the abundant presence of medical devices required for treatment25,26. It is paramount to monitor this population closely as the addition of a PI to an already complex situation is considered an additional co-morbidity that can possibly lead to mortality23.
Other Considerations for Risk
Devices and objects
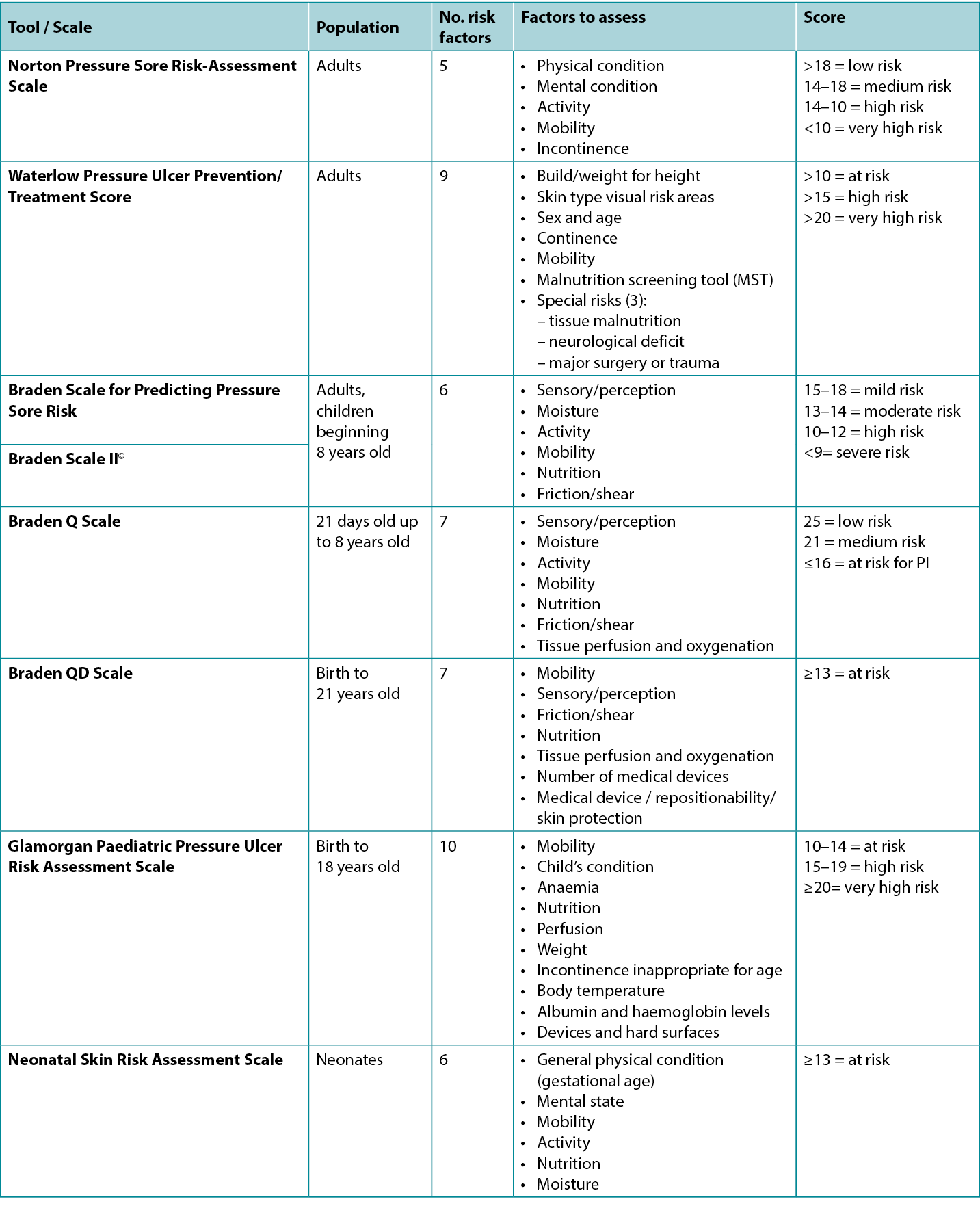
Medical devices and other objects such as eyeglasses and bottle caps can cause PIs27–29. Medical devices are the most frequent aetiology for medical device-related pressure injuries (MDRPI) in neonates and children1,16–18,30 (Figure 1); therefore, consideration for using the Braden QD Scale16 and the Glamorgan Scale18, which both include assessments for medical devices, is warranted.
