Volume 1 Issue 2
Randomised controlled trials in peripheral vascular access catheters: a scoping review
Mari Takashima, Gillian Ray-Barruel, Samantha Keogh, Claire M Rickard
Abstract
Randomised controlled trials (RCTs) are the “gold standard” for evaluating effectiveness of interventions, as they provide the most reliable evidence with minimal bias compared to other study designs. However, if the number and quality of RCTs are poor, studies may give inconsistent results and small effect sizes, creating difficulties in generalising the findings to a broader population. This scoping review investigated the current evidence for the insertion and management of peripheral vascular devices, including intravenous, midline, and arterial catheters. We searched Pubmed, Cochrane Central Register of Controlled Trials, and CINAHL between 1 January 2005 and 30 June 2015. The final review included 128 RCTs (94 peripheral intravenous catheters, 2 midline catheters, and 32 arterial catheters). Catheter insertion strategies and analgesia methods have been comprehensively studied, particularly for peripheral intravenous catheters, but more RCTs are needed to address post-insertion care and maintenance, including dressings and securement, flushing practices and infection prevention strategies such as skin preparation and hub decontamination. This peripheral vascular catheter scoping review will enable clinicians and researchers to identify the gaps in evidence and prioritise areas needing further research.
Keywords: Scoping review, peripheral vascular device, peripheral intravenous catheter, peripheral arterial catheter, midline catheter, randomised controlled trial.
INTRODUCTION
The majority of hospital patients require at least one peripheral vascular device for blood tests or for the delivery of intravenous fluids and medications during their hospital stay. These catheters include peripheral intravenous, midline, and arterial catheters. Despite the ubiquity of peripheral catheters in hospital care, the rate of complications and failure of these devices is reported to be as high as 34%1-3, demonstrating the need for a much greater investment in research to reduce associated patient discomfort, delays in necessary medical treatments, and prolonged length of hospital stay. The Alliance for Vascular Access Teaching and Research (AVATAR) group, based at Griffith University in Brisbane, Australia, has a proven track record in conducting robust research into all facets of vascular access. With time and resource limitations a reality, the research agenda needs to incorporate targeted strategies so that studies can be conducted to best address patient needs and minimise duplication and costs.
Well-designed and executed randomised controlled trials (RCTs) provide reliable evidence with minimal bias compared to other study designs and are therefore considered the “gold standard” for evaluating the effectiveness of interventions4. Systematic reviews evaluate the combined results of RCTs, analyse for bias, and provide an even higher level of evidence. Clinical guideline developers and health care staff rely on quality RCTs and systematic reviews to guide decision-making in clinical practice, but the evidence in many areas is insufficient, with few RCTs, small sample sizes, poor reporting, and a lack of strong effect, meaning that findings cannot be generalised to a broader population5.
A scoping study or review is an excellent methodology for mapping the extent, range, and nature of existing literature in a current topic area6,7. Less comprehensive and time-consuming than a systematic review, a scoping study is ideally suited to mapping the existing research in a given field and highlighting the gaps in evidence. A scoping study generally examines all literature published in a given field, regardless of study design6, and in this review we sought to discover which topics in peripheral vascular devices have been well-researched, and to identify areas lacking in high-level evidence. Results from this review may provide unique insights that are useful for vascular access clinicians and researchers.
Aims of the scoping review
The review focused on answering the following research questions: What RCTs have been conducted with peripheral vascular devices in the past decade? What patient populations have been included? Which types of interventions have been studied? What are the outcome measures of these RCTs?
METHODS
Review framework
The scoping review was conducted along the following framework, outlined by Arksey and O’Malley6 and modified by the Cochrane Public Health Group7: 1. Identify the research question; 2. Identify relevant studies; 3. Select studies for inclusion; 4. Sort, collate and analyse data; and 5. Summarise and report results. The investigators engaged in a reiterative consultation process of the scoping framework and inclusion/exclusion criteria to ensure consistency in decision making.
Identifying relevant studies
The search strategy was developed with the assistance of a university health sciences librarian. Inclusion and exclusion criteria were developed at the outset of the search. We included RCTs (including quasi-randomised trials) and systematic reviews published in English between 1 January 2005 and 30 June 2015 that focused on peripheral vascular devices, including peripheral intravenous catheters (PIVC), midline catheters (MC), and arterial catheters (AC). We included all participant ages and settings (inpatient and ambulatory). Systematic reviews were included in the search terms so we could examine the reference lists to identify potentially relevant RCTs. We excluded non-randomised controlled trials, secondary analysis of RCTs, and RCTs pertaining solely to central access catheters. Databases searched were Pubmed, Cochrane Central Register of Controlled Trials, and CINAHL. (See Appendix A for search terms.)
Study selection and data extraction
Titles and abstracts were initially screened for relevance. If the abstract was considered to meet the inclusion criteria, or if the reviewers were uncertain about inclusion, full text articles were then obtained and evaluated. Two reviewers read the full text of each article included in the final analysis.
Data sorting, collating and analysis
We used an Endnote library to sort the references into catheter type and then created a Microsoft Excel file to organise the data into the following headings: author(s); year of publication; study location; first author profession; study population (inpatient/ambulant, neonates/paediatrics/adults, clinical specialities); sample size; intervention and comparator; outcome measures; and grant funding. Two researchers (MT and GRB) independently reviewed each article for themes and met on several occasions to discuss the findings and achieve consensus. Unlike a systematic review, a scoping study does not seek to assess the quality of evidence6. While other researchers have argued that a quality analysis is an important component of a scoping study8, we did not analyse the quality of the evidence because the purpose of this review was to create a snapshot of the RCTs already conducted with peripheral vascular catheters and to point the way forward for further research. Authors were not contacted for further information.
Summarising and reporting results
After the data was organised into themes, we produced some preliminary tables. This enabled us to identify areas that had been the focus of RCTs and areas where the evidence was lacking.
RESULTS
Database searching identified 2528 studies and reference list searching identified a further 27 studies. (See Figure 1 for the flow chart of articles screened for inclusion in the scoping review.) After duplicates were removed, we screened the remaining abstracts for the inclusion and exclusion criteria. We reviewed the full text of 281 articles, of which 128 RCTs (94 PIVC, 2 MC, 32 AC) met the criteria and were included in the final review.
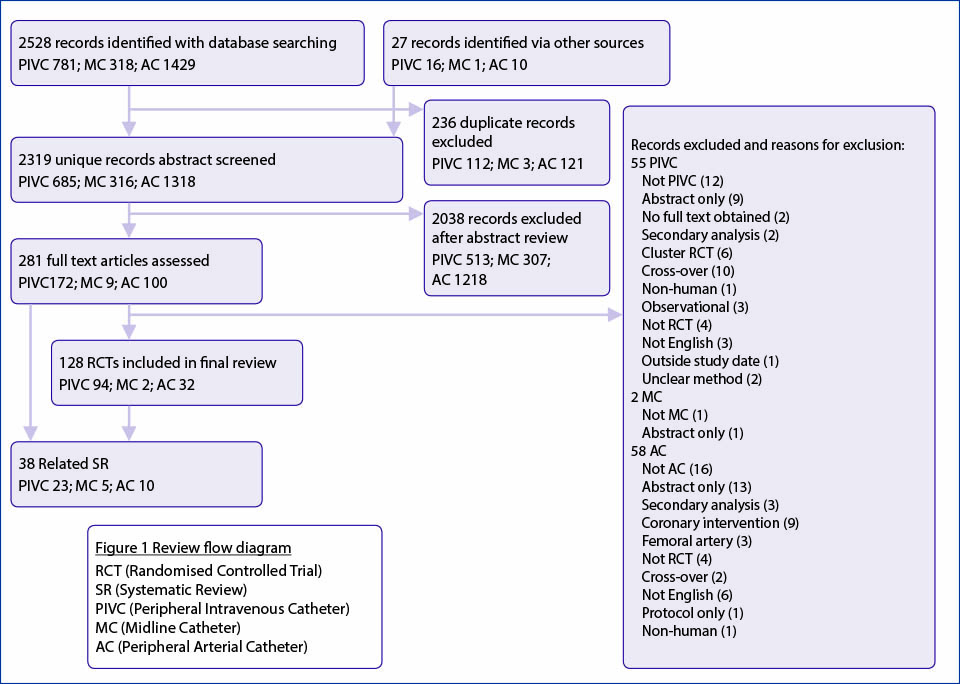
Figure 1. Flow chart of articles screened for inclusion in the scoping review
Peripheral vascular catheter RCTs were most commonly conducted in adults (75.2%) and inpatient settings (84%) (see Table 1). Countries conducting the most RCTs included the USA (44 trials), Australia (15 trials), Turkey (10 trials), Spain (8 trials), France (6 trials) and Japan (6 trials); these comprised 69.5% of the RCTs (see Figure 2). Nine multi-centre RCTs were identified (7 PIVC, 2 AC), with the remainder of studies being conducted in a single site. Six stated pilot RCTs were identified (3 PIVC, 3 AC). Although the majority of studies focused on a single catheter type (86 PIVC, 1 MC, 29 AC), a handful included more than one catheter type in the research protocol, so these were only included if results were presented separately for each catheter type.
