Volume 31 Number 2
Economic evaluation of compression therapies in the treatment of venous leg ulcers: a systematic review protocol
Ana C Fuhrmann, Fernanda P Cordova, Elizabeth Dennett, Lisiane MG Paskulin,Jeffrey Johnson
Keywords systematic review, Venous ulcer, compression bandage, cost-benefit analysis, health economics
For referencing Fuhrmann AC et al. Economic evaluation of compression therapies in the treatment of venous leg ulcers: a systematic review protocol. Wound Practice and Research 2023; 31(2):82-86.
DOI
https://doi.org/10.33235/wpr.31.2.82-86
Submitted 14 February 2023
Accepted 25 April 2023
Abstract
Background Venous leg ulcers (VLU) are an important public health issue, impacting individuals’ lives and representing a societal economic burden. Compression therapy is considered the best treatment for VLU, but there is no conclusive evidence whether the health benefits outweigh the costs.
Objectives To identify and describe economic evaluations relating to compression therapy for the treatment of VLU and to evaluate the quality of these studies.
Method We will conduct a systematic review of the MEDLINE, EMBASE, Cochrane Central, CINAHL, Scopus, Web of Science and LILACS databases and Google Scholar. We will include randomised controlled trials, pragmatic clinical trials, cohort studies, case-control studies and quasi-experimental studies which also describe an economic evaluation of compression therapy for VLU, published in English, Portuguese or Spanish. No time restriction will be applied. The screening and assessment will be done by two independent reviewers, supported by Covidence Software. ROB-2 and ROBINS-I tools will be used to assess risk of bias, and the CHEERS tool will support the assessment of the quality of economic evaluations.
Discussion This systematic review will contribute to expand the knowledge about the topic. In addition, it may support health professionals’ clinic decision making, assist managers regarding the allocation of resources, and improve the quality of life of individuals with VLU.
Registration PROSPERO CRD42023393289.
Introduction
Venous leg ulcers (VLU) are an important public health issue. They greatly impact an individual’s life, causing discomfort, social isolation and disability. They also represent a societal economic burden, as they can lead to loss of productivity, frequent visits to healthcare services and recurrent hospitalisations1,2. Systematic reviews have demonstrated varied approaches to VLU management in developed countries (Australia, France, Germany, Italy, Spain, the UK, and the USA), but all treatments have high costs3. Countries are spending 3–6% of their total health costs treating chronic wounds4. Furthermore, the average recurrence rate for VLU is 70%5, meaning they have an important impact on the quality of life of these individuals.
Compression therapy is considered the gold standard treatment for VLU management3,6 and there is evidence of moderate certainty that compression therapy is better than no compression on VLU healing6,7. A systematic review comparing compression systems to no compression published in 2021 included 14 studies (with 32 publications) and a total of 1391 participants with VLU7. It concluded that using compression systems rather than non-compression resulted in a shorter time to complete healing and a greater number of VLU which completely healed7. A more recent meta-review included 12 published systematic reviews with a total of 71 trials and 7,141 participants with VLU. The comparison of compression vs no compression included 10 trials and 768 participants, and the superiority of compression was identified as moderate certainty evidence (RR:1.5; 95% CI 1.43–1.78, p<.00001), where 61% (n=236/385) of the participants in the compression group healed while only 39% (n=151/383) of VLU healed in the no compression group6.
Different types of compression systems are available – inelastic and elastic compression bandages, simple layer and multilayer bandages, compression hosiery, and intermittent pneumatic compression systems3,6. The compression systems can be classified according to the pressure applied – light (14–17mmHg), moderate (18–24mmHg), high (25–35mmHg) and extra-high (up to 60mmHg). In the same way, hosiery can be classified as light-support (14–17mmHg), medium-support (18–24mmHg) and strong-support (25–35mmHg)7. Among the different forms of compression therapy, the recent meta-review suggested no conclusion as to the best approach from the available studies, largely due to missing information about parameters of elasticity/inelasticity of the compression bandages used in the studies6.
