Volume 43 Number 2
Delving into skin and soft tissue infections (SSTI). Part III: focus on cellulitis
Caley Shukalek, Vidhi Desai, Brandon Christensen, Christopher Lata, Ranjani Somayaji
Keywords soft tissue infection, case-based review, cellulitis
For referencing Shukalek C et al. Delving into skin and soft tissue infections (SSTI). Part III: focus on cellulitis. WCET® Journal 2023;43(2):24-28
DOI
https://doi.org/10.33235/wcet.43.2.24-28
Submitted 27 July 2022
Accepted 6 June 2023
Abstract
In this third part of a series of articles (Part I WCET® Journal Volume 36 Number 2 – April/June 2016: PP29-34; Part II WCET® Journal Volume 37 Number 3 – July/September 2017: PP20-24) on skin and soft tissue infections (SSTI), cellulitis syndromes are explored. A case-based approach to the diagnosis and management of cellulitis for clinicians is discussed.
Introduction
Skin and soft tissue infections (SSTI) represent a spectrum of diseases, from mild superficial infection such as erysipelas and cellulitis to deep fascial infections as seen in necrotising fasciitis. The presentations vary but are common both within primary and acute care settings. The burden of SSTI is vast, with rates rising through the late 1990s–2000s, attributed to increasing age and comorbidities such as obesity1. However, there are no significant differences between men and women2. A US-based study in 2010 showed SSTI to be two-times more common than UTI and 10 times more common than pneumonia, with rates as high as 48.5 cases per 1000 person years3. Similarly, a study examining rates of cellulitis in the US between 1998–2013 demonstrated the rates of acute hospitalisation were nearly double, with costs totalling nearly US$3.74 billion4. At the same time, a challenging aspect of cellulitis and other SSTI is diagnosis, with a reliance on clinical history and physical examination. The absence of objective microbiological or laboratory testing allows for non-infectious aetiologies to be mistakenly diagnosed as SSTI. This too is costly to the system, with one study showing up to 30% of patients admitted with lower limb cellulitis were misdiagnosed, with an estimated cost of between US$195–515 million5.
Endeavours to develop aids in the diagnosis of cellulitis have been undertaken; however, challenges remain around developing ‘gold standard’ diagnostics and appropriate comparators, given the heterogeneity of alternative diagnoses. A 2019 systematic review found several tools to aid in diagnosis; however, none were adequately validated for lower limb cellulitis6.
As previously discussed, SSTI often result from minor superficial trauma to the skin barrier7,8. Trauma can come in the form of external damage to the skin, chronic venous insufficiency, or inflammation4,9,10.
Cellulitis Overview
Clinical manifestations
Cellulitis is a rapidly progressive SSTI involving the dermis and subcutaneous tissues11. Symptoms typically include acute onset redness, warmth, oedema and pain, but can occasionally include systemic symptoms such as fevers and rigours. Most commonly, cellulitis is found on the lower extremities, with rates as high as 39.9% of all cellulitis12. As mentioned previously, damage to the skin surface through trauma, inflammation or oedema typically precedes infection. Less commonly, cellulitis can occur due to spread of an infection from the bloodstream or a contiguous source (i.e. abscess in the fat tissue expanding outward)2.
Numerous risk factors exist for the primary/first episode of cellulitis, including homelessness, advanced age, obesity, skin breakdown (ulcers, inflammation, trauma), oedema/lymphoedema, toe web infections (fungal, bacterial) venous insufficiency and previous venectomy among others13. At the same time, risk factors for recurrent cellulitis include obesity, tinea pedis, oedema/lymphoedema and venous insufficiency, but also smoking, malignancy and previous cellulitis1. Recurrence rates of cellulitis following a primary episode are high but ranges vary depending on the study, with some reporting ~8–20%12 while others show between 22–49% when risk factors are present1.
Non-necrotising and non-purulent infections rarely cause mortality1. However, the estimated overall mortality rate for cellulitis is reported to be 1.1%, although infection itself may only be the culprit in up to one third of cases1,14. The vast majority of infections are caused by Staphylococcus aureus and streptococci and, in one study, where microbiologic diagnoses were confirmed, these two pathogenic groups were cultured 97% of the time13.
Several scoring systems have been developed, including the ERON15 and the modified Dundee classification, which have been included in the UK CREST guidelines16. However, these criteria have not been widely adopted and have been criticised for being overly simplified or not clinically robust in distinguishing severity11,17.
Interestingly, recent studies have shown the incidence of cellulitis can vary by season. One such study out of Denver showed a trend toward higher rates of admissions for primary cellulitis in warmer months, with July having 66.63% higher odds of infection compared to the colder winter months18. At the same time, a study out of southwestern Taiwan showed rates of lower extremity cellulitis increased in the days immediately following a typhoon, suggesting climates prone to floods and excessive precipitation may place occupants at risk of cellulitis with enteric, gram-negative and water-borne organisms due to exposure to contaminated water19. One explanation may be soaking of the extremities for prolonged periods, thus impairing natural host defence systems and facilitating a portal of entry through the skin surface19. Furthermore, during climate disasters, bites from animals and insects may also contribute to increased rates of infection18.
Pathogenesis
Once superficial damage occurs to the skin surface, bacterial contamination with the patient’s own skin flora can occur. This explains why staphylococcal and streptococcal species are the most prevalent organisms in cellulitis. Successful infection occurs in three steps – the bacteria must first adhere to the host’s cells, then invade the tissue while evading the host’s defences, and finally utilise its toxigenic factors19. A cytokine and neutrophil response are triggered after bacteria penetrate the skin. This epidermal response results in antimicrobial peptide production and keratinocyte proliferation, both of which induce the characteristic examination findings of cellulitis2. The portal of entry is not always evident, particularly as cellulitis can occur with seemingly intact skin in the context of other risk factors. In these instances, microscopic cracks occur in skin; these become irritated or inflamed, thus facilitating bacterial migration inward20.