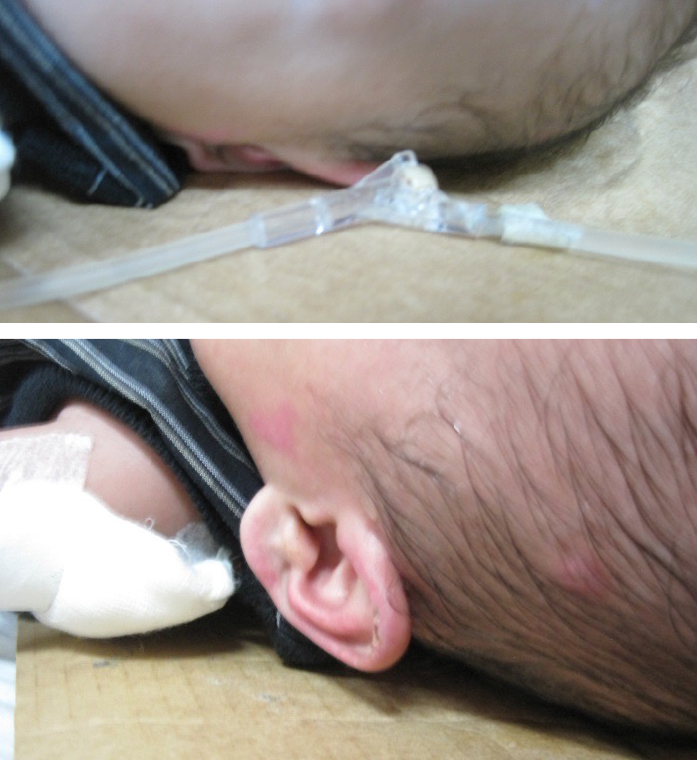
Figure 1. PI that developed from baby lying on IV tubing [©EA Ayello 2015, used with permission]
MDRPI also occur in adults27 (Figure 2). Currently, none of the adult PI risk assessment scales assess for MDRPI even though the 2019 International Guideline does address PI from devices – medical and other sources27. Therefore, raising awareness of devices as an aetiology for device-related PIs in adults is of great importance1,27–29. Consider using the SORE mnemonic to alert staff to medical and other devices that can cause PIs28. Research has supported that MDRPI occur 3 days sooner than other PIs, so staff need to be vigilant in assessing patients who have medical devices29. Remember to keep track of your facility’s MDRPI incidence28. Also, MDRPI on the lip cannot be staged as mucosa, does not keratinise and therefore cannot be staged using the NPIAP staging classification system1,27,28.
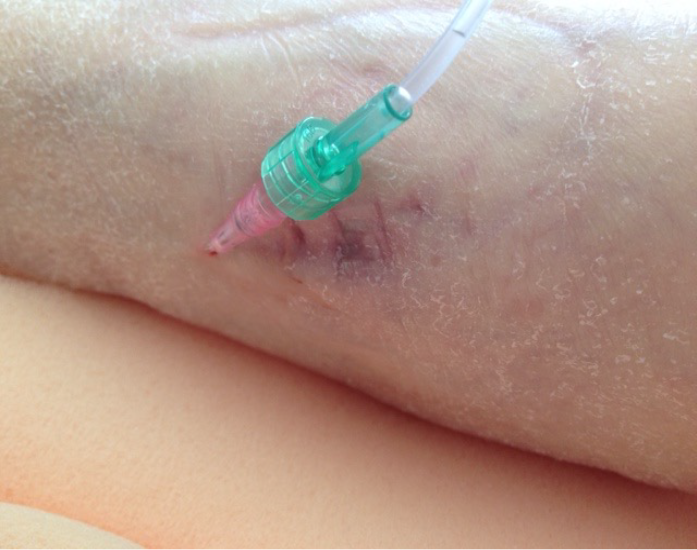
Figure 2. PI as a result of an intravenous (IV) hub that was secured directly to the skin. Notice the imprint on the skin that matches the design of the IV hub [©Delmore 2015, used with permission]
Specific anatomical areas at risk
Heels
Heels are believed to be the second most common anatomical site for PIs31. Due to the anatomy of the heel and limited tissue by the calcaneus, the heel is a particularly vulnerable to risk of a PI31–36 (Figure 3). Two research studies32,33 have provided evidence that patient co-morbidities, specifically diabetes mellitus and vascular disease along with immobility, are risk factors for developing heel PIs and should be considered with assessing a person’s risk for heel PIs along with a validated risk assessment instrument32–34.
Figure 3. Deep tissue pressure injury (DTPI) of the right heel. Unlike Stage 1 PIs that are intact and a lighter red/pink, DTPIs are intact but have a deeper discolouration indicating a deeper level of damage. These full-thickness PIs often evolve to a Stage 3 or 4, or an unstageable PI [©B Delmore & EA Ayello, 2020, used with permission]
In the main analysis (n=337) in one hospital, the predictor variables for heel PIs were diabetes, vascular disease, immobility and Braden Scale <1832. The study was expanded to other hospitals by using data from the New York State Statewide planning and Research Cooperative system (SPARCS)33. The main analysis had 1,697 patients (323 patients who had heel PIs and 1,374 who did not). There were seven significant and independent predictors – diabetes, vascular disease, perfusion issues, impaired nutrition, age >65 years, mechanical ventilation and surgery. Based in part from these two studies, the authors concluded that patient comorbidities, in this instance both diabetes and vascular disease, should be considered as risk factors along with results of formal risk assessment instruments33. Clinicians may find our heels algorithm helpful in their practice34 (Figure 4).
