Volume 31 Number 2
Re-imagining wound dressing trolleys and disposable wound dressing packs
Clarissa A Young
Keywords wound dressing trolleys, stainless steel, dressing packs
For referencing Young CA. Re-imagining wound dressing trolleys and disposable wound dressing packs. Wound Practice and Research 2023; 31(2):68-73.
DOI
https://doi.org/10.33235/wpr.31.2.68-73
Submitted 13 December 2022
Accepted 20 February 2023
Abstract
This article describes the pathway used to re-imagine the design of both the wound dressing trolley (WDT) and one key component of a commercially available disposable wound dressing pack used in healthcare settings. A chance observation of equipment led to the development of WDT specifications using the plan-do-study-act (PDSA) quality improvement cycle. This included auditing all ward and clinic WDTs, excluding the operating room, and carrying out consultations with experienced co-workers. The subsequent redesign and specifications for the WDT coincided with an opportunity to remedy a long-standing oversight of the size of a wound dressing pack sterile field. The two issues are inter-connected and presented an opportunity for practical solutions and outcomes in a mid-sized acute health facility and across all health settings.
Introduction
Stainless steel wound dressing trolleys (WDTs) have been used in acute care and non-acute healthcare settings for over 4 decades. Stainless steel manufacturers have responded to requests or initiated design features fabricating a variety of styles dependent on the proposed end use, setting, historical purchasing patterns and preference of the purchaser.
However, a review of the national and international literature has revealed an absence of guidelines or standards related to the construction, materials, dimensions and infection control considerations for WDTs used in healthcare settings. The absence of guidelines or specifications has resulted in ad-hoc purchasing decisions over many years, with eight different WDT styles in our facility.
Similarly, the disposable wound dressing pack has evolved over time and is a key component in contemporary wound management. However, there is no historical narrative to describe their style or components and they have remained largely unchanged for decades. One small change of increasing the size of the sterile wound field in the wound dressing packs has the potential to enhance aseptic technique in wound management.
Stainless steel
Stainless steel was first manufactured in 1913 and is a corrosion-resistant alloy of iron, chromium and, in some cases, nickel and other metals1,2. Stainless steel has undergone huge developments in manufacture, with approximately 100 grades of stainless steel produced for commercial use2. The classification of stainless steel is based on the arrangement of its molecules or crystalline structure, and the grades come under four family groups, with austenitic stainless steel grades 304 and 316 used the most3. Stainless steel is chemically inert, non-toxic and can be manufactured into smooth non-absorbent surfaces, some of which can be thoroughly cleaned, disinfected and sterilised without degradation or corrosion; it is non-magnetic and can recoat itself in the presence of oxygen4,5.
Commonly used descriptions of stainless steel include commercial, surgical and medical grades. However, there is no formal definition on the constitution of surgical stainless steel, and product manufacturers and distributors often apply the term to any grade of corrosion-resistant stainless steel6. Commercial grade 304 stainless steel is used in industrial and commercial kitchens and is the most common, versatile and widely used stainless steel in the healthcare environment. It can resist chemical compounds such as iodine, bleach, peroxide, dyes, human tissue, blood and bodily fluids7.
Stainless steel in healthcare
Surgical and medical grade stainless steel may also be referred to as marine grade 316. This grade exhibits greater corrosion resistance, has relative strength, and is used in biomedical applications, the manufacture of medical surgical instruments7, and the manufacture and handling of pharmaceutical and food products6. It is considered ideal in pharmaceutical and medical applications as sterilisation processes in these industries combine strong disinfectants, high temperatures, or both, to prevent contamination4. Grade 316 has better resistance to chemicals and chlorides (like salt) than grade 3048 but it is not fully resistant to sea water9.
Stainless steel surfaces are reported to have a greater hygiene value and require lower concentrations of disinfectant to achieve the level of hygiene required8. One weakness of grade 304 stainless steel is its susceptibility to pitting and localised areas of corrosion due to high levels of exposure to chloride solutions or saline environments. As little as 25ppm of chlorides can cause pitting corrosion to begin7.
The continuing safety of using stainless steel in healthcare environments was confirmed in a 2020 study4. Researchers from Manchester Metropolitan University and Agro Paris Tech found that there was no discernible difference between the efficiency of disinfection across the range of grades and finishes, and whether the stainless steel was new or aged. This confirmed the effectiveness of disinfecting stainless steel against bacteria associated with healthcare-acquired infections and its ongoing suitability as a material for use in clinical environments4,8.
