Volume 29 Number 3
Measurement of toe systolic pressures: a technique paper
Peta Tehan, Martin Fox and Joseph L Mill
Keywords vascular assessment, toe systolic pressure, toe-brachial index, technique, podiatry, woundcare
For referencing Tehan P et al. Measurement of toe systolic pressures: a technique paper. Wound Practice and Research 2021; 29(3):148-153.
DOI
https://doi.org/10.33235/wpr.29.3.148-153
Submitted 2 June 2021
Accepted 30 June 2021
Abstract
Background Toe systolic pressures (TSP) are an essential part of a lower limb wound assessment. They assist wound clinicians ascribe aetiology, and predict wound healing capacity, assisting with triage and ongoing care planning.
TSP method The toe cuff should be placed around the base of the hallux securely. The photoplethysmography (PPG) probe should be placed on the distal pulp of the hallux so that the probe is flush with the skin surface, affixed with tape. Wait for a strong cyclical signal from the probe to appear on the Doppler screen. Next, inflate the cuff, taking care not to overinflate. Once a flat line with the PPG signal is obtained on the screen, inflate a further 20mmHg. Then, slowly deflate the cuff at a rate of 2–5mmHg per second. The first regular cyclical waveform on the screen is the point at which the pressure in the artery is greater than the pressure in the cuff, and is considered the TSP.
Discussion TSP are a valuable measure to include in wound practice. TSP are an important part of wound staging systems, can predict wound healing capacity and help guide ongoing management.
Background
Toe systolic pressures (TSP) are an essential part of a thorough lower limb wound assessment1. They assist wound clinicians ascribe aetiology, and predict wound healing capacity, both of which assist with triage and ongoing care planning1. TSP are normally performed in combination with other vascular testing methods, such as pulse palpation, continuous wave Doppler waveform analysis, and ankle-brachial indices (ABI). TSP were first described by Lezack and Carter in 19702 and are now included in international guidelines for wound management3, lower extremity vascular assessment4, and wound grading and prediction systems such as WIfI (wound, ischaemia, foot infection)5.
TSP are considered advantageous compared to ABI in specific patient cohorts because digital arteries are less likely impacted by medial arterial calcification (MAC)6,7. MAC is the formation of hydroxyapatite in the tunica media of the artery which leads to stiffening and loss of elasticity in the vessel and can render them incompressible8. This most commonly occurs in the calf vessels and is known to interfere with the accuracy of lower limb vascular testing methods, and also makes some surgical interventions more challenging9. In subgroups of people with diabetes, such as those with severe renal disease and advanced age (>75 years), MAC is more prevalent10–12; therefore, TSP are generally preferred as predictors of wound healing over ankle pressures in these populations1.
TSP are generally considered to be approximately 6–10mmHg less than a brachial pressure due to the increased distance from the heart compared to brachial pressures and the smaller size of the digital arteries13. TSP are also used in calculations of toe-brachial indices (TBIs), which have been demonstrated to be a valuable adjunctive vascular testing method for identifying peripheral artery disease (PAD)14.
TSP of less than 50mmHg are generally indicative of reduced healing capacity in foot wounds, with lower values associated with worse outcomes, including higher likelihood of non-healing and amputation15. Chronic limb threatening ischaemia (CLTI) is considered when a patient presents with tissue loss, ischaemic rest pain, and a TSP of less than 60mmHg16. However, there is little data available on what a normal TSP should be, with estimates ranging from 70–100mmHg17,18, and since an upper limit has not yet been established, it is currently unclear what the TSP of a digit affected by MAC would be. Generally, TSP values of greater than 140mmHg in the absence of hypertension could be considered elevated and clinicians could hold a high suspicion of MAC in the digital arteries. However, it should be noted that there is a clear lack of evidence to guide practice on this particular aspect of TSP interpretation, so interpretation of high values should be done with caution. TSP should always be correlated with the pedal and digital arterial waveforms, and interpreted in the context of other vascular testing methods, the patient, and their wound or other presenting clinical picture.