Furthermore, the majority of compression therapy studies indicate the clinical effectiveness of these treatments on VLU healing – time-to-complete wound healing (HR:2.17; 95% CI 1.52–3.10) and proportion of wounds completely healed (RR:1.77; 95% CI 1.41–2.21), pain improvement (mean difference (MD) –1.39; 95% CI –1.79 to –0.98), and better patients’ health-related quality of life (MD –6.87; 95% CI –13.10 to –0.64) – but there is no evidence whether the health benefits outweigh the costs7. Evidence about cost and effectiveness of different compression systems are important to inform the allocation of scarce resources and to expand knowledge. Thus, the following question will guide our systematic review: what is the evidence in the literature on the economic evaluation of compression therapies in the treatment of VLU?
Objective
This protocol aims to provide a detailed overview of the process of developing the systematic review, promoting a transparent process. The systematic review aims to identify and describe economic evaluations relating to compression therapies for the treatment of VLU and to analyse the quality of these studies.
Methods
This protocol was registered with The International Prospective Register of Systematic Reviews (PROSPERO) – CRD42023393289 and was developed according to the Preferred Reporting Items for Systematic review and Meta-Analysis Protocol (PRISMA-P) recommendations8.
Eligibility criteria
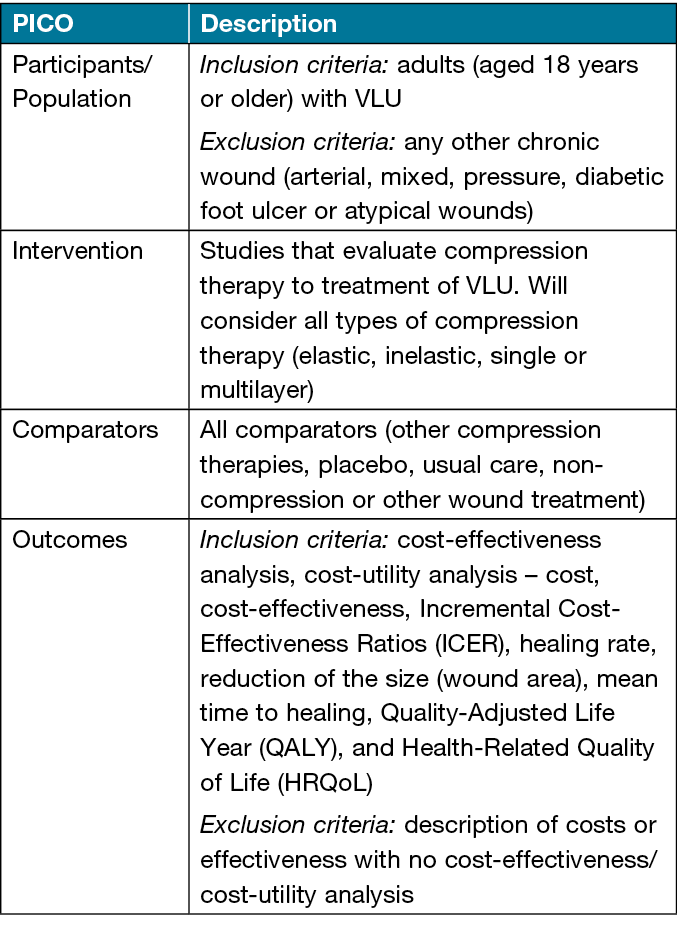
The inclusion criteria are framed by PICO strategy, as presented in Table 1.
Regarding study design, we will include randomised controlled trials (RCTs), pragmatic clinical trials, cohort studies, case-control studies and quasi-experimental studies which describe a cost-effectiveness analysis and/or a cost-utility analysis. Other designs, such as systematic reviews, will be excluded. Besides that, no restriction will be adopted on timeframe. We will consider available studies published in English, Portuguese and Spanish.
Table 1. Inclusion criteria framed by PICO strategy
Information sources
Searches will be conducted in the following electronic databases: Ovid MEDLINE, EMBASE, Cochrane Central, Cumulative Index of Nursing and Allied Health Literature (CINAHL), Scopus, Web of Science, the Latin American and Caribbean of Health Sciences Information System (LILACS) and Google Scholar.