Clinical approach / microbiology
Cellulitis can be classified into non-purulent and purulent forms based on the clinical presentation. Non-purulent cellulitis, classically caused by streptococci, presents as a unilateral, poorly demarcated, warm and red area lacking purulent discharge or abscess. Conversely, purulent cellulitis, classically caused by staphylococci, generally develops around wounds, collections or carbuncles. In both, there is surrounding oedema and tenderness to palpation which can expand rapidly as the infection progresses. Other local features can include local necrosis and abscess formation (subsequent to cellulitis process) based on the bacterial species and infection severity.
S. aureus is more frequently associated with purulent cellulitis, although both bacterial species are capable of severe infectious features based on the virulence factors of the infecting strain. Other streptococci that are also implicated in cellulitis include Group B, C and G streptococcus – these are more common in persons with diabetes or vascular disease. As a wound becomes chronic, there is a transition of skin flora to one that is polymicrobial with colonisation by enteric gram-negatives, anaerobes or environmental pathogens. Following a similar pathogenesis, these organisms can cause infection, often in those with untreated wounds, poor circulation, or diabetic foot ulcers21.
More atypical organisms can be involved in cellulitis, including those seen in animal bite, fresh/salt water, or aquarium exposures. These are often identified with careful history and require broader spectrum therapies which are beyond the scope of this review.
Differential diagnosis
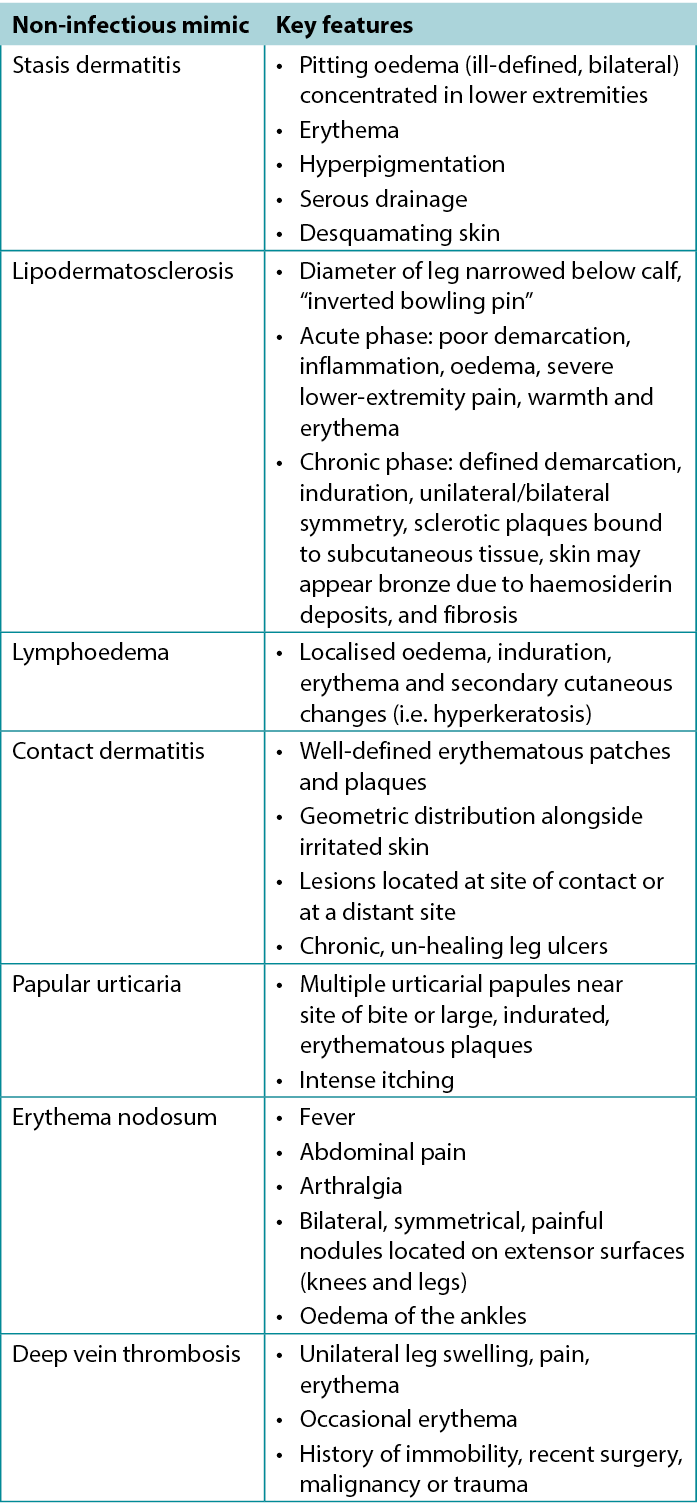
Given the wide spectrum of dermatologic conditions, the largely subjective nature of history and physical examination, and the non-specific symptoms (i.e. tenderness, erythema, oedema) seen in the skin, cellulitis is frequently misdiagnosed22. Syndromes that mimic cellulitis include statis dermatitis, lipodermatosclerosis and lymphoedema; these are summarised in Table 122. Stasis dermatitis is the most common mimic of cellulitis, although it tends to be slower onset and more often bilateral. However, stasis dermatitis and other mimics are risk factors for SSTI and, as such, infection should remain on the differential. Lymphoedema refers to oedema resulting from abnormal lymphatic flow of any cause and presents most commonly as a unilateral non-pitting oedema. There can be associated erythema due to inflammation, but pain and warmth may not be present. Other conditions that can mimic infections include contact dermatitis and papular urticaria, both relating to a dermal sensitivity reaction to an allergen or insect bite22. Generally, addressing other factors such as systemic signs, laboratory tests and occasionally biopsy can assist in making a diagnosis in more challenging cases23.