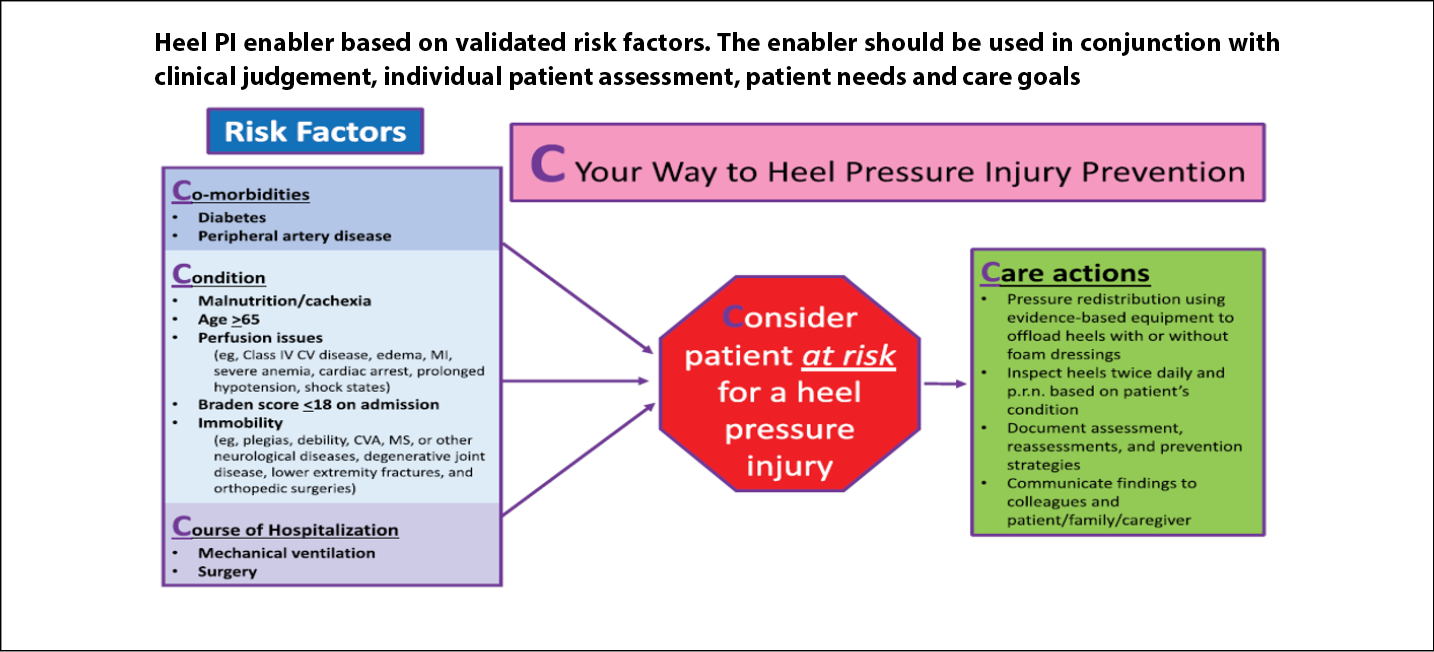
Figure 4. Heel pressure injury algorithm [©Delmore, Ayello & Smart 2020, used with permission]32–34
CV – cardiovascular; CVA – cerebrovascular accident; MI – myocardial infarction; MS – multiple sclerosis
Foot position may also be a risk factor. In another study of 10 healthy male volunteers, there was more strain on the heel tissue when the foot was in external rotation rather than upright (90°)36. Our recent clinical practice point may be helpful to clinicians as to proper foot positioning to help prevent heel PIs34.
Sacrum/coccygeal/ischial tuberosities
The sacrum is the most common anatomical site for PIs. Some research suggests that a patient’s skeletal morphology may be an intrinsic non-modifiable risk for Pl. The work of Gefen37 provides knowledge about changes in persons with spinal cord injury that increase risk for PI. This includes skeletal muscle atrophy, fat infiltration into muscles, bone shape loss leading to flattening of the tips of the ischial tuberosities and thinning of the skin around the ischial tuberositites37.
A recent retrospective case-control study by Delmore and colleagues compared the skeletal sacrococcygeal region of 15 patients with full-thickness PIs to 15 patients without full-thickness PIs using MRIs38. The premise of this study was to determine if the skeletal sacrococcygeal region may act as a possible intrinsic PI risk factor. Findings revealed that patients with full-thickness PIs did have different morphology and morphometry, resembling patients with other conditions. This study also noted that PIs in this region were more located in the coccyx region.
Risk Assessment Instruments and Technology
There is some growing debate in the literature about use of risk assessment scales as they may not capture all important risk factors, so do think about patient comorbidities that may not be captured on a risk assessment scale. There is research to study identification of additional risk factors and/or early indicators for PI including skin temperature39,40 as well as subdermal moisture and imaging41–43. It will be interesting to see how various technologies will reduce cost43. The future of PI risk assessment may include a systematic risk assessment including a valid and reliability scale, patient characteristics such as comorbidities, and technology that will impact on reducing PI incidence.
Conclusion and summary
There are several valid and reliable PI risk assessment scales available for use in practice. Although research continues to provide evidence as to which are the best in terms of predictive ability, identification of patient co-morbidities as well as technology may be additional data to help clinicians identify persons at risk for PIs. It is most important to remember that PI assessment is a process with the care goal of implementing a care plan in a timely manner to prevent avoidable PI44 from occurring.