Search strategy
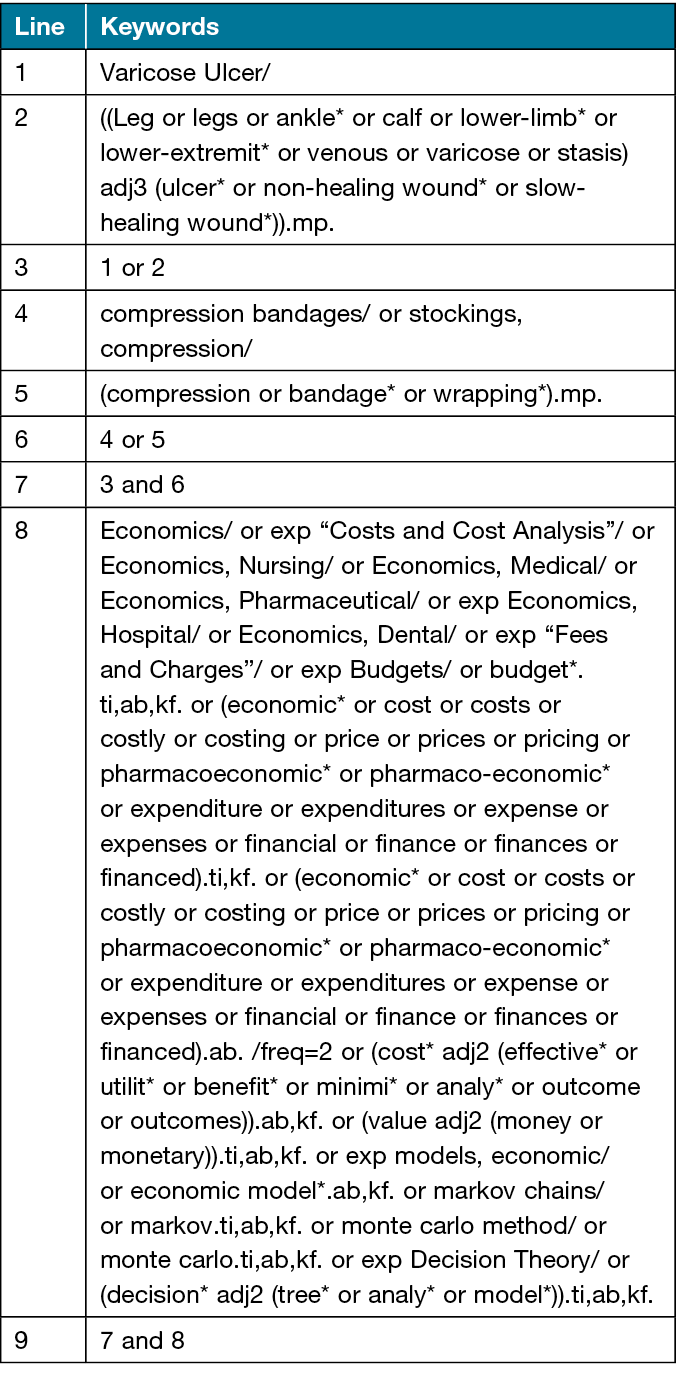
Search terms will include controlled vocabulary such as medical subject headings (MeSH terms) and free text terms related to compression therapy to treat VLU. The search strategy will be designed and conducted in collaboration with an experienced librarian from the University of Alberta (UofA) (ED), and reviewed by another librarian from UofA using the Peer Review of Electronic Search Strategies (PRESS) checklist9. Table 2 shows the search strategy for the ovid MEDLINE electronic database which used the economic evaluations filter from CADTH10.
Table 2. Search strategy for the Ovid MEDLINE electronic database
Study records: data management, selection and data collection process
All references will be imported into Covidence Systematic Review Software which will support the screening process. All duplicate titles will be removed, and two reviewers (ACF and FPC) will independently screen titles and abstracts, according to the inclusion criteria. Included studies will then be assessed based on full text reading by the same reviewers independently. Any disagreement will be resolved by discussion, and if necessary, by a third reviewer.
A PRISMA flow chart (Figure 1) will be constructed according to the search process and included/excluded studies.