Table 1. Population and setting in included RCTs
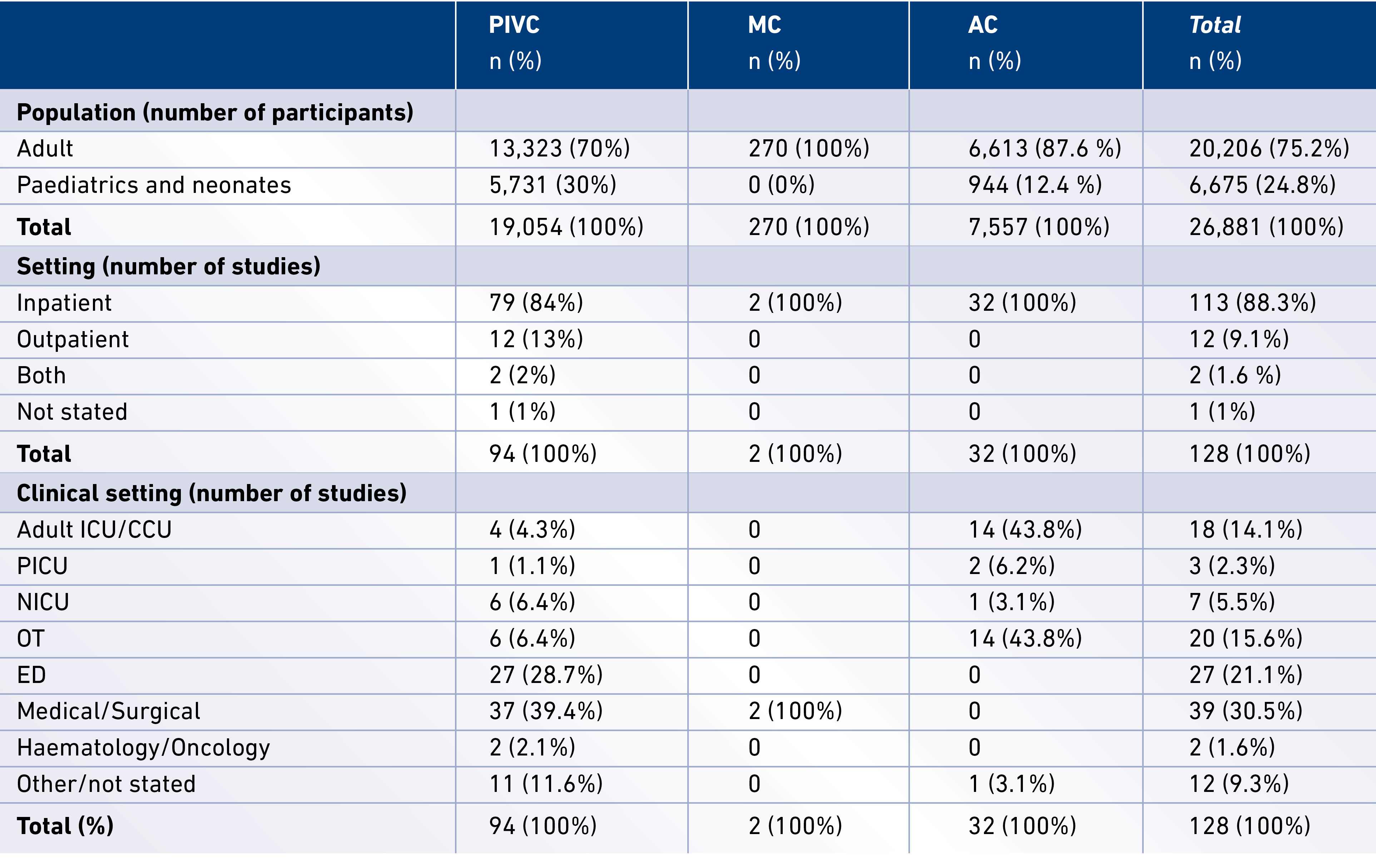
Figure 2. Number of published RCTs per country (January 2005 – June 2015)
Templates from http://www.amcharts.com/
The characteristics of the included RCTs, including catheter type, study topic, and clinical specialty, are presented in Table 2. Predominant topics studied in 94 PIVC RCTs included technology-guided catheter insertion (n=21, 22.3%) and analgesia pre-insertion (n=30, 32%), followed by add-on devices including needleless connectors and reflux valves (n=12, 12.8%), flushing solutions (n=8, 8.5%), catheter indwell time (n=7, 7.4%), and dressings and securement methods (n=5, 5.3%). Predominant study topics in 32 AC RCTs included cannulation strategies (n=13, 40.1%) and flush solutions (n=7, 21.8%), followed by blood conservation (n=4, 12.5%) and dressings (n=4, 12.5%). The 2 RCTs in MCs respectively studied needleless connectors and anti-thrombolytic medications added to parenteral nutrition.
Table 2. Characteristics of the included RCTs
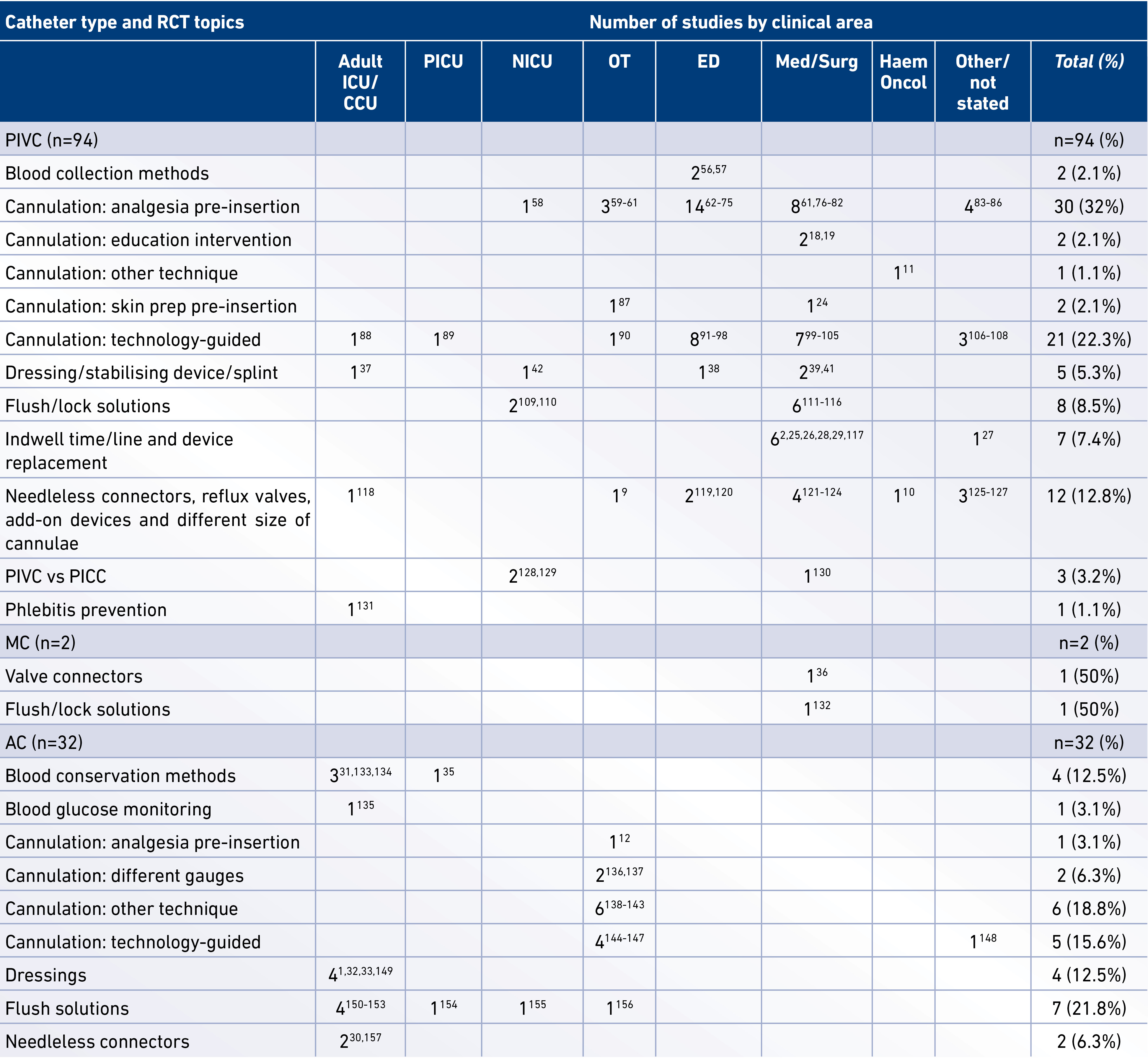
RCT (randomised controlled trial), PIVC (peripheral intravenous catheter), MC (midline catheter), AC (peripheral arterial catheter), PICC (peripherally inserted central catheter), ICU (intensive care unit), PICU (paediatric intensive care unit), NICU (neonatal intensive care unit), OT (operating theatre), ED (emergency department), Med/Surg (medical and/or surgical wards), Haem/Oncol (haematology and/or oncology wards).