Methods
The Launceston General Hospital (LGH) is the second largest public hospital in the island state of Tasmania, Australia. The facility provides inpatient and ambulatory services in the north of the state, providing most specialties, and is an accredited teaching hospital with over 300 inpatient beds.
In June 2021 a chance observation of ‘rust-like streaks’ on the predominately vertical surfaces of six of 21 (29%) WDTs in the surgical outpatient clinic raised concerns regarding the potential effects of cleaning agents and the quality of stainless steel in the WDTs (Figure 1). To explore the issues, in consultation with the infection prevention and control unit (IPCU), a quality improvement framework applying the elements of the plan-do-study-act (PDSA) cycle was registered. The PDSA cycle is widely accepted in healthcare improvement as a method for rapidly testing a change by planning it, trying it, observing results and acting on the learnings10. The key principle behind the PDSA cycle is to test on a small scale and to test quickly11.
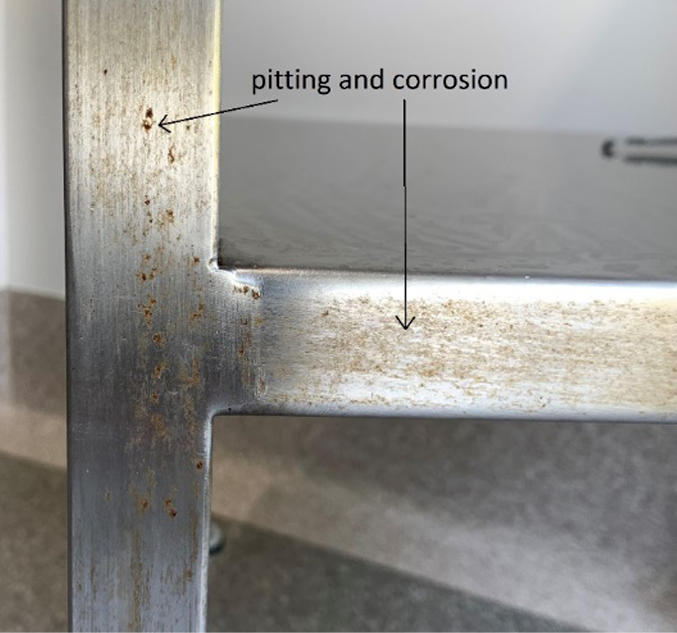
Figure 1. Corrosion on both vertical and horizontal surfaces following the grain of the stainless steel
The quality improvement project was delivered from June 2021 to July 2022 and included:
- Facility-wide audit of the existing stainless steel WDTs.
- Literature review exploring WDTs and stainless steel in healthcare.
- Development of specifications to guide future purchasing.
- Engagement with industry to produce WDTs built to specifications.
- On site testing and refinement of bespoke WDTs based on our specifications.
The project was undertaken under the auspices of the facility group and input from external key clinicians, with fluid membership required for stages of the PDSA cycle.
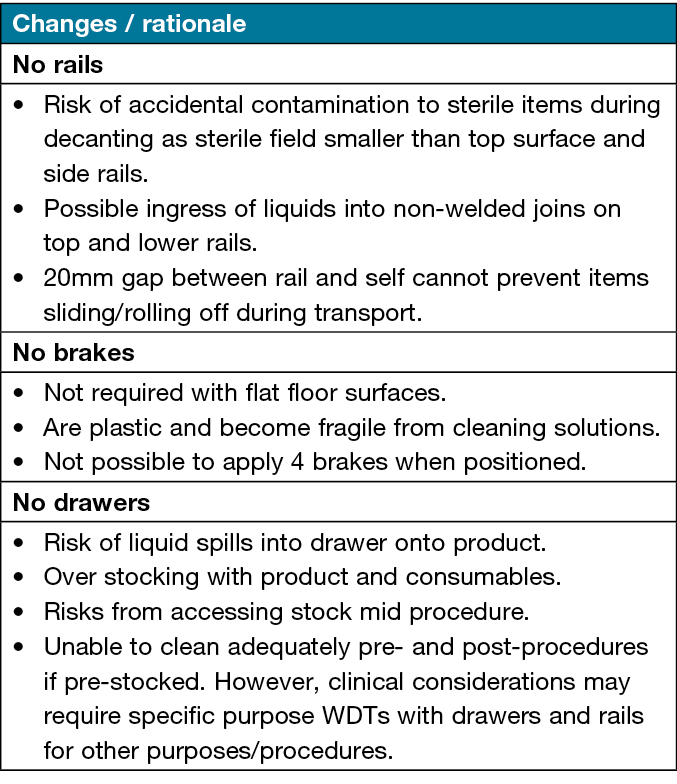
Audit
The facility-wide audit identified 63 WDTs, with a variety of seven styles and sizes in use (Table 1). Key issues identified included signs of corrosion on 40% of the galvanised forks/castors (Figure 2), brittle plastic brakes, and the possible effects of chloride-based cleaning products on vertical surfaces on 10% of WDTs. There were a smaller number of WDTs with one or more drawers, partially or fully filled with consumables, with rails on the top and the second shelf. There were no labels identifying the grade of the stainless steel or manufacture date on any of the WDTs; this appears to be a universal practice.