Measurement of TSP has been demonstrated to have good levels of inter- and intra-tester reliability19,20. TSP are also good predictors of wound healing capacity in diabetic foot ulceration21 and chronic foot ulceration19, and predicting outcomes post minor amputation22. As the evidence for their use continues to grow, TSP are increasingly being recognised for their clinical utility; however, many clinicians remain uncertain on the measurement technique23,24. This narrative review aims to clarify the technical aspects of TSP measurement for clinicians who manage lower limb wounds.
TSP measurement technique
Test conditions
There are a number of environmental and physical factors which need to be observed in order to optimise accuracy of TSP measurement. The room temperature should be controlled, and ideally be between 22–24˚Celsius25. Skin blood flow is influenced by temperature, with cold temperatures inducing vasoconstriction which will unduly influence the TSP, leading to an inaccurate reading. The patient should also be rested in a supine position for a minimum of 10 minutes prior to the measurement being taken to stabilise blood pressure26. Maintaining this supine position during measurement is also important to ensure the toes are at the same level as the heart.
Other factors known to influence accuracy of measurement include smoking, caffeine intake, and exercise in the 2 hours prior to measurement27, therefore these should also be avoided to optimise the accuracy of the measurement. Limb tremor, sudden movement and hyperaemia are also known to interfere with the photoplethysmography (PPG) signal28. Finally, vaso-neural disorders such as Raynaud’s phenomenon are known to influence TSP measurement, with structural and functional changes in the arteries29 leading to potential reductions in TSP readings.
Equipment
Specialised equipment is required to measure TSP and has been cited as one of the barriers to measurement by clinicians23,24. Several different types of equipment can be used to measure TSP, including PPG mercury strain gauge, laser Doppler, and continuous wave Doppler. The most commonly used is the PPG which is an infra-red probe which uses opacity changes, and changes in blood volume, to produce a waveform30.
A sphygmomanometer and digital pneumatic cuff are also required, and care should be taken to choose the right sized cuff for the digit being measured. The cuff should be approximately 1.5 times the diameter of the digit. Cuffs that are too small will result in falsely high pressures and cuffs that are too large will result in erroneously low pressures31. Readily available cuffs come in three sizes, 1.5, 1.9 and 2.5cm, with 2.5cm suitable for most halluces and 1.5cm suitable for most other digits32. A Doppler unit that is compatible with the probe is required, and a range of equipment is currently available. Manual measures are currently preferred33; however, some automated devices have been validated and shown to have adequate levels of reliability and accuracy34. With practice, a manual TSP measurement should take less than 5 minutes to perform including the application and removal of testing equipment.
Technique
Normal hand hygiene should be followed and wearing of gloves is recommended, particularly in wounded patients. The cuff should be placed around the base of the hallux carefully, ensuring that no excess pressure is exerted on the digit whilst not inflated, however sufficiently firm to keep it in place. Care should also be taken with placement of the PPG lead to minimise any effect of lead movement or sway on the signal. In the absence of a hallux, or if a wound prevents placement of the probe, the second digit can be used as a substitute and has been shown to be equivalent32.
There is currently a lack of evidence for the accuracy of other lesser digits; however, smaller digits make cuff position and PPG placement challenging. In these cases, other methods of non-invasive vascular assessment can be considered, such as ankle pressures or continuous wave Doppler, or further information from vascular imaging and/or a vascular consultant could be sought, particularly if the limb appears threatened with the overall clinical presentation.
The PPG probe should be placed on the distal pulp of the hallux so that the probe is flush with the skin surface to ensure that no light can enter the underside of the probe (Figure 1). The PPG can be taped into place with hypoallergenic tape. It is important to ensure that the leads are positioned well, so as not to be moved during inflation of the cuff. The PPG probe is very sensitive to movement, so movement from the patient and also from an inflating cuff can result in interference to the waveform on the screen.