Table 1. Characteristics of non-infectious mimics of cellulitis
The differentiation between erysipelas and cellulitis is often challenging, but often not clinically relevant. Erysipelas, by definition, involves the superficial epidermis, whereas cellulitis involves the dermis and subcutaneous tissues24. Cellulitis and erysipelas both have similar clinical presentations; however, cellulitis usually presents as a flat, erythematous patch. Erysipelas, however, may be raised and tends to be more well demarcated than cellulitis, with clear margins between infected and uninfected skin25. Additionally, erysipelas is more classically described in the face25. In light skinned individuals, lesions also differ in colour, with cellulitis being more pink and erysipelas being described classically as ‘salmon-red’. Clinically, both erysipelas and cellulitis are treated with similar agents and for similar duration24.
A final important differential consideration are necrotising SSTI, including necrotising fasciitis. While erythematous skin changes are common to both, necrotising fasciitis tends to be exquisitely painful, beyond what the clinician would expect of the skin changes present. In contrast to cellulitis, there are often systemic symptoms, including fever, hypotension, tachycardia or altered level of consciousness, but these findings may be late in the disease process26. Additionally, there may be blisters, bullae, skin discolouration, crepitus (presence of gas under the skin), pain, and rapid extension of erythema within hours26.
Therapy
The degree of clinical severity determines the type of treatment that is needed for cellulitis; a guideline detailing treatment approaches can be found elsewhere24. Cases of cellulitis that lack systemic signs of infection (i.e. fever, tachycardia) can be treated with an oral antimicrobial agent that is active against streptococci alone (mild cases). Moderate–severe cases may require intravenous antimicrobials initially, with a subsequent step down to oral antibiotics after a period of improvement. For severe infections, empiric coverage against methicillin-resistant S. aureus (MRSA) may be considered based on the location of infection, risk factors, and local MRSA prevalence. In purulent cellulitis, incision and drainage may be indicated alongside antimicrobial therapies.
Although classical descriptions exist to differentiate streptococcal and staphylococcal cellulitis, the distinctions are not generally clear and, as such, agents with activity against both are often used. For treatment, penicillins with staphylococcal activity or cephalosporins are frequently used, with the latter also used in cases of penicillin allergy – for severe reactions other classes will be considered. Clinical improvements often lag antimicrobial therapy by 24–48 hours and at times erythema can extend27. In these cases, it is often appropriate to continue with therapy and reassess at 72 hours, when the body’s inflammatory response begins to subside. In the absence of improvement at 72 hours, the diagnosis or choice of therapy may need to be reassessed.
Prevention
As described above, recurrence is a common and costly in cellulitis, with each additional episode causing more inflammatory damage to the lymphatic system, thus perpetuating the problem28. Non-pharmacological prevention options include regular moisturisation, prevention of toe-space infections (tinea pedis), weight loss, regular exercise, and lower leg compression therapy (e.g. compression stockings29). While there is no evidence for topical solutions to prevent cellulitis, topical antibiotic ointments have been shown to reduce infection in acute lacerations and wounds28,30. After initiating the non-pharmacological options above, if recurrent cellulitis remains an issue, low dose suppressive penicillin has been shown to be effective in preventing recurrent cellulitis27.
Case studies
Case 1
Ms Lee is a 35-year-old otherwise healthy woman who presents with a 2-day history of fever, redness, pain and swelling around her left ear. There was no recent trauma or injury. There is no previous history of dermatologic ailments in the head or neck, including eczema. Physical examination reveals a fever of 38.5˚C, heart rate of 90 beats per minute, and blood pressure of 95/60 (normal). Examination of the left ear itself reveals a normal tympanic membrane with no drainage or lesions. There is marked erythema and induration around the left ear with tender pre-auricular nodes. Note is made of an ‘ear pit’ or preauricular sinus proximal to the tragus of the left ear (Figure 1).
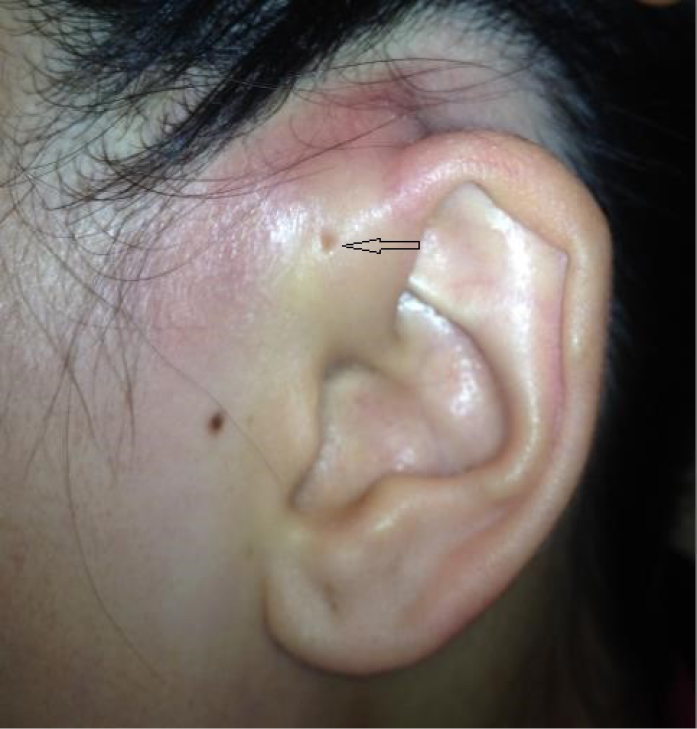
Figure 1. Case study 1.
On further questioning, Ms Lee reveals that her mother had a similar sinus which became infected in her 30s and required surgical removal. Ms Lee is initiated on cefazolin 2g IV every 8 hours for 72 hours via home parenteral pump after which she has a 40% improvement. She is stepped down to cephalexin 500mg PO four times daily for 4 days to complete a total 7-day course. She is also referred to the otolaryngology service for consideration of surgical removal of the sinus once her symptoms are resolved.