The reported outcome measures were sorted into broad categories (see Table 3): 1. Patient outcomes (pain, anxiety, satisfaction, length of hospital stay, survival/death); 2. Catheter insertion outcomes (cannulation success rate, time to catheterisation, number of attempts, ease of cannulation, insertion difficulties); 3. Catheter complications (unplanned removal, dislodgement, extravasation, infiltration, obstruction, rupture, skin reactions, thrombosis); 4. Other catheter outcomes (catheter dynamics and flow rate, dwell time, patency, venous reflux); 5. Infective outcomes (CRBSI, colonisation, local infection, phlebitis, thrombophlebitis); 6. Blood sampling (blood loss, haemolysis of samples, sampling techniques); 7. Flushing and lock solution (heparin, manual flushing); and 8. Health services outcomes (cost-effectiveness). We also located a range of systematic reviews and one meta-analysis examining RCTs in peripheral catheter and these are displayed in Table 4.
Table 3. Outcome measures studied per catheter type*
*Most RCTs included more than one outcome measure. RCT (randomised controlled trial), PIVC (peripheral intravenous catheter),
MC (midline catheter), AC (peripheral arterial catheter)
Table 4. Systematic review topic per catheter type

*Meta-analysis
CRBSI Catheter-related bloodstream infection
In the 128 RCTs reviewed, the primary author was a medical doctor in 73 (57.0%) studies and a nurse in 37 (28.9%) studies. Other first author professions included other professor/researcher (4, 3.1%), dentist (2, 1.6%), pharmacist (2, 1.6%), other (2, 1.6%), or not stated (8, 6.2%). Grant funding for studies was reported for 73 (57.0%) studies, and sources included a mixture of commercial (20, 15.6%), non-commercial (50, 39.1 %), and a combination of the two (3, 2.3 %).
DISCUSSION
This scoping review has revealed gaps in recent research relating to peripheral vascular devices. Firstly, paediatrics and neonates are still understudied across all peripheral catheter types, with much of the evidence being extrapolated from adult studies, and therefore likely not to be relevant for this population. However, although the absolute number of paediatric studies was small, sample sizes were not; paediatric patients comprised 24.8% of all study participants. Secondly, the majority (55%) of studies centred on cannulation (56 PIVC, 14 AC), with very little evidence available to guide maintenance care, such as catheter securement, dressing, and patency. Despite the importance of maintenance care in preventing catheter failure and infection, the only RCT examining catheter hub decontamination was conducted in the operating theatre9, and no RCT examined flushing volume or frequency.
As expected, the highest proportion of PIVC RCTs was conducted in the emergency department (28.7%) or medical/surgical wards (39.4%). Studies of ACs mostly took place in the intensive care setting (43.8%) or operating theatre (43.8%). We found only two RCTs conducted in a haematology/oncology setting10,11, which was particularly concerning as this patient cohort is likely to receive a high number of cannulations and experience venous depletion. We identified nine multi-centre RCTs, but the majority of studies were conducted in a single site, with small sample sizes, making the findings less amenable to generalisation. None of the identified pilot studies were followed up with larger definitive RCTs at the time of writing.
Recent evidence for peripheral catheter insertion has focused on pre-insertion analgesia and technology-guided insertion. Analgesia for catheter insertion featured in 31 (24.2%) RCTs, primarily in PIVCs (n=30), but only one RCT studied this topic in ACs12 and there were no relevant trials in MCs. Despite the prevalence of studies concerning pre-insertion analgesia, we did not find any systematic review of this topic. Technology-guided insertion featured in 26 (20.2%) of the 128 RCTs reviewed (21 PIVC, 5 AC). Three systematic reviews of ultrasound-guided cannulation of PIVCs have been published13-15, but other insertion technology strategies, such as transmitted light devices, have yet to be comprehensively reviewed. Both the Centers for Disease Control and Prevention16 and the Infusion Nurses Society17 guidelines support ultrasound guidance for central catheter insertion, but neither has recommendations for the use of technology in peripheral device insertion.
We found no RCT that examined the impact of skilled inserters in the past 10 years. Two educational intervention RCTs targeted interns18,19, but we found no RCTs of education programs for nurses. As nurses deliver the bulk of catheter care post-insertion, this is concerning and should be the focus for future research.
Strategies for the prevention of catheter-related bloodstream infection (CRBSI) historically have focused on central vascular access devices, but recent research has brought attention to the risk of infection with peripheral devices20-22. As the number of patients receiving peripheral catheters is much greater than central devices, it is surprising that we did not find more RCTs focusing on infection prevention. Skin preparation prior to cannulation is an understudied topic. Only two RCTs examined skin preparation solutions for PIVC insertion23,24, and there were no studies on skin preparation before cannulation for ACs and MCs. A handful of RCTs examined post-cannulation preventative action for CRBSI in PIVCs, such as catheter hub disinfection stations in the operating theatre9 and catheter replacement policies2,25-29. The prevention of CRBSI or colonisation of ACs has been examined in recent RCTs conducted in adult ICUs30-34, with only one study addressing the paediatric intensive care population35. Prevention of CRBSI related to needleless connectors was examined in one study in MCs36.
In the adult population, two RCTs have examined the comparative effectiveness of PIVC dressing types37 and securement38, and two recent pilot RCTs in adults compared a range of dressings and securement methods in reducing catheter failure in PIVCs39 and ACs1, but no large multi-centre trials have been published. A meta-analysis in ACs concluded the benefits of chlorhexidine dressings in preventing catheter colonisation and CRBSI in high-risk adult and paediatric patients40. Dressing and securement studies are notably scarce in paediatrics. One RCT compared dressing types in this population41, and one RCT found that limb immobilisation in neonates was ineffective in preventing PIVC failure42. As paediatric patients have different anatomy and may require different dressing techniques to secure the vascular device, studies in this area are needed and could add significant findings. We did not find any RCT that examined dressing and securement in MCs.
In this review of peripheral catheter RCTs, over two-thirds of research in this area was led by medical authors. This is perhaps not surprising because, firstly, medical staffs often have more access to paid research time and, secondly, the insertion of peripheral devices, particularly in patients with difficult vascular access, remains a medical responsibility in many hospitals. However, as nurses provide the bulk of catheter care post-insertion, and the catheter failure rate continues to be around 34%1-3, we argue that it is essential to have more nurses undertake research in this area to determine why so many peripheral catheters fail, and test potential strategies for prevention in RCTs. For this to occur, nurses would need to be funded to incorporate research into their work, or health facilities would need to employ nurse researchers.
Sadly, only a small percentage of Australian National Health and Medical Research Funding is awarded to nursing studies. In the last five years, just 10 out of 2189 grants (0.35%) were awarded to applications coded under the nursing field of research43. This is disproportionately low, and disappointing to say the least. There are over 320,000 nurses working in Australia, and one per cent work in research roles44. Both nurses and the research system need to prioritise nurse-led research if it is to attract adequate funding to investigate and propose answers to clinical questions.
We used the Arksey and O’Malley framework for scoping studies to gain an understanding of the general landscape of recent RCTs in peripheral vascular catheters. This framework is well suited for this purpose. Some authors have criticised this methodology for not assessing the quality of evidence and determining the generalisability of evidence, but at this stage, there is no standardised method to assess the quality component of scoping reviews45. However, with respect to RCTs, this is the domain of a systematic review, and therefore analysis of bias of the included studies was beyond the scope of this review.
The designated time frame of the past decade is a potential limitation of the study, but the review sought to capture the current state of the evidence, rather than earlier and possibly outdated strategies for catheter insertion and care. For instance, technology-guided cannulation is a recent innovation and different kinds of catheters, dressings, securements and add-on devices are now in daily use. For practical reasons, research published in languages other than English was excluded because of the cost and time involved in translating material, and this may have screened out some relevant studies.
A major strength of this study is the limitation to RCTs and systematic reviews, as these provide the highest level of evidence to inform clinical practice4. Throughout the study, however, we anecdotally identified some poorly reported or conducted RCTs. Although RCTs are considered a “gold standard” of clinical evidence, if bias is evident or sample sizes are too small, the results may be inconsistent, lack strong effect, or fail to be generalisable to the specific clinical population5. Systematic reviews of various interventions for peripheral vascular devices repeatedly show that there is a need for more robust RCTs to be conducted in order to demonstrate powerful clinical effects46-55.
CONCLUSION
This peripheral vascular catheter scoping review identified RCTs published in the past decade to enable clinicians and researchers to identify the gaps in evidence and prioritise areas needing further research. Although many RCTs examined catheter insertion strategies and analgesia methods, particularly for PIVCs, there were surprisingly few studies about the post-insertion care and maintenance of peripheral catheters, including dressings and securement, particularly in the paediatric population. More RCTs in this area are needed, as well as studies on flushing practices and infection prevention strategies such as skin preparation and hub decontamination. As nurses provide the bulk of post-insertion catheter care, RCTs examining nursing education for catheter care are a priority. Evidence-based care will remain an elusive goal until the evidence base comprises quality RCTs to support daily clinical practice.
AUTHOR DISCLOSURES
Nil.