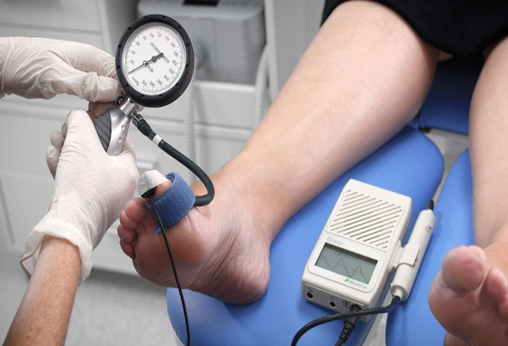
Figure 1. PPG probe placement and PPG waveform
Wait for a strong cyclical signal from the probe to appear on the Doppler unit screen (Figure 1). The PPG waveform can be interpreted qualitatively, with dampening, delay and diminishment of the PPG waveform occurring with increasing severity of PAD35. Next, inflate the cuff, taking care not to overinflate as the smaller sized cuff does not take much effort to inflate and can easily burst if overinflated. Do not inflate the cuff greater than 250mmHg to avoid damaging the cuff. The waveform should generally flatten between 150–200mmHg; this may be lower in a severely diseased patient. Once a flat line with the PPG signal is obtained on the screen, inflate a further 20mmHg. Then, slowly deflate the cuff at a rate of 2–5mmHg per second. The first regular cyclical waveform (blip) on the screen (Figure 2) is the point at which the pressure in the artery is greater than the pressure in the cuff, and is considered the TSP.
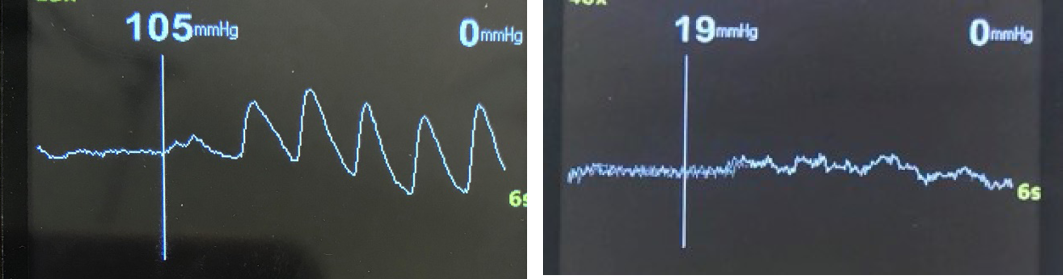
Figure 2. Two examples of the return of the PPG waveform – a TSP of 105mmHg and a TSP of 19mmHg
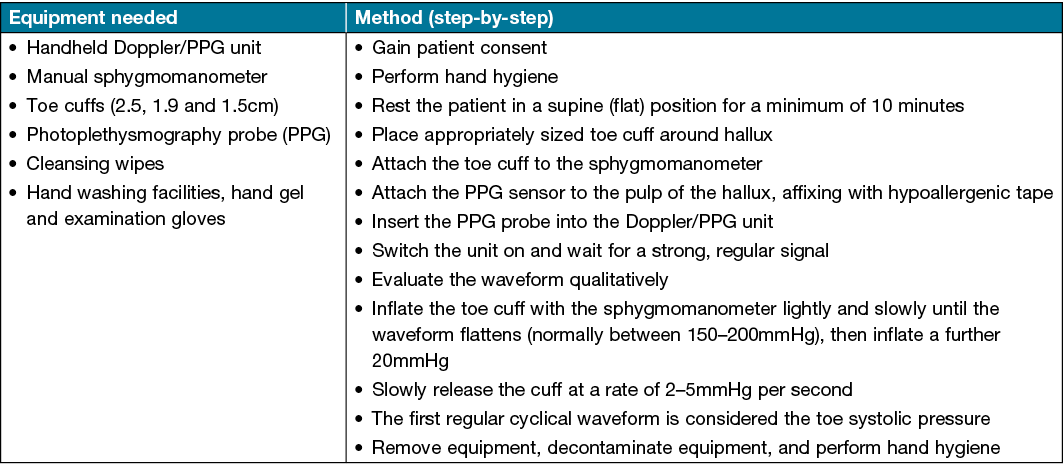
This is often quite small and subtle, and commonly missed by novices. Another common error is waiting for the waveform to return to its baseline size and shape – this will result in an erroneous measure (value lower than the actual TSP). If an error is made, and the pressure needs to be re-measured, the clinician should wait 3 minutes before taking a subsequent measure. This will avoid a hyperaemic response which will artificially increase the TSP value temporarily36. Once satisfied with the TSP measurement, release the sphygmomanometer pressure and remove equipment, ensuring that all equipment is decontaminated with an alcohol or neutral wipe at the end of the testing procedure. A brief step-by-step guide for TSP measurement, including equipment required, is located in Table 1.