Conflict of Interest
Dr Ayello was a member of the small working group on medical device-related pressure injuries for the 2019 EPUAP/NPIAP/PPPIA Prevention and treatment of pressure ulcers/injuries1. Dr Delmore is a Board Member of the National Pressure Injury Advisory Panel and is on the Editorial Board for Advances in Skin and Wound Care. She was a member of a small working group on heel pressure injuries for the 2019 EPUAP/NPIAP/PPPIA Prevention and treatment of pressure ulcers/injuries1.
Funding
The authors received no funding for this study.
压力性损伤的风险评估
Elizabeth A Ayello and Barbara A Delmore
DOI: https://doi.org/10.33235/wcet.42.4.31-37
摘要
本文重点讨论了常用的压力性损伤(PI)风险评估工具(量表)以及在日常实践中临床医生应考虑的其他因素,以确定患者是否存在发生PI的风险。
引言
每年11月,许多专业组织都会参加“压力性损伤/压疮预防日”活动。它提供了一个机会来提高公众以及其他医疗保健专业人员对压力性损伤(PI)的认识。预防PI是临床医生日常实践的重要组成部分。本文的目的是简要总结一些常用的PI风险评估工具(量表)以及其他患者特征,以作为综合风险评估过程一部分。
风险评估概述
风险评估的目的是确定一个人是否存在PI的风险,如果存在,则实施个体化预防计划,尤其要考虑可改变和不可改变的风险因素1。2019版国际指南及其实施建议1为临床医生在任何护理环境下针对PI风险个体采取最佳实践提供了帮助。风险评估是预防PI时需要考虑的关键组成部分之一。这是一个系统的过程,至少包括检查患者皮肤的任何变化、了解任何器械(包括可能导致压力的医疗器械和其他物品)、评估已知为风险因素的个体患者特征,以及使用经过验证/可靠的风险评估工具(量表)和临床医生的临床判断进行评估。
风险评估工具
有效且可靠的风险评估工具(量表)有多种(表1),因此使用能够反映您的人群年龄和实践环境的工具非常重要。一些风险评定工具有使用说明手册或术语表。临床医生应了解工具中使用的术语定义,以便知道如何根据所选工具中概述的每个风险因素准确地评估患者。
对于何时应进行风险评估,大多数实践环境都有具体的政策或指南。风险评估一般首先在患者入住机构时进行,例如医院、长期护理/疗养院、康复机构、门诊(例如诊所)或家庭护理任务。后续的风险评估基于临床环境。例如,在急症护理机构中,临床医生每天都会在转移到另一个护理病房、患者病情发生变化和出院时进行风险评估。在疗养院或长期护理机构,临床医生倾向于每周和出院时进行风险评估。在家庭护理机构中,临床医生倾向于在每次访视时进行风险评估,与在门诊环境中类似。毫无疑问,临床医生应该遵循他们工作环境的政策,并根据工具中的每个风险因素对患者进行准确的评估。值得注意的是,临床医生还应在使用风险评估工具之外对患者的PI风险进行判断。
以下部分简要介绍了比较常用的风险评估工具。
表1.常用PI风险评估工具(量表)[˝Delmore & Ayello 2022]
成人风险评估工具
Norton压疮风险评估量表
公认的第一个已知量表是Norton压疮风险评估量表2。它于1962年在英国由Doreen Norton创建。它有五个类别(表1),根据描述水压疮预防/治疗评分词术语分配数字评分。将数字相加,低风险被确定为>18,中等风险为14-18,数字越低表明风险越高,<10被认为是极高风险3。
Waterlow压疮预防/治疗评分
Waterlow评分由英国的Judy Waterlow于1985年创建,并于2005年由Queensland Health4进行了修订。如表1所示,它有六个类别。此外,使用营养不良筛查工具(MST)在该量表上评估患者的营养状况。还有一节标题为“特殊风险”。将评分相加,当评分>10时,认为患者存在风险,评分>15时为高风险,评分>20时为极高风险。量表卡背面有预防策略的简短总结以及欧洲压疮咨询委员会(EPUAP)的分类定义;更多详情见judy-waterlow.co.uk网站5。
Braden压疮风险预测量表
该量表被许多人称为Braden量表,由Barbara Braden博士和Nancy Bergstrom博士在美国根据他们在1987年发表的概念模式创建6-8。该量表有六个评估风险因素Å\Å\感觉/感知、潮湿、活动度、移动能力、营养摄入、摩擦力/剪切力(表1)。随后发表了几篇关于该量表的验证的早期出版物8-11。多年来,该量表已在世界各地使用,并进行了大量研究来验证其在不同肤色人群中的使用12。它适用于8-100岁以上的人群。15-18分为是轻度风险,13-14分为中度风险,10-12分为高度风险,<9分是重度风险。
Braden量表II˝
用于预测压疮风险的Braden量表最初发表于20世纪80年代后期6-8。自2021年4月起,Braden量表版权现在归Health Sense Ai所有,并称之为Braden II˝13。已与原始量表开发人员Barbara Braden博士和Nancy Bergstrom博士合作将其更新为Braden量表