APPENDIX A: SEARCH TERMS
(((((((“arteries”[MeSH Terms] OR “arteries”[All Fields] OR “arterial”[All Fields]) AND (“catheters”[MeSH Terms] OR “catheters”[All Fields] OR “catheter”[All Fields])) OR (“vascular access devices”[MeSH Terms] OR (“vascular”[All Fields] AND “access”[All Fields] AND “devices”[All Fields]) OR “vascular access devices”[All Fields] OR (“arterial”[All Fields] AND “line”[All Fields]) OR “arterial line”[All Fields])) OR ((“arteries”[MeSH Terms] OR “arteries”[All Fields] OR “arterial”[All Fields]) AND (“equipment and supplies”[MeSH Terms] OR (“equipment”[All Fields] AND “supplies”[All Fields]) OR “equipment and supplies”[All Fields] OR “device”[All Fields]))) OR ((“haemodynamic”[All Fields] OR “hemodynamics”[MeSH Terms] OR “hemodynamics”[All Fields] OR “hemodynamic”[All Fields]) AND monitoring[All Fields])) AND (“blood vessels”[MeSH Terms] OR (“blood”[All Fields] AND “vessels”[All Fields]) OR “blood vessels”[All Fields] OR “vascular”[All Fields])) AND peripheral[All Fields]) AND (randomized controlled trial[pt] OR controlled clinical trial[pt] OR randomized[tiab] OR randomised[tiab] OR randomization[tiab] OR randomisation[tiab] OR “clinical trials as topic”[MeSH Terms:noexp] OR placebo[tiab] OR randomly[tiab] OR (“clinical trials as topic”[MeSH Terms] OR (“clinical”[All Fields] AND “trials”[All Fields] AND “topic”[All Fields]) OR “clinical trials as topic”[All Fields] OR “trial”[All Fields])) AND ((Randomized Controlled Trial[ptyp] OR systematic[sb]) AND (“2005/01/01”[PDAT] : “2015/06/30”[PDAT]) AND “humans”[MeSH Terms]) AND ((systematic[sb] OR Randomized Controlled Trial[ptyp]) AND “loattrfull text”[sb])
((midline[All Fields] OR (midline[All Fields] AND (“catheters”[MeSH Terms] OR “catheters”[All Fields] OR “catheter”[All Fields]))) AND ((Randomized Controlled Trial[ptyp] OR systematic[sb]) AND (“2005/01/01”[PDAT] : “2014/12/31”[PDAT]) AND “humans”[MeSH Terms])) AND (randomized controlled trial[pt] OR controlled clinical trial[pt] OR randomized[tiab] OR randomised[tiab] OR randomization[tiab] OR randomisation[tiab]) AND ((Randomized Controlled Trial[ptyp] OR systematic[sb]) AND “loattrfull text”[sb] AND (“2005/01/01”[PDAT] : “2015/06/30”[PDAT]) AND “humans”[MeSH Terms] AND English[lang])(((((((peripheral[All Fields] AND intravenous[All Fields] AND (“catheters”[MeSH Terms] OR “catheters”[All Fields] OR “catheter”[All Fields])) OR (peripheral[All Fields] AND intravenous[All Fields] AND (“equipment and supplies”[MeSH Terms] OR (“equipment”[All Fields] AND “supplies”[All Fields]) OR “equipment and supplies”[All Fields] OR “device”[All Fields]))) OR PIV[All Fields]) OR (IV[All Fields] AND (“catheters”[MeSH Terms] OR “catheters”[All Fields] OR “catheter”[All Fields]))) OR PIV[All Fields]) OR IVC[All Fields]) OR IVD[All Fields]) AND (randomized controlled trial[pt] OR controlled clinical trial[pt] OR randomized[tiab] OR randomised[tiab] OR randomization[tiab] OR randomisation[tiab] OR “clinical trials as topic”[MeSH Terms:noexp] OR placebo[tiab] OR randomly[tiab] OR (“clinical trials as topic”[MeSH Terms] OR (“clinical”[All Fields] AND “trials”[All Fields] AND “topic”[All Fields]) OR “clinical trials as topic”[All Fields] OR “trial”[All Fields])) AND ((Randomized Controlled Trial[ptyp] OR systematic[sb]) AND “loattrfull text”[sb] AND (“2005/01/01”[PDAT] : “2015/06/30”[PDAT]) AND “humans”[MeSH Terms] AND English[lang])
Author(s)
Mari Takashima* RN, BN, Grad Cert ICU, MEpi Research Assistant, Alliance for Vascular Access Teaching and Research (AVATAR) group, NHMRC Centre of Research Excellence in Nursing (NCREN), Centre for Health Practice Innovation, Menzies Health Institute Queensland, Griffith University, Nathan, Qld 4111, Australia Email: mari.takashima@griffithuni.edu.au Gillian Ray-Barruel RN, BSN, BA(Hons), Grad Cert ICU Nursing Senior Research Assistant, Alliance for Vascular Access Teaching and Research (AVATAR) group, NHMRC Centre of Research Excellence in Nursing (NCREN), Centre for Health Practice Innovation, Menzies Health Institute Queensland, Griffith University, Nathan, Qld 4111, Australia Email: g.ray-barruel@griffith.edu.au Samantha Keogh RN, IC Cert, BSc(Hons), PhD Senior Research Fellow, Alliance for Vascular Access Teaching and Research (AVATAR) group, NHMRC Centre of Research Excellence in Nursing (NCREN), Centre for Health Practice Innovation, Menzies Health Institute Queensland, Griffith University, Nathan, Qld 4111, Australia Email: s.keogh@griffith.edu.au Claire M Rickard RN, BN, GradDip N(CritCare), PhD, FACN Professor of Nursing, Alliance for Vascular Access Teaching and Research (AVATAR) group, NHMRC Centre of Research Excellence in Nursing (NCREN), Centre for Health Practice Innovation, Menzies Health Institute Queensland, Griffith University, Nathan, Qld 4111, Australia Email: c.rickard@griffith.edu.au *Corresponding author
References
- Reynolds H, Taraporewalla K, Tower M, Mihala G, Tuffaha HW, Fraser JF et al. Novel technologies can provide effective dressing and securement for peripheral arterial catheters: A pilot randomised controlled trial in the operating theatre and the intensive care unit. Aust Crit Care 2015: 204–5.
- Rickard CM, Webster J, Wallis MC, Marsh N, McGrail MR, French V et al. Routine versus clinically indicated replacement of peripheral intravenous catheters: a randomised controlled equivalence trial. Lancet 2012; 380(9847):1066–74.
- Wallis MC, McGrail M, Webster J, Marsh N, Gowardman J, Playford EG et al. Risk factors for peripheral intravenous catheter failure: a multivariate analysis of data from a randomized controlled trial. Infect Control Hosp Epidemiol 2014; 35(1):63–8.
- Akobeng AK. Understanding randomised controlled trials. Arch Dis Child 2005;90(8):840–4.
- Garg AX, Hackam D & Tonelli M. Systematic review and meta-analysis: when one study is just not enough. Clin J Am Soc Nephrol 2008; 3(1):253–60.
- Arksey H & O’Malley L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol 2005; 8(1):19–32.
- Armstrong R, Hall BJ, Doyle J & Waters E. ‘Scoping the scope’ of a cochrane review. J Public Health 2011; 33(1):147–50.
- Levac D, Colquhoun H & O’Brien KK. Scoping studies: advancing the methodology. Implement Sci 2010;5:69.
- Loftus RW, Brindeiro BS, Kispert DP, Patel HM, Koff MD, Jensen JT et al. Reduction in intraoperative bacterial contamination of peripheral intravenous tubing through the use of a passive catheter care system. Anesth Analg 2012; 115(6):1315–23.
- Barreras F, Cabeza M & Collantes de Teran L. Clinical efficacy and safety of Securflux(R), an anti-reflux device for intravenous infusion. J Vasc Access 2013; 14(1):77–82.
- Fink RM, Hjort E, Wenger B, Cook PF, Cunningham M, Orf A et al. The impact of dry versus moist heat on peripheral IV catheter insertion in a hematology-oncology outpatient population. Oncol Nurs Forum 2009; 36(4):E198–204.
- Ruetzler K, Sima B, Mayer L, Golescu A, Dunkler D, Jaeger W et al. Lidocaine/tetracaine patch (Rapydan) for topical anaesthesia before arterial access: a double-blind, randomized trial. Br J Anaesth 2012; 109(5):790–6.
- Egan G, Healy D, O’Neill H, Clarke-Moloney M, Grace PA & Walsh SR. Ultrasound guidance for difficult peripheral venous access: systematic review and meta-analysis. Emerg Med J 2013; 30(7):521–6.
- Liu YT, Alsaawi A & Bjornsson HM. Ultrasound-guided peripheral venous access: a systematic review of randomized-controlled trials. Eur J Emerg Med 2014; 21(1):18–23.
- Heinrichs J, Fritze Z, Klassen T & Curtis S. A systematic review and meta-analysis of new interventions for peripheral intravenous cannulation of children. Pediatr Emerg Care 2013; 29(7):858–66.
- O’Grady NP, Alexander M, Burns LA, Dellinger EP, Garland J, O’Heard S et al. Guidelines for the prevention of intravascular catheter-related infections. Clin Infect Dis 2011; 52(9):e162–93.
- Infusion Nurses Society. Infusion nursing standards of practice. J Infus Nurs 2011; 34(1S):S1–S110.
- Gaies MG, Morris SA, Hafler JP, Graham DA, Capraro AJ, Zhou J et al.. Reforming procedural skills training for pediatric residents: a randomized, interventional trial. Pediatrics 2009; 124(2):610–9.
- Kessler DO, Arteaga G, Ching K, Haubner L, Kamdar G, Krantz A et al. Interns’ success with clinical procedures in infants after simulation training. Pediatrics 2013; 131(3):e811–20.