Table 1. Brief instructions for TSP measurement
Use of TSP in international guidelines
TSP are a useful clinical test and as such are included in multiple international guidelines for wound and vascular assessment. The International Working Group for the Diabetic Foot (IWGDF) guidelines suggest clinical vascular examination of all patients with diabetes and foot ulceration be conducted in order to identify PAD3, with TSP of less than or equal to 30mmHg necessitating urgent imaging and revascularisation. Other international guidelines, such as the American Heart Association guidelines for lower extremity PAD4, stipulate the use of TSP as a useful adjunct test to identify significant arterial occlusive disease. Wound grading and prediction systems such as WIfI5 use and prefer TSP measurement to fulfil the system’s risk stratifications including risk of amputation and benefit of revascularisation. The global guidelines on CLTI mandate assessment of foot perfusion in patients with diabetic foot ulcer and/or those suspected of having CLTI (presence of PAD in combination with rest pain, gangrene or lower limb ulceration >2 weeks’ duration)16. These guidelines suggest that assessment should include TSP and TBI, with TBI values of less than 0.7 considered abnormal, and TSP values less than 30mmHg typically associated with advanced ischaemia. This recommendation was made to ensure that CLTI is not missed in such patients for whom a foot ulcer may be a precursor to amputation. As the evidence for TSP and TBI continue to grow, the uptake into clinical guidelines will also likely increase.
Care planning and onward referral
Ideally, foot wound and vascular care would be provided as part of an integrated, multidisciplinary limb salvage team, but this is not possible or practical in all clinical settings. This is particularly the case in areas of rural and remote Australia, where specialised integrated services are lacking. Pulse examination alone is necessary but insufficient to evaluate perfusion in the wounded lower limb9. A validated limb-staging system such as WIfI should be used to stage the limb and its predicted risk of amputation, as well as to guide initial therapy. In patients with active foot wounds, the preferred measurement of foot perfusion is TSP, although ABIs may be used in situations where they can be expected to be reliable, such as in patients who have compressible vessels without MAC.
WIfI staging is based on grading the wound, the perfusion, and the presence and severity of wound infection. The combination of grades in each of these three domains is used to place the patient into clinical stages (1 to 4) that correlate with 1-year amputation risk. Details of this system are available in the original publications5,16 and a free mobile application is available to calculate WIfI grades and clinical stages. Any patient with a foot ulcer and ischaemia Grade 2 or 3, as well as any patient with WIfI Clinical Stage >2 should be referred urgently to a vascular surgeon/specialist for further assessment of PAD. Where the wound assessment takes place in remote areas, locations far away from vascular departments, during times such as the recent COVID pandemic, or when the clinician is unsure of severity, a timely telemedicine consultation with the vascular team, including any WIfI results on hand, may be helpful to assist decision-making, triage, and avoidance of amputations.
Clinical Stage 1 patients, and those patients with Grade 0 ischaemia based on TSP >60mmHg, will usually heal with offloading and optimal wound care. However, even in these cases, vascular referral should be considered if wound healing stalls, or fails to occur, after 4–6 weeks of optimal care. In addition, it should be noted that TSP reflects toe and forefoot perfusion. There are patients with regional malperfusion of the foot, so these measurements should be interpreted with caution in patients with midfoot and hindfoot ulcers, particularly if the posterior tibial artery may be severely stenosed or occluded37, which may present as a low posterior tibial artery systolic pressure or an absent or a dampened monophasic Doppler waveform signal38.