Table 1 describes the variations in design of WDTs from the facility wide audit.
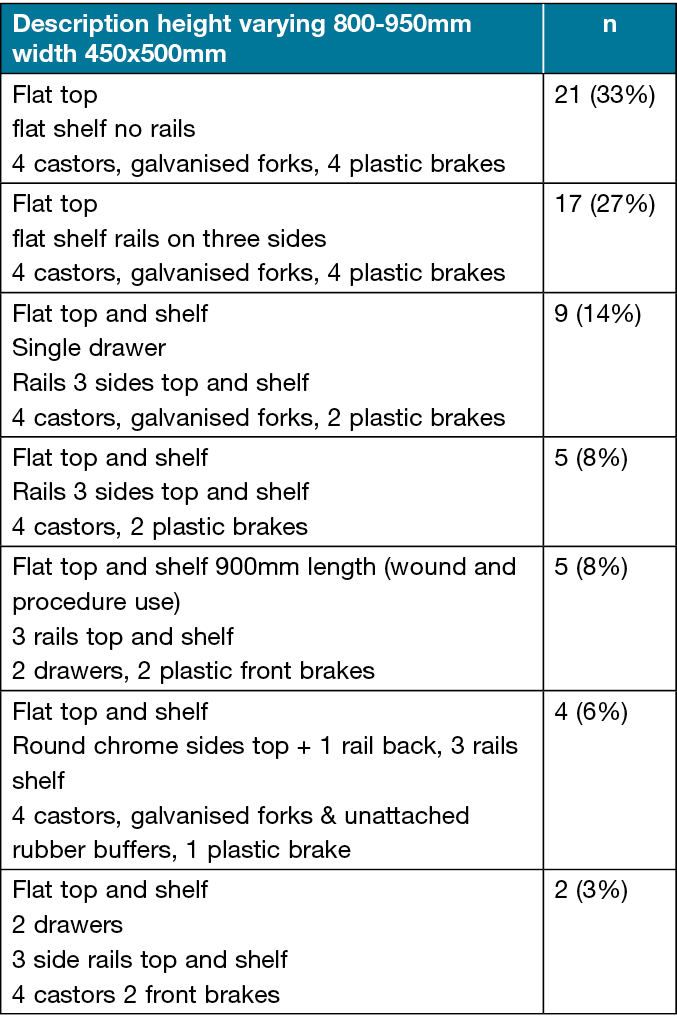

Figure 2. Rust/corrosion on galvanised forks and attachment point to WDT
Literature review
A comprehensive search of the literature revealed only two papers documenting WDT design for use in acute care facilities. The infection prevention and control section of the current Australasian Health Facility Guidelines12 suggest surfaces in patient areas need to be smooth, impervious and easily cleaned. In describing some features of a desirable WDT, Al Aboud13 suggested ‘the design of WDTs should not be left to factories’ but should be designed to a standard to meet the need of the health workforce. Some suggested criteria included easily cleanable surfaces, of appropriate height, and with quiet wheels; however, WDT’s material was undefined13. Similarly, Golvardi Yazdi14 presented an undefined grade of stainless steel for a rather complex automated WDT with an optional fibreglass construction.
Drafting specifications
Due to the absence of specifications and guidelines within the literature, a search for of any specifications including grade of stainless steel was sought from the commercial and industrial sectors. The height, width and depth of shelf, were developed by consulting experienced nurses and listening to advice from the onsite mechanical engineers and members of IPCU. Key features (Table 2) included the elimination of all rails from the WDT as a potential source of fluid contamination into the unsealed insertion points on the WDT legs. The rails on the platform also pose a risk from accidental contamination when decanting items onto the WDT as they are not covered by the current small sized sterile wound field. The 20mm gap between the rails and shelves does not prevent items rolling or falling from the WDT during transport. The innovation of flipping the second shelf to a tray with a 20mm high edges on four sides was proposed to prevent items, especially light small products or polyamps, rolling and falling during transport.