- Hadaway L. Short peripheral intravenous catheters and infections. J Infus Nurs 2012; 35(4):230–40.
- Pujol M, Hornero A, Saballs M, Argerich MJ, Verdaguer R, Cisnal M et al. Clinical epidemiology and outcomes of peripheral venous catheter-related bloodstream infections at a university-affiliated hospital. J Hosp Infect 2007; 67(1):22–9.
- Trinh TT, Chan PA, Edwards O, Hollenbeck B, Huang B, Burdick N et al. Peripheral Venous Catheter-Related Staphylococcus aureus Bacteremia. Infect Control Hosp Epidemiol 2011; 32(6):579–83.
- Small H, Adams D, Casey AL, Crosby CT, Lambert PA & Elliott T. Efficacy of adding 2% (w/v) chlorhexidine gluconate to 70% (v/v) isopropyl alcohol for skin disinfection prior to peripheral venous cannulation. Infect Control Hosp Epidemiol 2008; 29(10):963–5.
- van der Mee-Marquet NL. Efficacy and safety of a two-step method of skin preparation for peripheral intravenous catheter insertion: a prospective multi-centre randomised trial. BMC Anesthesiol 2007; 7:1.
- Nishanth S, Sivaram G, Kalayarasan R, Kate V & Ananthakrishnan N. Does elective re-siting of intravenous cannulae decrease peripheral thrombophlebitis? A randomized controlled study. Natl Med J India 2009; 22(2):60–2.
- Rickard CM, McCann D, Munnings J & McGrail MR. Routine resite of peripheral intravenous devices every 3 days did not reduce complications compared with clinically indicated resite: a randomised controlled trial. BMC Med 2010; 8:53.
- Van Donk P, Rickard CM, McGrail MR & Doolan G. Routine replacement versus clinical monitoring of peripheral intravenous catheters in a regional hospital in the home program: A randomized controlled trial. Infect Control Hosp Epidemiol 2009; 30(9):915–7.
- Webster J, Clarke S, Paterson D, Hutton A, van Dyk S, Gale C et al. Routine care of peripheral intravenous catheters versus clinically indicated replacement: randomised controlled trial. BMJ 2008; 337:a339.
- Webster J, Lloyd S, Hopkins T, Osborne S & Yaxley M. Developing a Research base for Intravenous Peripheral cannula re-sites (DRIP trial). A randomised controlled trial of hospital in-patients. Int J Nurs Stud 2007; 44(5):664–71.
- Esteve F, Pujol M, Limon E, Saballs M, Argerich MJ, Verdaguer R et al. Bloodstream infection related to catheter connections: a prospective trial of two connection systems. J Hosp Infect 2007; 67(1):30–4.
- Oto J, Nakataki E, Hata M, Tsunano Y, Okuda N, Imanaka H et al. Comparison of bacterial contamination of blood conservation system and stopcock system arterial sampling lines used in critically ill patients. Am J Infect Control 2012; 40(6):530–4.
- Timsit JF, Mimoz O, Mourvillier B, Souweine B, Garrouste-Orgeas M, Alfandari S et al. Randomized controlled trial of chlorhexidine dressing and highly adhesive dressing for preventing catheter-related infections in critically ill adults. Am J Respir Crit Care Med 2012; 186(12):1272–8.
- Timsit J-F, Schwebel C, Bouadma L, Geffroy A, Garrouste-Orgeas M, Pease S et al. Chlorhexidine-impregnated sponges and less frequent dressing changes for prevention of catheter related sepsis in critically ill adults: A randomized controlled trial. JAMA 2009; 301(12):1231–41.
- Yebenes JC, Vidaur L, Serra-Prat M, Sirvent JM, Batlle J, Motje M et al. Prevention of catheter-related bloodstream infection in critically ill patients using a disinfectable, needle-free connector: a randomized controlled trial. Am J Infect Control 2004; 32(5):291–5.
- Tang M, Feng M, Chen L, Zhang J, Ji P & Luo S. Closed blood conservation device for reducing catheter-related infections in children after cardiac surgery. Crit Care Nurse 2014; 34(5):53–61.
- Khalidi N, Kovacevich DS, Papke-O’Donnell LF & Btaiche I. Impact of the positive pressure valve on vascular access device occlusions and bloodstream infections. JAVA — Journal of the Association for Vascular Access 2009; 14(2):84–91.
- Chico-Padron RM, Carrion-Garcia L, Delle-Vedove-Rosales L, Gonzalez-Vargas CS, Marrero-Perera M, Medina-Chico S et al. Comparative safety and costs of transparent versus gauze wound dressings in intravenous catheterization. J Nurs Care Qual 2011; 26(4):371–6.
- Bausone-Gazda D, Lefaiver CA & Walters SA. A randomized controlled trial to compare the complications of 2 peripheral intravenous catheter-stabilization systems. J Infus Nurs 2010; 33(6):371–84.
- Marsh N, Webster J, Flynn J, Mihala G, Hewer B, Fraser J et al. Securement methods for peripheral venous catheters to prevent failure: a randomised controlled pilot trial. J Vasc Access 2015; 16(3):237–44.
- Safdar N, O’Horo JC, Ghufran A, Bearden A, Didier ME, Chateau D et al. Chlorhexidine-impregnated dressing for prevention of catheter-related bloodstream infection: a meta-analysis*. Crit Care Med 2014; 42(7):1703–13.
- Machado AF, Pedreira MLG & Chaud MN. Adverse events related to the use of peripheral intravenous catheters in children according to dressing regimens. Rev Lat Am Enfermagem 2008; 16(3):362–7.
- Dalal SS, Chawla D, Singh J, Agarwal RK, Deorari AK & Paul VK. Limb splinting for intravenous cannulae in neonates: a randomised controlled trial. Arch Dis Child Fetal Neonatal Ed 2009; 94:F394–6.
- National Health and Medical Research Council. Funding Facts Book 2013. Canberra: NHMRC, 2014.
- Australian Institute of Health and Welfare. Nursing and midwifery workforce 2012. Canberra: AIHW, 2013.
- Levac D CH, & O’Brien KK. Scoping studies: advancing the methodology. Implement Sci 2010; 5:69.
- Ainsworth S, Clerihew L & McGuire W. Percutaneous central venous catheters versus peripheral cannulae for delivery of parenteral nutrition in neonates. Cochrane Database Syst Rev 2007; 3.
- Flint A, McIntosh D & Davies MW. Continuous infusion versus intermittent flushing to prevent loss of function of peripheral intravenous catheters used for drug administration in newborn infants. Cochrane Database Syst Rev 2005; (4):CD004593.
- Hadaway L. Short peripheral intravenous catheters and infections. J Infusion Nurs 2012; 35(4):230–40.
- Helder O, van den Hoogen A, de Boer C, van Goudoever J, Verboon-Maciolek M & Kornelisse R. Effectiveness of non-pharmacological interventions for the prevention of bloodstream infections in infants admitted to a neonatal intensive care unit: A systematic review. Int J Nurs Stud 2013; 50(6):819–31.
- Niel-Weise BS, Stijnen T & van den Broek PJ. Should in-line filters be used in peripheral intravenous catheters to prevent infusion-related phlebitis? A systematic review of randomized controlled trials. Anesth Analg 2010; 110(6):1624–9.
- Niël-Weise BS, Daha TJ & van den Broek PJ. Is there evidence for recommending needleless closed catheter access systems in guidelines? A systematic review of randomized controlled trials. J Hosp Infect 2006; 62(4):406–13.
- Ray-Barruel G, Polit DF, Murfield JE & Rickard CM. Infusion phlebitis assessment measures: a systematic review. J Eval Clin Pract 2014; 20(2):191–202.
- Daud A, Cooke M & Rickard C. Replacement after Standard Versus Prolonged use of Administration Set for Arterial Catheter in an Australian Intensive Care Unit: A Feasibility RCT. J Vasc Access 2014; 15(3):193–239.
- Robertson-Malt S, Malt Greg N, Farquhar V & Greer W. Heparin versus normal saline for patency of arterial lines. Cochrane Database Syst Rev 2014; 5.
- LeMaster C, Agrawal A, Hou P & Schuur J. Systematic review of emergency department central venous and arterial catheter infection. Int J Emerg Med 2010; 3(4):409–23.
- Paoloni R, Taghizadeh M, Kouzios D & Janu M. Blood withdrawn through a cannula valve connector does not result in clinically significant haemolysis. Emerg Med Australas 2010; 22(4):310–5.
- Stauss M, Sherman B, Pugh L, Parone D, Looby-Rodriguez K, Bell A et al. Hemolysis of coagulation specimens: a comparative study of intravenous draw methods. J Emerg Nurs 2012; 38(1):15–21.
- Chen HC, Tzeng CM, Liu WS, Huang YF & Chen YY. Topical Xylocaine spray for reducing the pain of venipuncture in neonates. Clin Neonatol [Internet] 2006; 13(2):38–41. Available from: http://onlinelibrary.wiley.com/o/cochrane/clcentral/articles/582/CN-00641582/frame.html
- Deguzman ZC, O’Mara SK, Sulo S, Haines T, Blackburn L & Corazza J. Bacteriostatic normal saline compared with buffered 1% lidocaine when injected intradermally as a local anesthetic to reduce pain during intravenous catheter insertion. J Perianesth Nurs 2012; 27(6):399–407.