In people with chronic lower limb oedema, ABI has long been suggested as part of the holistic assessment to determine safety prior to considering the use of compression bandaging or hosiery39, with most guidelines stipulating an ABI of greater than or equal to 0.8 is required to proceed with high-level compression40. TBI has also been advocated where ABI is difficult due to the presence of oedema or in cases of MAC41. Despite these recommendations, TBI seems to be rarely used by lower limb clinicians to date, despite barriers to ABI performance, such as limb oedema, being common in people admitted in hospital for foot ulceration42. Despite little published research to date in this area, TSP may therefore have an important role in the assessment and management of chronic limb oedema, particularly where ABI is unsuitable for the reasons discussed above.
Conclusion
TSP are a valuable vascular testing method to include in wound assessment practice, particularly in patients with active foot ulceration, diabetes or renal disease. TSP are an important part of wound staging systems, and can predict wound healing capacity and help guide ongoing management. TSP play an important part of a comprehensive wound and lower limb vascular assessment and, once learned, are quick and easy to perform.
Conflicts of interest
None of the authors have any conflicts of interest to declare.
Ethics statement
Not applicable.
Funding
The authors received no funding for this study.
Author(s)
Peta Tehan* PhD, FFPM RCPS (Glasg)
Senior Lecturer Podiatry, School of Health Sciences, College of Health, Medicine and Wellbeing, BE130 Health Precinct, University of Newcastle, Ourimbah, NSW 2258 Australia
Email Peta.Tehan@newcastle.edu.au
Martin Fox FFPM RCPS (Glasg), FCPodM
Vascular Specialist Podiatrist, Manchester Leg Circulation Service, Manchester Local Care Organisation, Harpurhey Health Centre
United Kingdom
Joseph L Mills MD, FACS
Professor and Chief, Division of Vascular Surgery and Endovascular Therapy, Michael E DeBakey Department of Surgery, Baylor College of Medicine Houston, Texas, United States of America
* Corresponding author
References
- Tehan PE, Barwick AL, Casey SL, Lanting SM, Chuter VH. Accurate noninvasive arterial assessment of the wounded lower limb: a clinical challenge for wound practitioners. Int J Lower Extrem Wounds 2020;19(3):215–226.
- Lezack J, Carter S. Systolic pressures in the extremities of man with special reference to the toes. Canadian J Physiol Pharmacol 1970;48(7):469–474.
- Hinchliffe RJ, Forsythe RO, Apelqvist J, Boyko EJ, Fitridge R, Hong JP, Katsanos K, Mills JL, Nikol S, Reekers J et al. Guidelines on diagnosis, prognosis, and management of peripheral artery disease in patients with foot ulcers and diabetes (IWGDF 2019 update). Diabetes/Metabolism Res Rev 2020;36 Suppl 1:e3276.
- Rooke TW, Hirsch AT, Misra S, Sidawy AN, Beckman JA, Findeiss LK, Golzarian J, Gornik HL, Halperin JL, Jaff MR et al. 2011 ACCF/AHA focused update of the guideline for the management of patients with peripheral artery disease (updating the 2005 guideline): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am College Cardiol 2011;58(19):2020–2045.
- Mills JL, Sr., Conte MS, Armstrong DG, Pomposelli FB, Schanzer A, Sidawy AN, Andros G. The Society for Vascular Surgery lower extremity threatened limb classification system: risk stratification based on wound, ischemia, and foot infection (WIfI). J Vasc Surg 2014;59(1):220–234.e222.
- Leskinen Y, Salenius JP, Lehtimäki T, Huhtala H, Saha H. The prevalence of peripheral arterial disease and medial arterial calcification in patients with chronic renal failure: requirements for diagnostics. Am J Kidney Dis 2002;40(3):472–479.
- Young M, Adams JE, Anderson GF, Boulton AJM, Cavanagh PR. Medial arterial calcification in the feet of diabetic patients and matched non-diabetic control subjects. Diabetologia 1993;36:615–621.