II˝。您可以通过访问他们的网站(www.bradenscale.com13)、填写许可使用表并支付费用来申请Braden量表II˝的版权许可。
Braden量表II˝具有与原始Braden量表相同的六个风险评估因素Å\Å\感觉/感知、潮湿、活动度、移动能力、营养摄入、摩擦力/剪切力。Braden量表II˝的更新包括更新语言,使该量表符合当前使用的分类法,例如将压疮变更为压力性损伤。此外,还更新了小节描述,以方便用户使用工具进行准确评分。判定患者存在风险的临界值分数没有变化,但计划在患者护理计划中处理评分较高的子量表,即使总量表评分并未表明患者处于风险中。Braden量表II˝有英语、法语和西班牙语版本。
为了帮助临床医生使用量表进行评分,创建了术语表,并可在您获得版权使用许可后使用。Health Sense AI/HD Nursing还提供了多种资源材料,可帮助临床医生了解Braden量表II˝,,包括说明如何正确使用该量表的案例13。Braden量表II˝术语表和培训模块现在组成了Braden量表II工具包˝,当您获得Braden量表II˝许可时,该工具包将打包提供。这有助于确保工作人员接受正确培训,能够在直接患者护理中使用该量表。
儿科风险评估工具
Braden Q量表
Braden Q风险评估工具改编自Curley及其同事创建的Braden量表14,此后经常对其可靠性和有效性进行测试15。其在实践中预期用于年龄从21天(包括校正胎龄21天)至8岁的儿科患者。该工具包括与Braden量表的相同6个子量表,并増加了第7项Å\Å\组织灌注和氧合。25分为低风险,21分为中等风险,16分或以下为存在PI风险(表1)。
Braden QD量表
Braden QD是Curley及其同事创建的较新的风险评估工具之一16,该量表基于Braden Q量表。其预期用于早产儿至21岁的儿科患者。它包含Braden Q的5个项目(移动能力、感觉感知、摩擦力/剪切力、营养摄入、组织灌注和氧合)加上新増的医疗器械数量和位置变换/皮肤保护,后一项专门针对医疗器械(表1)。评分>13被认为存在PI风险17。
Glamorgan儿科压疮风险评估量表
该量表创建于2000年代后期,名为Glamorgan儿科压疮风险评估量表(简称为Glamorgan量表),被认为是首个将器械作为风险评估因素之一的儿科风险评估量表18。其他量表指标涉及移动能力、儿童状况、贫血、营养摄入、灌注、体重、与年龄不符的失禁、体温、白蛋白和血红蛋白水平、器械。10-14分被认为有风险,15-19分是高风险,≥20分被认为有极高的PI风险。
新生儿皮肤风险评估量表
该量表由Huffines和Logsdon于20世纪90年代后期基于Braden量表创建19。这是第一个针对新生儿人群进行信度和效度检验的量表。根据一般身体状况(胎龄)、精神状态、移动能力、活动度、营养摄入和潮湿对新生儿进行评分。评分≥13被认为存在风险。
存在风险的人群
老年人
高龄(>65岁)是PI的内在风险因素。大部分风险来自衰老过程导致的皮肤变化,例如表皮变薄和具有保护功能的脂肪组织的损失。此外,疾病负担和合并症的存在会在该人群中产生PI风险20,21。使用有效且可靠的量表评估风险只是评估老年人PI风险的一个组成部分。在这种情况下,应考虑量表未包括在内的风险因素(例如年龄、疾病负担)或反映疾病严重程度的风险因素(例如营养不良)20-22。
肥胖患者
根据2019版国际指南,肥胖患者被认为是需要进行严格PI风险评估的人群23。肥胖是一种未被充分认识的复杂疾病22。美国疾病控制与预防中心(CDC)根据身体质量指数(BMI)类别定义肥胖:1类,BMI为30-35 kg/m2;2类,BMI为35-40 kg/m2;3类,BMI为40 kg/m2或更高,被视为重度肥胖
24。在该人群中,PI的发生是多种因素造成的,如营养不良、与肥胖相关的疾病和病症以及由于器械不合适导致的器械相关性PI22,23。
手术患者
对2019版国际指南中研究文献的评估支持,从患者入院到接受手术的时间长短以及手术过程的时间长短可能是患者移动受限的标志1。此外,美国麻醉医师协会(ASA)身体状况分类可能是患者临床状况的标志22。所有这三种因素均应被视为接受手术患者的风险因素。
重症监护
重症患者是另一个应视为存在PI形成高风险的特殊人群,因此需要进行严格的PI风险评估23。这种高风险的原因在于该人群的危重疾病、环境本身以及治疗所需医疗器械的大量存在25,26。密切监测该人群至关重要,因为在已经很复杂的情况下,PI的増加被认为是可能导致死亡的额外合并症23。
其他风险考虑因素
器械和物品
医疗器械和其他物品(如眼镜、瓶盖)可能导致PI27-29。医疗器械是新生儿和儿童医疗器械相关压力性损伤(MDRPI)的最常见病因1,16-18,30(图1);因此,有必要考虑使用Braden QD量表16和Glamorgan量表18,两者均包括医疗器械评估。

图1.婴儿躺在静脉输液管上形成的PI [˝EA Ayello 2015,经许可使用]
MDRPI也发生在成人中27(图2)。目前,尽管2019版国际指南确实阐述了来自医疗器械Å\Å\医疗和其他来源的PI27,但成人PI风险评估量表均未评估MDRPI。因此,提高对器械是成人器械相关性PI病因的认识非常重要1,27-29。考虑使用SORE助记符来提醒工作人员注意可能导致PI的医疗器械和其他器械28。研究表明MDRPI比其他PI早3天发生,因此工作人员在评估使用了医疗器械的患者时需要保持警觉29。请记住跟踪您所在机构的MDRPI发生率28。此外,唇部的MDRPI不能作为粘膜分期,不会角质化,因此不能使用NPIAP分期分类系统进行分期1,27,28。

图2.静脉(IV)导管座直接固定在皮肤上导致的PI。注意皮肤上的印记与IV导管座的形状相匹配 [˝Delmore 2015,经许可使用]
存在风险的特定解剖区域
足跟