In case 1, we see an atypical presentation of cellulitis of the outer ear, with the likely risk factor being the anatomical variant described. Therapy targeting staphylococci and streptococci yield clinical improvement. To prevent recurrence, surgical consultation and intervention may be required.
Case 2
Mr Brown is a 56-year-old businessman with no past medical history and no obesity. He presents to the emergency department with a 48-hour history of swollen, erythematous and painful left lower leg after a month-long trip to Turkey. He has just returned home after an >8-hour flight. Pain began prior to the flight but has worsened in recent days. In the emergency department he is mildly tachycardic (HR105), normotensive and afebrile. Other haemodynamic markers are within normal limits. His blood work demonstrates a white blood cell count of 16,000 with elevated CRP. Other laboratorial parameters are within normal limits. A doppler ultrasound of the left leg rules out deep vein thrombosis.
There is no preceding trauma or injury, and no apparent risk factors for cellulitis. The ED physician makes a diagnosis of cellulitis based on the patients presenting clinical history of a swollen, painful erythematous lower leg and exclusion of DVT. He is started on cefazolin 2g IV every 8 hours and discharged home via home parenteral pump. He is followed up in clinic and after 5 days of parenteral therapy has not improved. Treatment is broadened with anti-MRSA therapy in the form of Doxycycline and 3 days later improvement is minimal. Additional history obtained elucidates frequent swimming in pools and fresh/saltwater lakes while abroad. The decision is made to discontinue parenteral therapy at the patient’s request. He is started on highly bioavailable oral ciprofloxacin for empiric gram-negative coverage in addition to the gram-positive/MRSA coverage provided by doxycycline. Five days later, the redness, erythema and swelling have reduced 80%. He completes a 7-day course of this combined therapy.
Case 2, on the other hand, introduces two unique considerations. The first is the need to rule out possible differentials, in this case deep vein thrombosis, given the history of long-haul flight. The second consideration are organisms beyond staphylococci and streptococci. As discussed, improvement with typical therapies should be seen within 72 hours. When this has not occurred, re-examining the history and differential is often important. Here, a history of multiple water exposures has been uncovered, leading the clinician to consider therapies targeting gram-negative and environmental pathogens. The ultimate improvement once on anti-gram-negative therapy confirms the diagnosis.