- Masud S, Wasnich RD, Ruckle JL, Garland WT, Halpern SW, Mee-Lee D et al. Contribution of a heating element to topical anesthesia patch efficacy prior to vascular access: results from two randomized, double-blind studies. J Pain Symptom Manage 2010; 40(4):510–9.
- Gupta D AA, Dhiraaj S, Tandon M, Kumar M, Singh RS et al. An evaluation of efficacy of balloon inflation on venous cannulation pain in children: A prospective, randomized, controlled study. Anesth Analg 2006; 102:1372–5.
- Anderson S, Cockrell J, Beller P, Murphy E, Nelson P, Hawkins M et al. Administration of local anesthetic agents to decrease pain associated with peripheral vascular access. J Infus Nurs 2010; 33(6):353–61.
- Arendts G, Stevens M & Fry M. Topical anaesthesia and intravenous cannulation success in paediatric patients: a randomized double-blind trial. Br J Anaesth 2008; 100(4):521–4.
- Kim do K, Choi SW & Kwak YH. The effect of SonoPrep® on EMLA® cream application for pain relief prior to intravenous cannulation. Eur J Pediatr 2012; 171(6):985–8.
- Hartstein BH & Barry JD. Mitigation of pain during intravenous catheter placement using a topical skin coolant in the emergency department. Emerg Med J 2008; 25(5):257–61.
- Hijazi R, Taylor D & Richardson J. Effect of topical alkane vapocoolant spray on pain with intravenous cannulation in patients in emergency departments: randomised double blind placebo controlled trial. BMJ (Clinical Research ed) 2009; 338:b215.
- Spanos S, Booth R, Koenig H, Sikes K, Gracely E & Kim IK. Jet Injection of 1% buffered lidocaine versus topical ELA-Max for anesthesia before peripheral intravenous catheterization in children: a randomized controlled trial. Pediatr Emerg Care 2008; 24(8):511–5.
- Page DE & Taylor DM. Vapocoolant spray vs subcutaneous lidocaine injection for reducing the pain of intravenous cannulation: a randomized, controlled, clinical trial. Br J Anaesth 2010; 105(4):519–25.
- Valdovinos NC, Reddin C, Bernard C, Shafer B & Tanabe P. The use of topical anesthesia during intravenous catheter insertion in adults: a comparison of pain scores using LMX-4 versus placebo. J Emerg Nurs 2009; 35(4):299–304.
- Waterhouse MR, Liu DR & Wang VJ. Cryotherapeutic topical analgesics for pediatric intravenous catheter placement: ice versus vapocoolant spray. Pediatr Emerg Care 2013; 29(1):8–12.
- Singer AJ, Weeks R & Regev R. Laser-assisted anesthesia reduces the pain of venous cannulation in children and adults: a randomized controlled trial. Acad Emerg Med 2006; 13(6):623–8.
- Singer AJ, Taira BR, Chisena EN, Gupta N & Chipley J. Warm lidocaine/tetracaine patch versus placebo before pediatric intravenous cannulation: a randomized controlled trial. Ann Emerg Med 2008; 52(1):41–7.
- Singer AJ, Regev R, Weeks R & Tlockowski DS. Laser-assisted anesthesia prior to intravenous cannulation in volunteers: a randomized, controlled trial. Acad Emerg Med 2005; 12(9):804–7.
- Skarbek-Borowska S, Becker BM, Lovgren K, Bates A & Minugh PA. Brief focal ultrasound with topical anesthetic decreases the pain of intravenous placement in children. Pediatr Emerg Care 2006; 22(5):339–45.
- Robinson PA, Carr S, Pearson S & Frampton C. Lignocaine is a better analgesic than either ethyl chloride or nitrous oxide for peripheral intravenous cannulation. Emerg Med Australas 2007; 19(5):427–32.
- Beck RM, Zbierajewski FJ, Barber MK, Engoren M & Thomas R. A comparison of the pain perceived during intravenous catheter insertion after injection with various local anesthetics. AANA J 2011; 79(4 Suppl):S58–61.
- Burke SD, Vercler SJ, Bye RO, Desmond PC & Rees YW. Local anesthesia before IV catheterization. Am J Nurs 2011; 111(2):40–5; quiz 6–7.
- Canbulat N, Ayhan F & Inal S. Effectiveness of external cold and vibration for procedural pain relief during peripheral intravenous cannulation in pediatric patients. Pain Manag Nurs 2015; 16(1):33–9.
- Jacobson AF. Cognitive-behavioral interventions for IV insertion pain. AORN J 2006; 84(6):1031–48.
- Jimenez N, Bradford H, Seidel KD, Sousa M & Lynn AM. A comparison of a needle-free injection system for local anesthesia versus EMLA for intravenous catheter insertion in the pediatric patient. Anesth Analg 2006; 102(2):411–4.
- Svensson M, Rosén S & Nilsson U. Local warming to reduce pain on peripheral intravenous cannula insertion: a randomised controlled study. Journal of Advanced Perioperative Care 2006; 2(3):107–11.
- Taddio A, Soin HK, Schuh S, Koren G & Scolnik D. Liposomal lidocaine to improve procedural success rates and reduce procedural pain among children: a randomized controlled trial. CMAJ 2005; 172(13):1691–5.
- Armagan E, Kocabas E, Koksal O, Simsek G & Bal H. Comparison of the efficacies of topical anaesthetics in the reduction of the pain during peripheral intravenous cannulation: A randomised trial. Hong Kong Journal of Emergency Medicine 2012; 19(3):183–8.
- Hall DL, Rezvan E, Tatakis DN & Walters JD. Oral clonidine pretreatment prior to venous cannulation. Anesth Prog 2006; 53(2):34–42.
- Kahre C, Fortune V, Hurley J & Winsett RP. Randomized controlled trial to compare effects of pain relief during IV insertion using bacteriostatic normal saline and 1% buffered lidocaine. J Perianesth Nurs 2011; 26(5):310–4.
- McCarthy AM HK, Zimmerman MB,Westhus N & Allen S. Impact of parent-provided distraction on child responses to an IV insertion. Child Health Care 2010; 39:125–41.
- Small H, Adams D, Casey AL, Crosby CT, Lambert PA & Elliott T. Efficacy of Adding 2% (w/v) Chlorhexidine Gluconate to 70% (v/v) Isopropyl Alcohol for Skin Disinfection Prior to Peripheral Venous Cannulation. Infect Control Hosp Epidemiol 2008; 29(10):963–5.
- Aponte H, Acosta S, Rigamonti D, Sylvia B, Austin P & Samolitis T. The use of ultrasound for placement of intravenous catheters. AANA J 2007; 75(3):212–6.
- Sun CY, Lee KC, Lin IH, Wu CL, Huang HP, Lin YY et al. Near-infrared light device can improve intravenous cannulation in critically ill children. Pediatr Neonatol 2013; 54(3):194–7.
- Hosokawa K KH, Kishi C, Kato Y & Shime N Transillumination by light-emitting diode facilitates peripheral venous cannulations in infants and small children. Acta Anaesthesiol Scand 2010; 54:957–61.
- Aulagnier J, Hoc C, Mathieu E, Dreyfus JF, Fischler M & Le Guen M. Efficacy of AccuVein to facilitate peripheral intravenous placement in adults presenting to an emergency department: a randomized clinical trial. Acad Emerg Med 2014; 21(8):858–63.
- Bair AE, Rose JS, Vance CW, Andrada-Brown E & Kuppermann N. Ultrasound-assisted peripheral venous access in young children: A randomized controlled trial and pilot feasibility study. West J Emerg Med 2008; 9(4):219–24.
- Chapman LL, Sullivan B, Pacheco AL, Draleau CP & Becker BM. VeinViewer-assisted intravenous catheter placement in a pediatric emergency department. Acad Emerg Med 2011; 18(9):966–71.
- Costantino TG, Parikh AK, Satz WA & Fojtik JP. Ultrasonography-guided peripheral intravenous access versus traditional approaches in patients with difficult intravenous access. Ann Emerg Med 2005; 46(5):456–61.
- Curtis SJ, Craig WR, Logue E, Vandermeer B, Hanson A & Klassen T. Ultrasound or near-infrared vascular imaging to guide peripheral intravenous catheterization in children: a pragmatic randomized controlled trial. CMAJ 2015; 187(8):563–70.
- Doniger SJ, Ishimine P, Fox JC & Kanegaye JT. Randomized controlled trial of ultrasound-guided peripheral intravenous catheter placement versus traditional techniques in difficult-access pediatric patients. Pediatr Emerg Care 2009; 25(3):154–9.
- Katsogridakis YL, Seshadri R, Sullivan C & Waltzman ML. Veinlite transillumination in the pediatric emergency department: a therapeutic interventional trial. Pediatr Emerg Care 2008; 24(2):83–8.
- Stein J, George B, River G, Hebig A & McDermott D. Ultrasonographically guided peripheral intravenous cannulation in emergency department patients with difficult intravenous access: a randomized trial. Ann Emerg Med 2009; 54(1):33–40.
- Avelar AF, Peterlini MA & Pedreira ML. Assertiveness and peripheral intravenous catheters dwell time with ultrasonography-guided insertion in children and adolescents. Rev Esc Enferm USP 2013; 47(3):539–46.