足跟被认为是第二经常发生PI的解剖部位31。由于足跟的解剖结构以及跟骨的组织有限,足跟特别容易受到PI风险的影响31-36(图3)。两项研究32,33提供的证据表明,患者的合并症(尤其是糖尿病和血管疾病)以及移动受限是发生足跟PI的风险因素,在使用经验证的风险评估工具评估患者的足跟PI风险时也应考虑这些因素32-34。

图3.右足跟部的深部组织压力性损伤(DTPI)。与完整且呈浅红色/粉红色的1期PI不同,DTPI完整但变色更深,表明损伤程度更深。这些全层PI通常进展为3期或4期,或无法分期的PI[˝B Delmore & EA Ayello,2020,经许可使用]
在一家医院的主要分析(n=337)中,足跟PI的预测变量为糖尿病、血管疾病、移动受限以及Braden量表评分<1832。该研究通过使用纽约州全州规划和研究合作系统(SPARCS)的数据扩展到其他医院33。主要分析包含1697名患者(323名患者有足跟PI,1374名患者没有足跟PI)。有7个显著且独立的预测因素Å\Å\糖尿病、血管疾病、灌注问题、营养不良、年龄>65岁、机械通气、手术。作者部分基于这两项研究得出结论,在这种情况下,患者合并症(糖尿病和血管疾病)应与正式风险评估工具的结果一起被视为风险因素33。临床医生可能会发现我们的足跟算法对他们的实践很有帮助34(图4)。

图4.足跟压力性损伤算法 [˝Delmore,Ayello & Smart 2020,经许可使用]32-34
CV-心血管;CVA-脑血管意外;MI-心肌梗死;MS-多发性硬化症
足部的位置也可能是一个风险因素。在另一项对10名健康男性志愿者的研究中,当足部处于外旋状态而不是直立状态(90Åã)时,足跟组织承受的压力更大36。我们最近的临床实践点可能有助于临床医生正确定位足部,以帮助预防足跟PI34。
骶骨/尾骨/坐骨结节
骶骨是最经常发生PI的解剖部位。一些研究表明,患者的骨骼形态可能是PI的内在不可改变风险。Gefen37的研究提供了关于脊髓损伤患者发生的变化的认识,而脊髓损伤会増加PI风险。这些变化包括骨骼肌萎缩、脂肪浸润肌肉、骨骼变形导致坐骨结节尖端变平以及坐骨结节周围皮肤变薄37。
Delmore及其同事最近进行的一项回顾性病例对照研究使用MRI比较了15名全层PI患者与15名非全层PI患者的骶尾部骨骼区域38。这项研究的前提是确定骶尾部骨骼区域是否可能作为内在PI风险因素。结果显示,全层PI患者确实具有不同的形态和形态测量值,与其他疾病的患者类似。这项研究还注意到,该区域的PI更多位于尾骨区域。
风险评估工具和技术
文献中关于使用风险评估量表的争论越来越多,因为量表可能无法涵盖所有重要的风险因素,因此请务必考虑风险评估量表可能无法涵盖的患者合并症。目前有研究关注PI的其他风险因素和/或早期指标的识别,包括皮肤温度39,40以及皮下水分和成像41-43。观察各种技术将如何降低成本将会很有趣
43。未来的PI风险评估可能包括系统性风险评估,包括有效和可靠的量表、患者特征(如合并症)以及将对降低PI发生率产生影响的技术。
结论和总结
在实践中有多种有效且可靠的PI风险评估量表可用。尽管研究持续提供证据证明哪种方法在预测能力方面表现最佳,但患者合并症的识别以及技术可能是帮助临床医生识别PI风险人群的额外数据。最重要的是要记住,PI评估是一个过程,其护理目标是及时实施护理计划,以防止可避免的PI44发生。
利益冲突声明
Ayello博士是2019版EPUAP/NPIAP/PPPIA《压疮/压力性损伤的预防和治疗》的医疗器械相关压力性损伤小型工作组的成员1。Delmore博士是美国国家压力性损伤咨询小组的委员会成员,是《Advances in Skin and Wound Care》的编委会成员。她是2019版 EPUAP/NPIAP/PPPIA《压疮/压力性损伤的预防和治疗》的足跟压力性损伤小型工作组的成员1。
资助
作者未因该项研究收到任何资助。
Author(s)
Elizabeth A Ayello*
PhD, RN, CWON, MAPWCA, FAAN
Co-Editor in Chief, Advances in Skin and Wound Care
Executive Editor Emeritus, WCET® Journal
New York, NY, USA
Barbara A Delmore
PhD, RN, CWCN, MAPWCA, IIWCC-NYU, FAAN
Senior Nurse Scientist, Center for Innovations in the Advancement of Care (CIAC), NYU Langone Health, New York, NY, USA
Clinical Assistant Professor, Hansjörg Wyss Department of Plastic Surgery, NYU Grossman School of Medicine, New York, NY, USA
* Corresponding author
References
- European Pressure Ulcer Advisory Panel, National Pressure Injury Advisory Panel and Pan Pacific Pressure Injury Alliance (EPUAP/NPIAP/PPPIA). Prevention and treatment of pressure ulcers/injuries: clinical practice guideline. The international guideline. Emily Haesler, editor. Perth, WA: Cambridge Media; 2019.