Conclusion
Cellulitis and SSTI are an increasing burden to the healthcare system world-wide, owing to the rise in age and comorbidities. Diagnosis and management present major challenges given the absence of gold standard, inter-clinician variability, and the large number of mimics. However, emerging evidence around prevention provides an unique opportunity to prevent morbidity and avoid additional healthcare costs.
Conflict of Interest
The authors declare no conflicts of interest.
Funding
The authors received no funding for this study.
深入研究皮肤和软组织感染(SSTI) 第三部分:关注蜂窝织炎
Caley Shukalek, Vidhi Desai, Brandon Christensen, Christopher Lata, Ranjani Somayaji
DOI: https://doi.org/10.33235/wcet.43.2.24-28
摘要
在本系列文章的第三部分(第一部分WCET®杂志第36卷第2期Å\Å\2016年4月/6月:PP29-34;第二部分WCET®杂志第37卷第3期Å\Å\2017年7月/9月:PP20-24)对皮肤和软组织感染(SSTI)、蜂窝织炎综合征进行了探讨。本文讨论了临床医生诊断和管理蜂窝织炎的基于病例的方法。
引言
皮肤和软组织感染(SSTI)代表了一系列疾病谱,从轻度浅表感染(如丹毒和蜂窝织炎)到深层筋膜感染(如坏死性筋膜炎)。其表现形式各不相同,但在初级和急症治疗机构中都很常见。SSTI的负担是巨大的,在20世纪90年代末至21世纪初期,由于年龄的増长和肥胖等合并症,SSTI的发病率不断上升1。然而,男性和女性之间没有显著差异2。2010年在美国开展的一项研究显示,SSTI的发病率是UTI的2倍,是肺炎的10倍,发病率高达每年48.5例/1000人3。同样,一项调查1998-2013年间美国蜂窝织炎发病率的研究表明,急性住院率几乎翻了一番,费用总额近37.4亿美元4。与此同时,蜂窝织炎和其他SSTI的一个挑战性层面是诊断,需要依赖于临床病史和体格检查。由于缺乏客观的微生物学或实验室检查,非感染性病原体被误诊为SSTI。这对系统来说成本也很高,一项研究显示,多达30%的下肢蜂窝织炎患者被误诊,估计成本在1.95-5.15亿美元之间5。
已经在努力开发诊断蜂窝织炎的辅助工具;然而,鉴于替代性诊断的异质性,在开发Åg金标准Åh诊断方法和适当的比较工具方面仍然存在挑战。2019年的一项系统综述发现几种有助于诊断的工具;但是,这些一个工具均未在下肢蜂窝织炎方面得到充分验证6。
如前所述,SSTI通常由皮肤屏障的轻微浅表创伤引起7,8。创伤的形式可以是皮肤的外部损伤,慢性静脉功能不全或炎症4,9,10。
蜂窝织炎概述
临床表现
蜂窝织炎是一种快速进展的SSTI,累及真皮和皮下组织11。症状通常包括急性发病的红肿、发热、水肿和疼痛,但偶尔也会出现全身症状,如发烧和寒战。最常见的是下肢蜂窝织炎,在所有蜂窝织炎中的比例高达39.9% 12。如前所述,感染发生之前通常有外伤、炎症或水肿造成的皮肤表面损害。较少见的蜂窝织炎可能是由于来自血流或邻近来源的感染扩散(即脂肪组织中的脓肿向外扩张)2。
原发性/首次发作蜂窝织炎存在许多风险因素,包括无家可归、高龄、肥胖、皮肤破溃(溃疡、炎症、外伤)、水肿/淋巴水肿、趾蹼感染(真菌、细菌)、静脉功能不全和既往静脉切除术等13。同时,复发性蜂窝织炎的风险因素包括肥胖、足癣、水肿/淋巴水肿和静脉功能不全,还包括吸烟、恶性肿瘤和既往蜂窝织炎1。原发性蜂窝织炎的复发率很高,但范围因研究而异,有些报告为8%-20% 12,而另一些报告显示,当存在风险因素时,复发率为22%-49% 1。
非坏死性和非化脓性感染很少引起死亡1。然而,据报告,蜂窝织炎的估计总死亡率为1.1%,尽管感染本身可能只是最多三分之一病例的罪魁祸首1,14。绝大多数感染是由金黄色葡萄球菌和链球菌引起的,在一项研究中,在微生物学诊断得到证实的情况下,这两个致病菌群的培养率为97% 13。
目前已经开发了多种评分系统,包括ERON15和改良的Dundee分类,这些系统已经被纳入英国CREST指南16。然而,这些标准尚未得到广泛采用,并且有人批评其过于简化或在区分严重程度方面缺乏临床稳健性11,17。
有趣的是,最近的研究表明,蜂窝织炎的发病率会因季节而异。在丹佛开展的一项研究表明,在温暖的月份,原发性蜂窝织炎的入院率有上升的趋势,与寒冷的冬季相比,7月份的感染几率要高66.63%18。同时,在台湾西南部开展的一项研究显示,在台风过后的几天内,下肢蜂窝织炎的发病率有所増加,这表明容易发生洪水和过多降水的气候可能会使居住者因暴露于被污染的水而面临由肠道、革兰氏阴性和水源性微生物引起的蜂窝织炎的风险19。一种解释可能是四肢长期浸泡,从而损害了天然的宿主防御系统,为通过皮肤表面进入身体提供了便利19。此外,在气候灾害期间,动物和昆虫的叮咬也可能导致感染率増加18。
发病机理