- Benkhadra M, Collignon M, Fournel I, Oeuvrard C, Rollin P, Perrin M et al. Ultrasound guidance allows faster peripheral IV cannulation in children under 3 years of age with difficult venous access: a prospective randomized study. Paediatr Anaesth 2012; 22(5):449–54.
- Kaddoum RN, Anghelescu DL, Parish ME, Wright BB, Trujillo L, Wu J et al. A randomized controlled trial comparing the AccuVein AV300 device to standard insertion technique for intravenous cannulation of anesthetized children. Paediatr Anaesth 2012; 22(9):884–9.
- Simhi E, Kachko L, Bruckheimer E & Katz J. A vein entry indicator device for facilitating peripheral intravenous cannulation in children: a prospective, randomized, controlled trial. Anesth Analg 2008; 107(5):1531–5.
- Szmuk P, Steiner J, Pop RB, Farrow-Gillespie A, Mascha EJ & Sessler DI. The VeinViewer vascular imaging system worsens first-attempt cannulation rate for experienced nurses in infants and children with anticipated difficult intravenous access. Anesth Analg 2013; 116(5):1087–92.
- Takeshita J, Nakayama Y, Nakajima Y, Sessler DI, Ogawa S, Sawa T et al. Optimal site for ultrasound-guided venous catheterisation in paediatric patients: an observational study to investigate predictors for catheterisation success and a randomised controlled study to determine the most successful site. Crit Care 2015; 19(15):1–9.
- Kim MJ, Park JM, Rhee N, Je SM, Hong SH, Lee YM et al. Efficacy of VeinViewer in pediatric peripheral intravenous access: a randomized controlled trial. Eur J Pediatr 2012; 171(7):1121–5.
- Pappas N. Ultrasound guidance as a rescue technique for peripheral intravenous cannulation. AANA J 2006; 74:464.
- Rose JS & Norbutas CM. A randomized controlled trial comparing one-operator versus two-operator technique in ultrasound-guided basilic vein cannulation. J Emerg Med 2008; 35(4):431–5.
- Yamazaki S, Tomita S, Watanabe M, Kawaai H & Shimamura K. Effects of a transmitted light device for pediatric peripheral venipuncture and intravenous cannulation. Medical Devices: Evidence and Research 2011; 4(1).
- Arnts IJ, Heijnen JA, Wilbers HT, van der Wilt GJ, Groenewoud JM & Liem KD. Effectiveness of heparin solution versus normal saline in maintaining patency of intravenous locks in neonates: a double blind randomized controlled study. J Adv Nurs 2011; 67(12):2677–85.
- Upadhyay A, Verma KK, Lal P, Chawla D & Sreenivas V. Heparin for prolonging peripheral intravenous catheter use in neonates: a randomized controlled trial. J Perinatol 2015; 35(4):274–7.
- Elgin K, Cozzi K, Fowler MA, Perry SA, Davis MS, Conaway MR et al. Maintaining patency with packed red blood cell infusions: comparison of IV normal saline infusion vs. normal saline syringe method. Medsurg Nurs 2011; 20(3):134–8.
- Mok E, Kwong TKY & Chan MF. A randomized controlled trial for maintaining peripheral intravenous lock in children. Int J Nurs Pract 2007; 13(1):33–45.
- Myrianthefs P, Sifaki M, Samara I & Baltopoulos G. The epidemiology of peripheral vein complications: evaluation of the efficiency of differing methods for the maintenance of catheter patency and thrombophlebitis prevention. J Eval Clin Pract 2005; 11(1):85–9.
- Patidar AB, Choudhary M & Bindu K. Comparative efficacy of heparin saline and normal saline flush for maintaining patency of peripheral intravenous lines: a randomized control trial. Int J Health Sci Res 2014; 4(3):159–66.
- Schreiber S, Zanchi C, Ronfani L, Delise A, Corbelli A, Bortoluzzi R et al. Normal saline flushes performed once daily maintain peripheral intravenous catheter patency: a randomized controlled trial. Arch Dis Child 2015; 100:700–3.
- Tripathi S, Kaushik V & Singh V. Peripheral IVs: factors affecting complications and patency: a randomized controlled trial. J Infus Nurs 2008; 31(3):182–8.
- Haddad FG, Waked CH & Zein EF. Peripheral venous catheter-related inflammation. A randomized prospective trial. J Med Liban 2006; 54(3):139–45.
- Elia F, Ferrari G, Molino P, Converso M, De Filippi G, Milan A et al. Standard-length catheters vs long catheters in ultrasound-guided peripheral vein cannulation. Am J Emerg Med 2012; 30(5):712–6.
- Ozsarac M, Dolek M, Sarsilmaz M, Sever M, Sener S, Kiyan S et al. The effect of cannula material on the pain of peripheral intravenous cannulation in the emergency department: A prospective, randomized controlled study. Turkiye Acil Tip Dergisi 2012; 12(4):151–6.
- Prunet B, Meaudre E, Montcriol A, Asencio Y, Bordes J, Lacroix G et al. A prospective randomized trial of two safety peripheral intravenous catheters. Anesth Analg 2008; 107(1):155–8.
- Fraser N, Snyman JR, Wessels F & Nel G. Intravenous fluid therapy: a randomized controlled trial to investigate the effectiveness of the IV2TM flow medical device. J Clin Nurs 2007; 16(9):1593–601.
- González López JL, Arribi Vilela A, Fernández del Palacio E, Olivares Corral J, Benedicto Martí C & Herrera Portal P. Indwell times, complications and costs of open vs closed safety peripheral intravenous catheters: a randomized study. J Hosp Infect 2014; 86(2):117–26.
- Idemoto BK, Rowbottom JR, Reynolds JD & Hickman Jr RL. The AccuCath intravenous catheter system with retractable coiled tip guidewire and conventional peripheral intravenous catheters: a prospective, randomized, controlled comparison. Journal of the Association for Vascular Access 2014; 19(2):94–102.
- Martinez JA, Piazuelo M, Almela M, Blecua P, Gallardo R, Rodriguez S et al. Evaluation of add-on devices for the prevention of phlebitis and other complications associated with the use of peripheral catheters in hospitalised adults: a randomised controlled study. J Hosp Infect 2009; 73(2):135–42.
- Onia R, Eshun-Wilson I, Arce C, Ellis C, Parvu V, Hassman D et al. Evaluation of a new safety peripheral IV catheter designed to reduce mucocutaneous blood exposure. Curr Med Res Opin 2011; 27(7):1339–46.
- Ruiz-Sternberg A, Velez-Van-Meerbeke A & Ruiz-Sternberg J. Clinical acceptability and ease of use of a safety IV catheter system. Curr Med Res Opin 2012; 28(8):1381–7.
- Schwab SA, Uder M, Anders K, Heinrich MC & Kuefner MA. Peripheral intravenous power injection of iodinated contrast media through 22G and 20G cannulas: can high flow rates be achieved safely? A clinical feasibility study. RöFo 2009; 181(4):355–61.
- Barria RM, Lorca P & Munoz S. Randomized controlled trial of vascular access in newborns in the neonatal intensive care unit. J Obstet Gynecol Neonatal Nurs 2007; 36(5):450–6.
- Wilson D, Verklan MT & Kennedy KA. Randomized trial of percutaneous central venous lines versus peripheral intravenous lines. J Perinatol 2007; 27(2):92–6.
- Periard D, Monney P, Waeber G, Zurkinden C, Mazzolai L, Hayoz D et al. Randomized controlled trial of peripherally inserted central catheters vs. peripheral catheters for middle duration in-hospital intravenous therapy. J Thromb Haemost 2008; 6(8):1281–8.
- Bagheri-Nesami M, Shorofi S, Hashemi-Karoie S & Khalilian A. The effects of sesame oil on the prevention of amiodarone-induced phlebitis. Iran J Nurs Midwifery Res 2015; 20(3):365–70.
- Catton JA, Davies J, Dobbins BM, Wood JM, McMahon MJ & Burke D. The effect of heparin in peripheral intravenous nutrition via a fine-bore midline: A randomised double-blind controlled trial. Clin Nutr 2006; 25(3):394–9.
- Harber CR, Sosnowski KJ & Hegde RM. Highly conservative phlebotomy in adult intensive care: a prospective randomized controlled trial. Anaesth Intensive Care 2006; 34(4):434–7.
- Mahdy S, Khan EI, Attia M, O’Brien BP & Seigne P. Evaluation of a blood conservation strategy in the intensive care unit: a prospective, randomised study. Middle East J Anaesthesiol 2009; 20(2):219–23.
- Raurell-Torredà M, Llano-Serrano Cd, Almirall-Solsona D & Nicolás-Arfelis JM. Arterial catheter setup for glucose control in critically ill patients: a randomized controlled trial. Am J Crit Care 2014; 23(2):150–9.
- Eker HE, Tuzuner A, Yilmaz AA, Alanoglu Z & Ates Y. The impact of two arterial catheters, different in diameter and length, on postcannulation radial artery diameter, blood flow, and occlusion in atherosclerotic patients. J Anesth 2009; 23(3):347–52.
- Kim SY, Lee JS, Kim WO, Sun JM, Kwon MK & Kil HK. Evaluation of radial and ulnar blood flow after radial artery cannulation with 20- and 22-gauge cannulae using duplex Doppler ultrasound. Anaesthesia 2012; 67(10):1138–45.