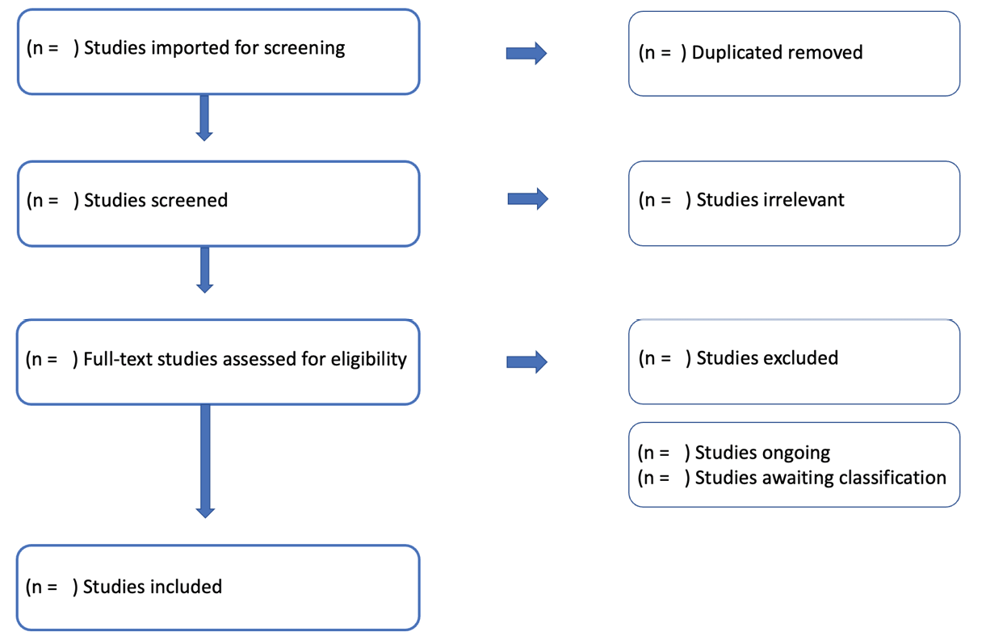
Figure 1. Flow chart for the systematic review process from the Covidence Systematic Review Software
Data items
We will extract the following information from the included studies:
- Study details: title, author, year, journal, country.
- Study methods: aims, design, setting, sample size, participants, length of follow-up, details about intervention and comparator, analysis performed.
- Outcome measurements.
- Costs (direct, indirect).
- Cost-effectiveness ratio.
- Sensitivity analyses (if done).
- Author conclusion.
- Conflicts of interest.
Risk of bias / quality assessment
The methodological quality of the included studies will be assessed by the two reviewers independently using tools regarding the type of studies. Any disagreement will be resolved by discussion, and if necessary, by a third reviewer. The Cochrane Risk of Bias (ROB-2) tool will be used to assess RCTs and the Risk Of Bias In Non-randomized Studies – of Interventions (ROBINS-I) tool will be used to assess cohort and case control studies. The ROB-2 evaluates the study as “low risk of bias, some concerns, and high risk of bias”, based on five domains; and the ROBINS-I indicates the study as “low risk of bias, moderate risk of bias, serious risk of bias, critical risk of bias, and no information on which to base a judgment about the risk of bias for this domain” by three domains11,12. Regarding the quality of economic evaluation studies, the Consolidated Health Economic Evaluation Reporting Standards (CHEERS) tool will be used by the reviewers13.
Data synthesis
The study characteristics and findings will be summarised using descriptive statistics where appropriate. Additionally, if possible, risk ratio (RR) and odd ratios (OR) for categorical outcome data or mean difference for continuous data, and 95% confidence interval (CI) will be calculated. For economic evaluation, incremental cost-effectiveness or cost/QALY ratios and ranges based on sensitivity analyses will be summarised.
Discussion
Our systematic review seeks to identify and describe economic evaluation studies relating to compression therapies for the treatment of VLU and to evaluate the quality of these studies. As demonstrated in the introduction, treatments for VLU are expensive and this pathology generates an important impact on individuals’ wellbeing. Thus, it is important to identify and synthesise the available evidence about the cost-effectiveness of different compression systems, in different contexts, to expand knowledge, to support clinical decisions of health professionals, and importantly, to inform the allocation of resources for the most effective therapies. Ultimately, the aim is to improve the outcomes and quality of life of individuals with VLU.
From a systematic review it is possible to facilitate the construction of knowledge in a transparent and rigorous process. In addition, assessing the methodological quality of the studies is essential to understand the factors that can influence the findings.