一旦皮肤表面发生浅表损伤,患者自身皮肤菌群就可能受到细菌污染。这也解释了为什么葡萄球菌和链球菌是蜂窝织炎中最常见的微生物。成功的感染需要三个步骤Å\Å\细菌必须首先粘附在宿主的细胞上,然后在躲避宿主防御的同时侵入组织,最后利用其产毒因子19。细菌侵入皮肤后,会触发细胞因子和中性粒细胞反应。这种表皮反应导致抗菌肽的产生和角质细胞的増殖,这两者都会导致蜂窝织炎的特征性检查结果2。细菌侵入门户并不总是明显的,特别是在有其他风险因素的情况下,蜂窝织炎发生时可能皮肤看似完好。在这些情况下,皮肤上会出现微小的裂缝;这些裂缝会受到刺激或发炎,从而为细菌向内迁移提供了便利20。
临床方法/微生物学
根据临床表现,蜂窝织炎可分为非化脓性和化脓性两种形式。非化脓性蜂窝织炎通常由链球菌引起,表现为单侧、边界不清的温暖红色区域,无脓性分泌物或脓肿。而化脓性蜂窝织炎通常由葡萄球菌引起,一般发生在在伤口、积液或痈周围。二者都会出现周围水肿和触痛,随着感染的进展,水肿和触痛范围会迅速扩大。其他局部特征可包括局部坏死和脓肿形成(蜂窝织炎过程之后),具体取决于细菌种类和感染严重程度。
金黄色葡萄球菌更常与化脓性蜂窝织炎有关,尽管根据感染菌株的毒力因子,这两种细菌都能够产生严重的感染性特征。其他与蜂窝组织炎有关的链球菌还包括B群、C群和G群链球菌Å\Å\这些链球菌在糖尿病患者或血管疾病患者中更为常见。当伤口变成慢性时,皮肤菌群就会转变为由肠道革兰氏阴性菌、厌氧菌或环境病原体定植的混合菌群。由于类似的发病机制,这些微生物可引起感染,通常发生在伤口未经治疗、血液循环不良或糖尿病足溃疡的患者中21。
蜂窝织炎可能涉及更多的非典型生物,包括在动物咬伤、淡水/盐水或水族馆接触中看到的微生物。这些通常通过详细的病史来确定,并且需要更广泛的治疗,这超出了本综述的范围。
鉴别诊断
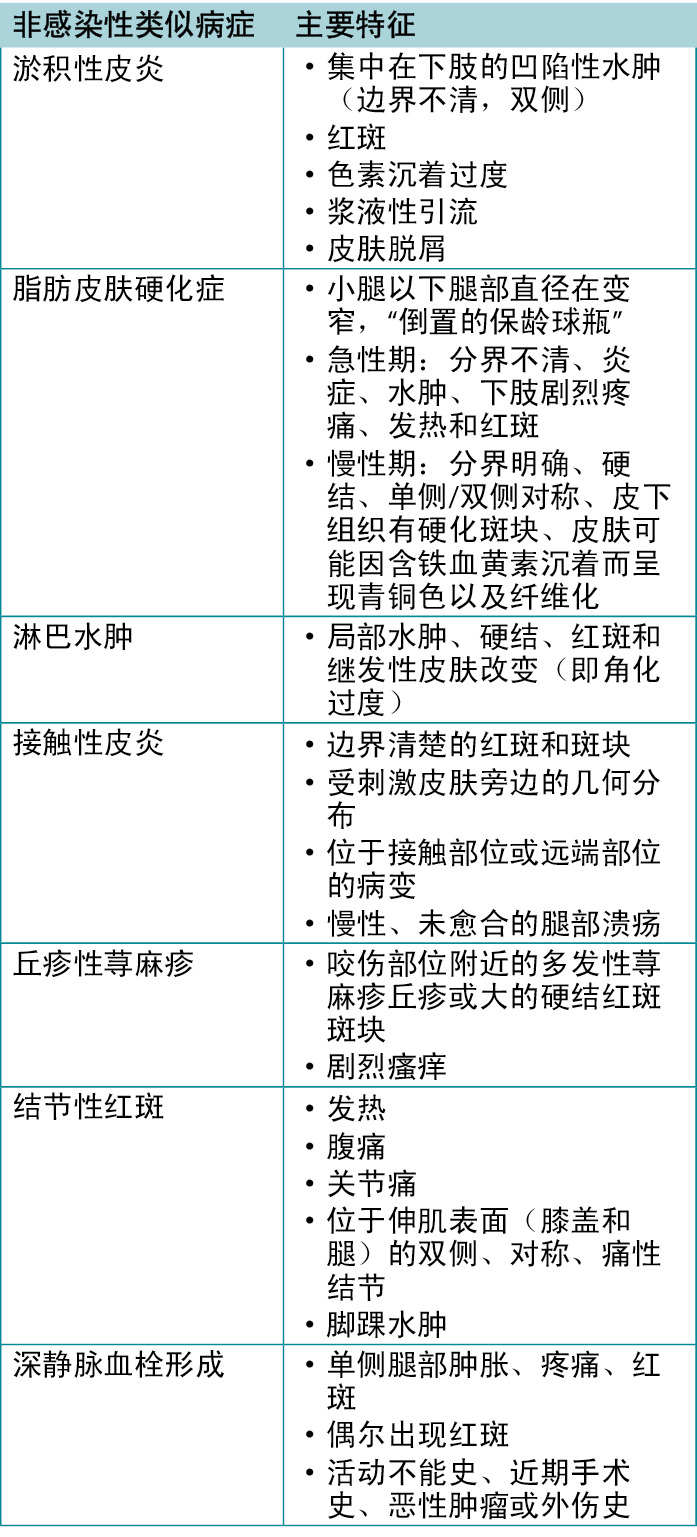
鉴于皮肤病的范围很广,病史和体格检查在很大程度上具有主观性,而且皮肤上会出现非特异性症状(即触痛、红斑、水肿),蜂窝织炎经常被误诊22。类似蜂窝织炎的综合征包括淤积性皮炎、脂肪皮肤硬化症和淋巴水肿;表122概述了这些症状。淤积性皮炎是最常见的蜂窝组织炎类似症状,但它往往发病较慢,而且多为双侧。然而,淤积性皮炎和其他类似症状是SSTI的风险因素,因此,感染应保持在鉴别诊断范围内。淋巴水肿是指由任何原因引起的淋巴流动异常导致的水肿,最常见的表现是单侧非凹陷性水肿。可能伴有炎症引起的红斑,但可能不伴有疼痛和发热。其他类似感染的情况包括接触性皮炎和丘疹性荨麻疹,两者均与皮肤对过敏原或昆虫叮咬的敏感反应有关22。一般而言,考虑其他因素,如全身体征、实验室检查和偶尔的活检,有助于在更具挑战性病例中做出诊断23。
表1.蜂窝织炎的非感染性类似病症的特征
丹毒和蜂窝组织炎的鉴别往往具有挑战性,但通常没有临床相关性。根据定义,丹毒累及表皮浅层,而蜂窝织炎累及真皮和皮下组织24。蜂窝织炎和丹毒的临床表现相似;但是,蜂窝织炎通常表现为扁平的红斑斑块。然而,丹毒可能会升高,而且往往比蜂窝织炎的界限更清楚,感染和未感染的皮肤之间有明显的界限25。此外,丹毒更典型地表现在面部25。在浅色皮肤的人群中,病变的颜色也有所不同,蜂窝组织炎多为粉红色,丹毒则通常被描述为Åg鲑鱼红色Åh。在临床上,丹毒和蜂窝织炎使用的治疗药物相似,治疗持续时间也相似24。
最后一个重要的鉴别考虑因素是坏死性SSTI,包括坏死性筋膜炎。虽然红斑性皮肤改变对两者来说都很常见,但坏死性筋膜炎往往会带来剧烈的疼痛,超出了临床医生对所出现的皮肤改变的预期。与蜂窝织炎相反,坏死性筋膜炎常常有全身症状,包括发热、低血压、心动过速或意识水平改变,但这些症状可能在疾病过程的后期才出现26。此外,还可能出现水泡、大疱、皮肤变色、压轧感(皮下有气体)、疼痛以及数小时内红斑迅速扩大26。
治疗
临床严重程度决定了蜂窝织炎所需的治疗类型;详细的治疗方法指南可在其他文章中找到24。无全身感染症状(即发热、心动过速)的蜂窝织炎病例可以用仅对链球菌有效的口服抗菌剂治疗(轻度病例)。中度至重度病例最初可能需要静脉注射抗菌药物,经过一段时间的改善后,逐渐改为口服抗生素。对于严重感染,可以根据感染的位置、风险因素和当地MRSA患病率,根据经验考虑抗耐甲氧西林的金黄色葡萄球菌(MRSA)治疗。在化脓性蜂窝织炎中,切开引流可能需要与抗菌治疗同时进行。
虽然有经典的描述来区分链球菌性和葡萄球菌性蜂窝织炎,但其区别通常并不明确,因此经常使用对两者都具有活性的药物。在治疗方面,经常使用具有葡萄球菌活性的青霉素类药物或头孢类药物,后者也用于青霉素过敏的情况Å\Å\对于严重的反应,将考虑使用其他类别的药物。临床改善通常滞后于抗菌治疗24-48小时,有时红斑会扩大27。在这些情况下,通常适合继续治疗并在72小时后重新评估,因为此时身体的炎症反应开始消退。如果在72小时内没有改善,则可能需要重新评估诊断或治疗方案。
预防
如上所述,复发对于蜂窝织炎而言很常见,而且代价很高,每一次额外的发作都会对淋巴系统造成更多的炎症损害,从而使问题长期存在28。非药物预防方案包括定期保湿、预防趾间感染(足癣)、减肥、定期锻炼和小腿加压疗法(如压力袜29)。虽然没有证据表明外用药水可以预防蜂窝组织炎,但外用抗生素软膏已被证明可以减少急性撕裂伤和伤口的感染28,30。在开始上述非药物治疗方案后,如果复发性蜂窝织炎问题仍然存在,低剂量抑制性青霉素已被证明可有效预防复发性蜂窝织炎27。
病例研究
病例1
李女士是一名35岁的健康女性,有两天的发烧史,左耳周围发红、疼痛、肿胀。最近无外伤或损伤。既往无头部或颈部皮肤病病史,包括湿疹。体格检查显示发烧38.5ÅãC,心率90次/分钟,血压95/60(正常)。对左耳本身的检查显示鼓膜正常,无引流或病变。左耳周围有明显的红斑和硬结,耳前结节有触痛。注意到左耳耳廓附近有一个Åg耳坑Åh或耳前窦(图1)。
在进一步的询问中,李女士透露,她的母亲也有一个类似的窦,在她30多岁时受到感染,需要手术切除。李女士开始通过家用注射泵接受头孢唑啉2 g静脉注射,每8小时一次,持续72小时,之后她的症状有40%的改善。她逐渐减量为头孢氨苄500 mg,每天四次,连续4天,完成总共7天的疗程。她还被转到耳鼻喉科,以便在症状得到解决后考虑通过手术切除窦。
在病例1中,我们看到外耳蜂窝织炎的非典型表现,可能的风险因素是所述的解剖学变化。针对葡萄球菌和链球菌的治疗获得了临床改善。为了防止复发,可能需要进行外科会诊和干预。