- De Oliveira GS, Jr., Beckmann K, Salvacion A, Kim J, Sherwani S & McCarthy RJ. The effect of the arterial catheter insertion technique on the success of radial artery cannulation: a prospective and randomized study. J Crit Care 2014; 29(3):475 e7–10.
- Karacalar S, Ceyhan M, Bayrak IK, Yegin S, Sarihasan B & Keceligil HT. Feasibility and safety of antegrade radial artery cannulation. Eur J Anaesthesiol 2009; 26(3):207–12.
- Karacalar S, Ture H, Baris S, Karakaya D & Sarihasan B. Ulnar artery versus radial artery approach for arterial cannulation: a prospective, comparative study. J Clin Anesth 2007; 19:209–13.
- Kucuk A, Yuce HH, Yalcin F, Boyaci FN, Yildiz S & Yalcin S. Forty-five degree wrist angulation is optimal for ultrasound guided long axis radial artery cannulation in patients over 60 years old: a randomized study. J Clin Monit Comput 2014; 28(6):567–72.
- Yildirim V, Ozal E, Cosar A, Bolcal C, Han Acikel C, Kilic S et al. Direct versus guidewire-assisted pediatric radial artery cannulation technique. J Cardiothorac Vasc Anesth [Internet] 2006; 20(1):48–50. Available from: http://onlinelibrary.wiley.com/o/cochrane/clcentral/articles/865/CN-00622865/frame.html
- Ohara Y, Nakayama S, Furukawa H, Satoh Y, Suzuki H & Yanai H. Use of a wire-guided cannula for radial arterial cannulation. J Anesth 2007; 21(1):83–5.
- Ganesh A, Kaye R, Cahill AM, Stern W, Pachikara R, Gallagher PR et al. Evaluation of ultrasound-guided radial artery cannulation in children. Pediatr Crit Care Med 2009; 10(1):45–8.
- Ishii S, Shime N, Shibasaki M & Sawa T. Ultrasound-guided radial artery catheterization in infants and small children*. Pediatr Crit Care Med 2013; 14(5):471–3.
- Peters C, Schwarz SKW, Yarnold CH, Kojic K, Kojic S & Head SJ. Ultrasound guidance versus direct palpation for radial artery catheterization by expert operators: a randomized trial among Canadian cardiac anesthesiologists. Can J Anaesth 2015.
- Ueda K, Puangsuvan S, Hove MA & Bayman EO. Ultrasound visual image-guided vs Doppler auditory-assisted radial artery cannulation in infants and small children by non-expert anaesthesiologists: a randomized prospective study. Br J Anaesth 2013; 110(2):281–6.
- Berk D, Gurkan Y, Kus A, Ulugol H, Solak M & Toker K. Ultrasound-guided radial arterial cannulation: long axis/in-plane versus short axis/out-of-plane approaches? J Clin Monit Comput 2013; 27(3):319–24.
- Edwards M, Rickard CM, Rapchuk I, Corley A, Marsh N, Spooner AJ et al. A pilot trial of bordered polyurethane dressings, tissue adhesive and sutureless devices compared with standard polyurethane dressings for securing short-term arterial catheters. Crit Care Resusc 2014; 16(3):175–83.
- Del Cotillo M, Grane N, Llavore M & Quintana S. Heparinized solution vs. saline solution in the maintenance of arterial catheters: a double blind randomized clinical trial. Intensive Care Med 2008; 34(2):339–43.
- Goh LJ, Teo HS & Masagoes M. Heparinised saline versus normal saline in maintaining patency of arterial and central venous catheters. Proc Singapore Healthcare 2011; 20(3):190–6.
- Hall KF, Bennetts TM, Whitta RK, Welman L & Rawlins P. Effect of heparin in arterial line flushing solutions on platelet count: a randomised double-blind study. Crit Care Resusc 2006; 8(4):294–6.
- Whitta RK, Hall KF, Bennetts TM, Welman L & Rawlins P. Comparison of normal or heparinised saline flushing on function of arterial lines. Crit Care Resusc 2006; 8(3):205–8.
- Witkowski MC, Moraes MA & Firpo CM. Lack of difference between continuous versus intermittent heparin infusion on maintenance of intra-arterial catheter in postoperative pediatric surgery: a randomized controlled study. Rev Paul Pediatr 2013; 31(4):516–22.
- Griffin MP & Siadaty MS. Papaverine prolongs patency of peripheral arterial catheters in neonates. J Pediatr 2005; 146(1):62–5.
- Tuncali BE, Kuvaki B, Tuncali B & Capar E. A comparison of the efficacy of heparinized and nonheparinized solutions for maintenance of perioperative radial arterial catheter patency and subsequent occlusion. Anesth Analg 2005; 100(4):1117–21.
- Yébenes JC, Sauca G, Solsona M, Martinez R, Serra-Prat M, Gil P et al. Safety of positive-pressure valve connectors in arterial catheters inserted into critically ill patients. J Hosp Infect 2008; 70(4):341–5.
- Dutt-Gupta J, Bown T & Cyna AM. Effect of communication on pain during intravenous cannulation: a randomized controlled trial. Br J Anaesth 2007; 99(6):871–5.
- Uman LS, Birnie KA, Noel M, Parker JA, Chambers CT, McGrath PJ et al. Psychological interventions for needle-related procedural pain and distress in children and adolescents. Cochrane Database Syst Rev 2013; 10:CD005179.
- Shiloh AL, Savel RH, Paulin LM & Eisen LA. Ultrasound-guided catheterization of the radial artery: a systematic review and meta-analysis of randomized controlled trials. Chest 2011; 139(3):524–9.
- Idvall E & Gunningberg L. Evidence for elective replacement of peripheral intravenous catheter to prevent thrombophlebitis: a systematic review. J Adv Nurs 2006; 55(6):715–22.
- Webster J, Osborne S, Rickard C & Hall J. Clinically-indicated replacement versus routine replacement of peripheral venous catheters. Cochrane Database Syst Rev 2010; 3:CD007798.
- Webster J, Osborne S, Rickard CM & New K. Clinically-indicated replacement versus routine replacement of peripheral venous catheters. Cochrane Database Syst Rev 2013; 4:CD007798.
- Gillies D, O’Riordan L, Wallen M, Morrison A, Rankin K & Nagy S. Optimal timing for intravenous administration set replacement. Cochrane Database Syst Rev 2005; 4:CD003588.
- Ullman AJ, Cooke ML, Gillies D, Marsh NM, Daud A, McGrail MR et al. Optimal timing for intravascular administration set replacement. Cochrane Database Syst Rev 2013; 9:Cd003588.
- Daud A, Rickard C, Cooke M & Reynolds H. Replacement of administration sets (including transducers) for peripheral arterial catheters: a systematic review. J Clin Nurs 2013; 22(3–4):303–17.
- Marsh NM, Webster J, Mihala G & Rickard Claire M. Devices and dressings to secure peripheral venous catheters to prevent complications (Review). Cochrane Database Syst Rev 2015; 6:CD011070.
- Foster J, Richards R & Showell M. Intravenous in-line filters for preventing morbidity and mortality in neonates. Cochrane Database Syst Rev 2006; 2:CD005248.
- Prayle Andrew P, Hurley Matthew N & Smyth Alan R. Percutaneous lines for delivering intravenous antibiotics in people with cystic fibrosis. Cochrane Database Syst Rev 2010; 11.
- Ainsworth SB, Clerihew L & McGuire W. Percutaneous central venous catheters versus peripheral cannulae for delivery of parenteral nutrition in neonates. Cochrane Database Syst Rev 2007; 3:CD004219.
- Maki DG, Kluger DM & Crnich CJ. The risk of bloodstream infection in adults with different intravascular devices: a systematic review of 200 published prospective studies. Mayo Clin Proc 2006; 81(9):1159–71.
- Phillips P, Cortina-Borja M, Millar M & Gilbert R. Risk-adjusted surveillance of hospital-acquired infections in neonatal intensive care units: a systematic review. Hosp Infect 2008; 70(3):203–11.
- O’Horo JC, Maki DG, Krupp AE & Safdar N. Arterial catheters as a source of bloodstream infection: a systematic review and meta-analysis*. Crit Care Med 2014; 42(6):1334–9.
- Zheng GH, Yang L, Chen HY, Chu JF & Mei L. Aloe vera for prevention and treatment of infusion phlebitis. Cochrane Database Syst Rev 2014; 6:N.PAG.
- Frampton GK, Harris P, Cooper K, Cooper T, Cleland J, Jones J et al. Educational interventions for preventing vascular catheter bloodstream infections in critical care: evidence map, systematic review and economic evaluation. Health Technol Assess 2014; 18(15):1–365.
- Ciofi Silva CL, Rossi LA, Canini SRMdS, Gonçalves N & Furuya RK. Site of catheter insertion in burn patients and infection: a systematic review. Burns (03054179) 2014; 40(3):365–73.
- Kumar M, Vandermeer B, Bassler D & Mansoor N. Low-dose heparin use and the patency of peripheral IV catheters in children: a systematic review. Pediatrics 2013; 131(3):e864–72.
- Robertson-Malt S, Malt Greg N, Farquhar V & Greer W. Heparin versus normal saline for patency of arterial lines. Cochrane Database Syst Rev 2014; 5.