- Ho CY, Shanahan CM. Medial arterial calcification: an overlooked player in peripheral arterial disease. Arterioscler, Thromb Vasc Biol 2016;36(8):1475–1482.
- Hinchliffe RJ, Brownrigg JR, Apelqvist J, Boyko EJ, Fitridge R, Mills JL, Reekers J, Shearman CP, Zierler RE, Schaper NC. IWGDF guidance on the diagnosis, prognosis and management of peripheral artery disease in patients with foot ulcers in diabetes. Diabetes Metab Res Rev 2016;32 Suppl 1:37–44.
- Suominen V, Rantanen T, Venermo M, Saarinen J, Salenius J. Prevalence and risk factors of PAD among patients with elevated ABI. Eur J Vasc Endovasc Surg 2008;35(6):709–714.
- Potier L, Halbron M, Bouilloud M, Dadon M, Le Doeuff J. Ankle-to-brachial ratio index underestimates the prevalence of peripheral occlusive disease in diabetic patients at high risk for arterial disease. Diabetes Care 2009;32(4):e44.
- Emanuele M, Buchanan B, Abraira C. Elevated leg systolic pressures and arterial calcification in diabetic occlusive vascular disease. Diabetes Care 1981;4(Mar-Apr):289–292.
- Nielsen P, Bell G, Lassen NA. The measurement of digital systolic blood pressure by strain gauge technique. Scand J Clin Lab Investigat 1972;29:371.
- Tehan PE, Bray A, Chuter VH. Non-invasive vascular assessment in the foot with diabetes: sensitivity and specificity of the ankle brachial index, toe brachial index and continuous wave Doppler for detecting peripheral arterial disease. J Diabetes Complicat 2016;30(1):155–160.
- Sonter J, Ho A, Chuter VH. The predictive capacity of toe blood pressure and the toe-brachial index for foot wound healing and amputation: a systematic review and meta-analysis. Wound Practice & Res 2014;22(4):208–217.
- Conte MS, Bradbury AW, Kolh P, White JV, Dick F, Fitridge R, Mills JL, Ricco J-B, Suresh KR, Murad MH. Global vascular guidelines on the management of chronic limb-threatening ischemia. Eur J Vasc Endovasc Surg 2019;58(1):S1-S109, e133.
- Tehan PE, Barwick AL, Sebastian M, Chuter VH. Diagnostic accuracy of resting systolic toe pressure for diagnosis of peripheral arterial disease in people with and without diabetes: a cross-sectional retrospective case-control study. J Foot Ankle Res 2017;10:58.
- Patry J, Laurencelle L, Bélisle J, Beaumier M. Vascular assessment in patients with a lower limb wound: a correlational study of photoplethysmography and laser Doppler flowmetry toe pressure techniques. J Diabetes Sci Technol 2020; https://doi.org/10.1177/1932296820979973.
- Sonter J, Chuter V, Casey S. Intra-tester and inter-tester reliability of toe pressures and toe brachial indices: a systematic review Manuscript submitted for publication; in press.
- Scanlon C, Park K, Mapletoft D, Begg L, Burns J. Interrater and intrarater reliability of photoplethysmography for measuring toe blood pressure and toe-brachial index in people with diabetes mellitus. J Foot Ankle Res 2012;5(13).
- Forsythe RO, Apelqvist J, Boyko EJ, Fitridge R, Hong JP, Katsanos K, Mills JL, Nikol S, Reekers J, Venermo M, et al. Performance of prognostic markers in the prediction of wound healing or amputation among patients with foot ulcers in diabetes: a systematic review. Diabetes/Metabolism Res Rev 2020;36 Suppl 1:e3278.
- Linton C, Searle A, Hawke F, Tehan PE, Sebastian M, Chuter V. Do toe blood pressures predict healing after minor lower limb amputation in people with diabetes? A systematic review and meta-analysis. Diabetes Vasc Dis Res 2020;17(3): doi: 10.1177/1479164120928868.
- Tehan PE, Fox M, Stewart S, Matthews S, Chuter VH. Lower limb vascular assessment techniques of podiatrists in the United Kingdom: a national survey. J Foot Ankle Res 2019;12(1):31.