图1.病例研究1。
病例2
布朗先生是一位56岁的商人,没有既往病史,也没有肥胖症。他在土耳其旅行一个月后,因左小腿肿胀、红斑和疼痛48小时而来到急诊科就诊。在经过8个小时以上的飞行后,他刚刚回到家中。疼痛坐飞机前就开始了,但在最近几天加重了。在急诊科,他有轻微的心动过速(HR105),血压正常,无发热。其他血液动力学指标均在正常范围内。他的血液检查结果显示白细胞计数为16,000,CRP升高。其他实验室检查参数均在正常范围内。左腿经多普勒超声检查排除了深静脉血栓形成。
既往无外伤或损伤,也无明显的蜂窝织炎风险因素。急诊科医生根据患者小腿肿胀、疼痛、红斑的临床病史以及排除深静脉血栓的情况,诊断为蜂窝织炎。他开始通过家用注射泵接受头孢唑啉2 g静脉注射,每8小时一次,并出院回家。他在诊所接受了随访,经过5天的注射治疗后,情况没有改善。用多西环素形式的抗MRSA治疗扩大了治疗范围,但3天后改善甚微。获得的其他病史表明,患者在国外时经常在游泳池和淡水/盐水湖中游泳。在患者的要求下,决定停止注射治疗。除了用多西环素进行抗革兰氏阳性菌/MRSA的治疗外,他还开始使用高生物利用度的口服环丙沙星,作为经验性的抗革兰氏阴性菌治疗。五天后,发红、红斑和肿胀已减少80%。他完成了为期7天的联合治疗疗程。
另一方面,病例2引入了两个独特的考虑因素。首先是需要排除可能的鉴别诊断,在本例中,考虑到长途飞行的历史,需要排除深静脉血栓形成。第二个考虑因素是葡萄球菌和链球菌以外的微生物。如上文所述,使用典型的治疗方法应该在72小时内看到改善。当没有改善时,重新检查病史和鉴别诊断往往很重要。在本例中,发现了多次接触水的历史,因此临床医生考虑针对革兰氏阴性和环境病原体的治疗。抗革兰氏阴性菌治疗后的最终改善证实了诊断。
结论
由于年龄的増长和合并症的増加,蜂窝织炎和SSTI对全世界的医疗系统来说是一个越来越大的负担。由于缺乏金标准、临床医生之间的差异性以及大量的类似病症,诊断和管理面临着重大挑战。然而,围绕预防的新证据提供了一个独特的机会来预防发病和避免额外的医疗费用。
利益冲突声明
作者声明无利益冲突。
资助
作者未因该项研究收到任何资助。
Author(s)
Caley Shukalek†
MD, MSc
Department of Medicine, University of Calgary, Calgary, AB, Canada
Vidhi Desai†
BSc student
University of Calgary, Calgary, AB, Canada
Brandon Christensen
MD
Department of Medicine, University of Calgary, Calgary, AB, Canada
Christopher Lata
MD, MSc
Department of Medicine, University of Calgary, Calgary, AB, Canada
Ranjani Somayaji*
MD, MPH
Assistant Professor, Departments of Medicine and Microbiology, Immunology & Infectious Disease, Cumming School of Medicine, University of Calgary, 3330 Hospital Drive NW,
Calgary, AB T2N 4N1, Canada
Email rsomayaj@ucalgary.ca
* Corresponding author †Co-first authors
References
- Raff AB, Kroshinsky D. Cellulitis: a review. JAMA 2016;316:325–37.
- Brown BD, Hood Watson KL. Cellulitis. Treasure Island (FL): StatPearls Publishing; 2022 [cited 2022 May 31]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK549770/
- Miller LG, Eisenberg DF, Liu H, Chang CL, Wang Y, Luthra R, et al. Incidence of skin and soft tissue infections in ambulatory and inpatient settings, 2005–2010. BMC Infect Dis 2015 Aug 21;15(1).
- Peterson RA, Polgreen LA, Cavanaugh JE, Polgreen PM. Increasing incidence, cost, and seasonality in patients hospitalized for cellulitis. Open Forum Infect Dis 2017 Jan 1;4(1).
- Weng QY, Raff AB, Cohen JM, Gunasekera N, Okhovat JP, Vedak P, et al. Costs and consequences associated with misdiagnosed lower extremity cellulitis. JAMA Dermatol 2017 Feb 1;153(2):141–6.
- Patel M, Lee SI, Akyea RK, Grindlay D, Francis N, Levell NJ, et al. A systematic review showing the lack of diagnostic criteria and tools developed for lower-limb cellulitis. Br J Dermatol 2019;181:1156–65.