On the other hand, due to differences in health system organisation, budgets and availability of products to treat VLU across countries, beyond the culture that can influence on the perception of quality of life, it may not be possible to conclude if one compression system is universally more cost-effective than others. However, the systematic review will contribute to identifying gaps in available evidence regarding types of compression systems, outcomes as to effectiveness, follow-up, sample size or context of studies. Such information can then help to inform more local decision making, taking into account those important broader considerations.
Acknowledgements
The first author received a scholarship called a doctoral sandwich from the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Brazil, to study during her PhD at the University of Alberta for 1 year.
Author contribution
All authors participated in the conception of this article, critically reviewing it and approving the final version to be published, agreeing to be responsible for all aspects of the work and ensuring that questions related to the accuracy and integrity of any part of this work are duly investigated and resolved.
Conflict of interest
The authors declare no conflicts of interest.
Ethics statement
This systematic review protocol does not require ethics approval as information will be obtained from publicly available databases.
Funding
The authors received no funding for this study.
Author(s)
Ana C Fuhrmann*1, Fernanda P Cordova2, Elizabeth Dennett3, Lisiane MG Paskulin12, Jeffrey Johnson4
1School of Nursing, Universidade Federal do Rio Grande do Sul, Porto Alegre, Brazil
2Hospital de Clínicas de Porto Alegre, Porto Alegre, Brazil
3Health Science Library, University of Alberta, Edmonton, Canada
4School of Public Health, University of Alberta, Edmonton, Canada
*Corresponding author Email acffuhrman@gmail.com
References
- Epstein DM, Gohel MS, Heatley F, et al. Cost-effectiveness analysis of a randomized clinical trial of early versus deferred endovenous ablation of superficial venous reflux in patients with venous ulceration. Br J Surg 2019;106(5):555–562. doi:10.1002/bjs.11082.
- Bonkemeyer Millan S, Gan R, Townsend PE. Venous ulcers: diagnosis and treatment. Am Fam Physician 2019;100(5):298–305.
- Kolluri R, Lugli M, Villalba L, et al. An estimate of the economic burden of venous leg ulcers associated with deep venous disease. Vasc Med 2022;27(1):63–72. doi:10.1177/1358863X211028298.
- Järbrink K, Ni G, Sönnergren H, et al. The humanistic and economic burden of chronic wounds: A protocol for a systematic review. Syst Rev 2017;6:15. doi:10.1186/s13643-016-0400-8.
- Finlayson KJ, Parker CN, Miller C, et al. Predicting the likelihood of venous leg ulcer recurrence: the diagnostic accuracy of a newly developed risk assessment tool. Int Wound J 2018;15:686–694. doi:10.1111/iwj.12911.
- Patton D, Avsar P, Sayeh A, et al. A meta-review of the impact of compression therapy on venous leg ulcer healing. Int Wound J 2022 Jul 18. doi:10.1111/iwj.13891.
- Shi C, Dumville JC, Cullum N, Connaughton E, Norman G. Compression bandages or stockings versus no compression for treating venous leg ulcers. Cochrane Database Syst Rev 2021;7(7):CD013397. doi:10.1002/14651858.CD013397.pub2.
- Shamseer L, Moher D, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ 2015;2:349. doi:10.1136/bmj.g7647.
- McGowan J, Sampson M, Salzwedel DM, Cogo E, Foerster V, Lefebvre C. PRESS peer review of electronic search strategies: 2015 guideline statement. J Clin Epidemiol 2016;75:40–6. doi:10.1016/j.jclinepi.2016.01.021.
- Economic Evaluations & Models – MEDLINE. In: CADTH search filters database. Ottawa: CADTH; 2023 [cited 2023 Jan 13]. Available from: https://searchfilters.cadth.ca/link/16
- Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 2019;366:l4898. doi:10.1136/bmj.l4898.
- Sterne JA, Hernán MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016;355:i4919. doi:10.1136/bmj.i4919
- Husereau D, Drummond M, Augustovski F, et al. Consolidated Health Economic Evaluation Reporting Standards 2022 (CHEERS 2022) statement: updated reporting guidance for health economic evaluations. BMJ 2022;376:e067975. doi:10.1136/bmj-2021-067975.