- Norton D, et al. An investigation of geriatric nursing problems in hospital. London, UK: National Corporation for the Care of Old People; 1962.
- Royal Commission into Aged Care Quality and Safety. The Norton Pressure Sore Risk-Assessment Scale Scoring System; n.d. Available from: https://agedcare.royalcommission.gov.au/system/files/2020-06/RCD.9999.0096.0460.pdf
- Waterlow J. Pressure sores: a risk assessment card. Nurs Times 1985;81(48):49–55.
- judy-waterlow.co.uk. The Waterlow Score; 2007. Available from: http://www.judy-waterlow.co.uk/waterlow_score.htm
- Braden B, Bergstrom N, Laguzza V, Homan A. The Braden Scale for Predicting Pressure Sore Risk. Nurs Res 1987;36(4):205–10.
- Braden B, Bergstrom N. A conceptual schema for the study of the etiology of pressure sores scale. Rehab Nurs 1987;12(1):8–12, 16.
- Bergstrom, N, Demuth PJ, Braden BJ. A clinical trial of the Braden Scale for Predicting Pressure Sore Risk. Nurs Clin North Am 1987;22:417–28.
- Bergstrom N, Braden B, Kemp M, Champagne M, Ruby E. Predicting pressure ulcer risk: a multisite study of the predictive validity of the Braden Scale. Nurs Res 1998;47(5):261–269. doi:10.1097/00006199-199809000-00005
- Braden B, Bergstrom N. Predictive validity of the Braden Scale for pressure sore risk in a nursing home population. Res Nurse Health 1994;17:459–70.
- Bergstrom N, Braden B. A prospective study of pressure sore risk among institutionalized elderly. J Am Geriatr Soc 1992;40:742–58.
- Lyder CH, et al. The Braden Scale for pressure ulcer risk: evaluating the predictive validity in Black and Latino/Hispanic elders. App Nurs Res 1999;12(2):60–8.
- Braden Scale II© Predicting Pressure Injuries. Available from: http://www.bradenscale.com.
- Curley MAQ, et al. Predicting pressure ulcer risk in pediatric patients: the Braden Q Scale. Nurs Res 2003;52(1):22–31.
- Noonan C, Quigley S, Curley MAQ. Using the Braden Q Scale to predict pressure ulcer risk in pediatric patients. J Pediatr Nurs 2011;26:566–75.
- Curley MAQ, Hasbani NR, Quigley SM, et al. Predicting pressure injury risk in pediatric patients: the Braden QD Scale. J Pediatr 2018;192:189–195.
- Chamblee TB, Pasek TA, Caillouette CN, et al. How to predict pediatric pressure injury risk with the Braden QD Scale. Am J Nurs 2018;118(11):34–43.
- Willock J, Baharestani MM, Anthony D. The development of the Glamorgan paediatric pressure ulcer risk assessment scale. J Wound Care 2009;18(1):17–21. Available from: http://www.ncbi.nlm.nih.gov/pubmed/19131913
- Huffines B, Logsdon MC. The Neonatal Skin Risk Assessment Scale for predicting skin breakdown in neonates. Issues Compr Pediatr Nurs 1997;20(2):103–114. doi:10.3109/01460869709026881
- European Pressure Ulcer Advisory Panel, National Pressure Injury Advisory Panel and Pan Pacific Pressure Injury Alliance (EPUAP/NPIAP/PPPIA). Chapter 4: Risk factors and risk assessment. In: Haesler E, editor. Prevention and treatment of pressure ulcers/injuries: clinical practice guideline. The international guideline. Perth, WA: Cambridge Media; 2019. p. 38–72.
- Jaul E, Barron J, Rosenzweig JP, Menczel J. An overview of co-morbidities and the development of pressure ulcers among older adults. BMC Geriatr 2018;18(305):1–11. doi:10.1186/s12877-018-0997-7
- Munoz N, Litchford M, Cox J, Nelson JL, Nie AM, Delmore B. National Pressure Injury Advisory Panel White Paper malnutrition and pressure injury risk in vulnerable populations: application of 2019 International Clinical Practice Guideline. Adv Skin Wound Care 2022;35(March):156–165. doi:10.1097/01.ASW.0000816332.60024.05
- European Pressure Ulcer Advisory Panel, National Pressure Injury Advisory Panel and Pan Pacific Pressure Injury Alliance (EPUAP/NPIAP/PPPIA). Chapter 3: Populations with specific pressure injury related needs. In: Haesler E, editor. Prevention and treatment of pressure ulcers/injuries: clinical practice guideline. The international guideline. Perth, WA: Cambridge Media; 2019. p. 28–37.