- Tehan PE, Chuter VH. Vascular assessment techniques of podiatrists in Australia and New Zealand: a web-based survey. J Foot Ankle Res 2015;8(1):71.
- Sawka A, Carter SA. Effect of temperature on digital systolic pressures in lower limb in arterial disease. Circulation 1992;85:1097–1101.
- Sadler S, Hawke F, Chuter V. Effect of pre-rest test duration on toe and ankle systolic pressure measurements. J Foot Ankle Res 2013;6(Suppl 1):13.
- Campbell N, Chockalingham A, Fodor JG, McKay DW. Accurate, reproducible measurement of blood pressure. Canadian Med Assoc J 1990;143(1):19–24.
- Pérez-Martin A, Meyer G, Demattei C, Böge G, Laroche J-P, Quéré I, Dauzat M. Validation of a fully automatic photoplethysmographic device for toe blood pressure measurement. Eur J Vasc Endovasc Surg 2010;40(4):515–520.
- Ho M, Belch JJF. Raynaud’s phenomenon: state of the art 1998. Scand J Rheumatol 1998;27(5):319–322.
- Rose SC. Noninvasive vascular laboratory for evaluation of peripheral arterial occlusive disease: Part I: hemodynamic principles and tools of the trade. J Vasc Interv Radiol 2000;11(9):1107–1114.
- Påhlsson H-I, Laskar C, Stark K, Andersson A, Jogestrand T, Wahlberg E. The optimal cuff width for measuring toe blood pressure. Angiol 2007;58(4):472–476.
- Bhamidipaty V, Dean A, Yap SL, Firth J, Barron M, Allard B, Chan ST. Second toe systolic pressure measurements are valid substitutes for first toe systolic pressure measurements in diabetic patients: a prospective study. Eur J Vasc Endovasc Surg 2015;49(1):77–82.
- The National Institute for Health and Care Excellence (NICE). Lower limb peripheral arterial disease: diagnosis and management (CG147); 2020. Available from: http://www.nice.org.uk/guidance/CG147
- Sonter J, Tehan P, Chuter V. Toe brachial index measured by automated device compared to duplex ultrasonography for detecting peripheral arterial disease in older people. Vascular 2017;25(6):612–617.
- Allen J. Photoplethysmography and its application in clinical physiological measurement. Physiolog Measure 2007;28(R1).
- Cracowski J-L, Minson CT, Salvat-Melis M, Halliwill JR. Methodological issues in the assessment of skin microvascular endothelial function in humans. Trends Pharmacol Sci 2006;27(9):503–508.
- Montero-Baker M, Rocha B, Armstrong D, Goshima K, Miller J. Diagnosis and endovascular management of segmental heel ischemia. Int J Clin Cardiol 2018;5:117.
- Kim ES, Sharma AM, Scissons R, Dawson D, Eberhardt RT, Gerhard-Herman M, Hughes JP, Knight S, Marie Kupinski A, Mahe G. Interpretation of peripheral arterial and venous Doppler waveforms: a consensus statement from the Society for Vascular Medicine and Society for Vascular Ultrasound. Vasc Med 2020;25(5):484–506.
- Kelechi TJ, Johnson JJ, Yates S. Chronic venous disease and venous leg ulcers: an evidence-based update. J Vascular Nurs 2015;33(2):36–46.
- Weller CD, Team V, Ivory JD, Crawford K, Gethin G. ABPI reporting and compression recommendations in global clinical practice guidelines on venous leg ulcer management: a scoping review. Int Wound J 2019;16(2):406–419.
- Doherty D, Morgan P, Moffatt C. Hosiery in lower limb lymphoedema. J Lymph 2009;1:30–37.
- Quéré I, Palmier S, Nøerregaard S, Pastor J, Sykorova M, Dring E, Franks PJ, Murray S, Keeley V, Bermark S. LIMPRINT: estimation of the prevalence of lymphoedema/chronic oedema in acute hospital in in-patients. Lymphat Res Biol 2019;17(2):135–140.