- Somayaji R. Approach to skin and soft tissue infections. WCET J 2016;36(2):20–4.
- Shukalek C, Parsons L, Somayaji R. Delving into skin and soft tissue infections (SSTI). Part II: Focus on superficial infections. WCET J 2017 Jul;37(3):20–4.
- Dupuy A, Benchikhi H, Roujeau JC, Bernard P, Vaillant L, Chosidow O, et al. Risk factors for erysipelas of the leg (cellulitis): case-control study. BMJ 1999 Jun 12;318:1591–4.
- Björndóttir S, Gottfredsson M, Thórisdóttir AS, Gunnarsson GB, Ríkardsdóttir H, Kristjánsson M, et al. Risk factors for acute cellulitis of the lower limb: a prospective case-control study. Clin Infect Dis 2005 Nov 15 [cited 2022 Oct 1];41(10):1416–22. Available from: https://pubmed.ncbi.nlm.nih.gov/16231251/
- Sullivan T, de Barra E. Diagnosis and management of cellulitis. Clin Med 2018 Apr 1 [cited 2022 Oct 1];18(2):160. Available from: /pmc/articles/PMC6303460/
- Ellis Simonsen SM, van Orman ER, Hatch BE, Jones SS, Gren LH, Hegmann KT, et al. Cellulitis incidence in a defined population. Epidemiol Infect 2006 Apr;134(2):293–9.
- Jenkins TC, Sabel AL, Sarcone EE, Price CS, Mehler PS, Burman WJ. Skin and soft-tissue infections requiring hospitalization at an academic medical center: opportunities for antimicrobial stewardship. Clin Infect Dis 2010 Oct 15 [cited 2022 Oct 1];51(8):895–903. Available from: https://pubmed.ncbi.nlm.nih.gov/20839951/
- Gunderson CG, Cherry BM, Fisher A. Do patients with cellulitis need to be hospitalized? A systematic review and meta-analysis of mortality rates of inpatients with cellulitis. J Gen Intern Med 2018;33:1553–60.
- Eron LJ, Lipsky BA, Low DE, Nathwani D, Tice AD, Volturo GA. Managing skin and soft tissue infections: expert panel recommendations on key decision points. J Antimicrob Chemother 2003 Nov [cited 2022 Oct 1];52 Suppl 1. Available from: https://pubmed.ncbi.nlm.nih.gov/14662806/
- Fulton R, Doherty L, Gill D. Guidelines on the management of cellulitis in adults. Northern Ireland: CREST; 2005.
- Ki V, Rotstein C. Bacterial skin and soft tissue infections in adults: a review of their epidemiology, pathogenesis, diagnosis, treatment and site of care. Can J Infect Dis Med Microbiol 2008 [cited 2022 Oct 1];19(2):173–84. Available from: https://pubmed.ncbi.nlm.nih.gov/19352449/
- Peterson RA, Polgreen LA, Sewell DK, Polgreen PM. Warmer weather as a risk factor for cellulitis: a population-based investigation. Clin Infect Dis 2017 Oct 1;65(7):1167–73.
- Lin PC, Lin HJ, Guo HR, Chen KT. Epidemiological characteristics of lower extremity cellulitis after a typhoon flood. PLoS One 2013 Jun 13;8(6).
- Habif TP. Cellulitis and erysipelas section of bacterial infections: In: Habif TP. Clinical dermatology: a color guide to diagnosis and therapy. 5th ed. Philadelphia, PA: Mosby; 2010. p. 342–350.
- Pitocco D, Spanu T, di Leo M, Vitiello R, Rizzi A, Tartaglione L, et al. Diabetic foot infections: a comprehensive overview. Eur Rev Med Pharmacol Sci 2019;23(2):26–37.
- Keller EC, Tomecki KJ, Alraies MC. Distinguishing cellulitis from its mimics. Cleve Clin J Med 2012;79:547–52.
- Bailey E, Kroshinsky D. Cellulitis: diagnosis and management. Dermatol Ther 2011;24:229–39.
- Stevens DL, Bisno AL, Chambers HF, Dellinger EP, Goldstein EJC, Gorbach SL, et al. Executive summary: practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin Infect Dis 2014 Jul 15;59(2):147–59.
- Stevens DL, Bryant AE. Streptococcus pyogenes: basic biology to clinical manifestations: impetigo, erysipelas and cellulitis. University of Oklahoma Health Sciences Center; 2016 [cited 2022 May 31]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK333408
- Bystritsky R, Chambers H. Cellulitis and soft tissue infections. Ann Intern Med 2018 Feb 6;168(3):ITC17–31.
- Thomas KS, Crook AM, Nunn AJ, Foster KA, Mason JM, Chalmers JR, et al. Penicillin to prevent recurrent leg cellulitis. N Engl J Med 2013 May 2 [cited 2022 Oct 1];368(18):1695–703. Available from: https://pubmed.ncbi.nlm.nih.gov/23635049/
- Dire DJ, Coppola M, Dwyer DA, Lorette JJ, Karr JL. Prospective evaluation of topical antibiotics for preventing infections in uncomplicated soft-tissue wounds repaired in the ED. Acad Emerg Med 1995 Jan 1 [cited 2022 Oct 1];2(1):4–10. Available from: https://onlinelibrary.wiley.com/doi/full/10.1111/j.1553-2712.1995.tb03070.x
- Webb E, Neeman T, Bowden FJ, Gaida J, Mumford V, Bissett B. Compression therapy to prevent recurrent cellulitis of the leg. New Eng J Med 2020 Aug 13;383(7):630–9.
- Singer AJ, Dagum AB. Current management of acute cutaneous wounds. N Engl J Med 2008 Sep 4 [cited 2022 Oct 1];359(10):1037–46. Available from: https://pubmed.ncbi.nlm.nih.gov/18768947/