- Centers for Disease Control and Prevention. Defining adult overweight and obesity; 2021 [cited 2022 Nov 23]. Available from: https://www.cdc.gov/obesity/adult/defining.html
- Cox J. Pressure injury risk factors in adult critical care patients: a review of the literature. Ostomy Wound Manag 2017;63(11):30–43.
- Alderden J, Rondinelli J, Pepper G, Cummins M, Whitney JA. Risk factors for pressure injuries among critical care patients: a systematic review. Int J Nurs Stud 2017;71:97–114. doi:10.1016/j.ijnurstu.2017.03.012
- European Pressure Ulcer Advisory Panel, National Pressure Injury Advisory Panel and Pan Pacific Pressure Injury Alliance (EPUAP/NPIAP/PPPIA). Chapter 11: Device related pressure injuries. In: Haesler E, editor. Prevention and treatment of pressure ulcers/injuries: clinical practice guideline. The international guideline. Perth, WA: Cambridge Media; 2019. p. 181–193.
- Delmore B, Ayello EA. Pressure injuries caused by medical devices and other objects: a clinical update. Am J Nurs 2017;117(12):36–45.
- Kayser SA, VanGilder CA, Ayello EA, Lachenbruch C. Prevalence and analysis of medical device-related pressure injuries: results from the International Pressure Ulcer Prevalence Survey. Adv Skin Wound Care 2018;31(6):276–285.
- Delmore B, Deppisch M, Sylvia C, Luna-Anderson C, Nie AM. Pressure injuries in the pediatric population: a National Pressure Ulcer Advisory Panel White Paper. Adv Skin Wound Care 2019;32(9):394–408. doi:10.1097/01.ASW.0000577124.58253.66
- European Pressure Ulcer Advisory Panel, National Pressure Injury Advisory Panel and Pan Pacific Pressure Injury Alliance (EPUAP/NPIAP/PPPIA). Chapter 9: Heel pressure injuries. In: Haesler E, editor. Prevention and treatment of pressure ulcers/injuries: clinical practice guideline. The international guideline. Perth, WA: Cambridge Media; 2019. p. 145–154.
- Delmore B, Lebovits S, Suggs B, Rolnitzky L, Ayello EA. Risk factors associated with heel pressure ulcers in hospitalized patients. JWOCN 2015;42(3):242–248.
- Delmore B, Ayello EA, Smith D, Rolnitzky L, Chu AS. Refining heel pressure injury risk factors in the hospitalized patient. Adv Skin Wound Care 2019:32(11):512–519.
- Delmore B, Ayello EA. Practice point: heel pressure injuries. Adv Skin Wound Care 2021;34(5):236-237.
- Gefen A. Why is the heel particularly vulnerable to pressure ulcers. Br J Nurs 2017;8;26(Sup20):S62-S74. doi:10.12968/bjon.2017.26.Sup20.S62.
- Tenenbaum S, Shabshin N, Levy A, Herman A, Gefen AJ. Effects of foot posture and heel padding devices on soft tissue deformations under the heel in supine position in males: MRI studies. J Rehabil Res Dev 2013;50(8):1149–56. doi:10.1682/JRRD/2012.10.0183.
- Gefen A. Tissue changes in patients following spinal cord injury and implications for wheelchair cushions and tissue loading: a literature review. Ostomy Wound Manage 2014;60(2):34–45.
- Delmore B, Sprigle S, Samim M, et al. Does sacrococcygeal skeletal morphology and morphometry influence pressure injury formation in adults. Adv Skin Wound Care 2022;35(11):586–95.
- Sprigle S, et al. Clinical skin temperature measurement to predict incipient pressure ulcers. Adv Skin Wound Care 2001;14(3):133–37.
- Langemo D, Spahn JG. A reliability study using long-wave infrared thermography device to identify relative tissue temperature variations of the body surface and underlying tissue. Adv Skin Wound Care 2017;30(3):109–119.
- Bates-Jensen BM, McCreath HE, Pongquan V. Subdermal moisture is associate with early pressure ulcer damage in nursing home residents with dark skin tones. JWOCN 2009;36(3):277–84.
- Ross G, Gefen A. Assessment of sub-epidermal moisture by direct measurement of tissue biocapacitance. Med Eng Physic 2019;73:92–99.
- Koerner, S, Adams D, Harper SL, Black JM, Langemo DK. Use of thermal imaging to identify deep-tissue pressure injury on admission reduces clinical and financial burdens of hospital-acquired pressure injuries. Adv Skin Wound Care 2019;32(7):312–20.
- Wound, Ostomy and Continence Nurses Society. (2017). WOCN Society position paper: Avoidable versus unavoidable pressure ulcers (injuries). Mt. Laurel, NJ: Author.