Table 2. The preferred features of WDTs from the key contributors
Recurrent themes from the key clinicians indicated brakes were not considered necessary as WDT, by definition, need to be mobile and in healthcare settings floor surfaces are usually level. Stainless steel forks and castors were advised to minimise corrosion to the galvanised sets usually affixed on purchase. Drawers were eliminated as they pose a risk from accidental fluid spills, inviting access mid-procedure, risking contact contamination and the overstocking of consumables. Removing rails and drawers allows for smooth flat surfaces for cleaning and decontamination of WDT pre- and post-dressing procedure in line with ANTT® guidelines15.
Specifications were sent to two local and one interstate manufacturer, and a local company was commissioned to produce one grade 304 stainless steel WDT in February 2022. The prototype allowed for the key clinicians and advisors to view, test and propose several changes to the specifications that became evident as we cycled through the PDSA. As a result of the review, changes for future WDTs included ensuring its height match the standard domestic bench of 900mm including the castors, increasing the height of the four sided tray to 40mm, and welding of waterfall joints in the tray. Rubber wheels/castors were selected as the original thermoplastic rubber was deemed unsuitable due to noise on movement over hard floor surfaces. The report confirming the suitability of the refined WDT was sent to and approved by the facilities Products Evaluation Committee (PEC) in April 2022.
Commercial wound dressing packs
Disposable wound dressing packs have also evolved over time based on the requests to manufacturers to meet changing clinical practice needs, or initiated by manufacturers themselves. Disposable dressing packs emerged in the early 1970s but there is no historical record of the development and specifications of the packs. A search of the academic literature identified an absence of any narrative or evidence of the evolution of the disposable wound dressing pack in wound practice.
Anecdotally, experienced health professionals remember changes to dressing pack items that include the replacement of cotton wool balls to woven cotton gauze, and then to non-woven gauze made from blended synthetic fibre during the late 1980s and early 1990s. Both these changes relate to the shedding of fibres into wounds and the potential for residual inflammatory changes16. It is unclear if the sterile field has become smaller and when, but it is perceived to have reduced dimensions over time.
Evolving practices in wound management and products have occurred along with practice changes in the application and principles of aseptic wound dressing procedures. Anecdotally, experienced wound professionals have identified the risk of contamination when decanting sterile items onto a small sterile field that exposes the uncovered top platform and/or the side rails of the WDT. The sterile/aseptic field should not be confused with the dressing towel inside the dressing pack. The average dimensions of stainless steel WDTs are between 480–520mm, and the current sterile field used in the THS and likely across the nation is 480mm with up to a 50mm uncovered edge/rail on the WDT platform. The anomaly between the undersized sterile field in the wound pack and the dimensions of the WDT has been apparent and unchecked for many years in Australia.
The 2021–22 THS state-wide wound product and related items tender afforded an opportunity to include dressing pack components, including sterile field specifications to meet this precise need. Based on an estimated 350,00 packs used in the THS in 2020–21, it is evident that millions of wound dressing packs are used each year nationally and internationally.
Only one company met the criteria of customising the pack with a sterile field measuring 600mm x 600mm that will cover the top and rails of the current usable and future WDTs. The ‘Tas custom pack’ is due for use across all Tasmanian government health sites in early 2023 and would be available to other services through normal procurement processes.
Discussion
It has been reported in the trade literature9 that, over time, stainless steel can become stained or discoloured. This effect has been reported on structural stainless steel most commonly within 5kms of coastal areas and progressively worsens closest to the marine source, and in the presence of surface corrosives9,17. This discolouration of surface stainless steel tends to follow the ‘grain’ of the finished surface and is referred to as ‘tea staining’. The presence of tea staining indicates corrosion of the stainless steel, is rarely seen as a problem indoors unless exposure to corrosive deposits, and is considered a cosmetic rather than a structural issue17.
Within the health sector there is little data in the literature of the rate of stainless steel corrosion on clinical furniture like the WDT. It is, however, known to occur when lower grade stainless steel is subject to heat and chemical disinfection, or both, in sterilising departments9. In the clinical environment sodium dichloroisocyanurate (NaDCC), a proprietary tablet diluted in water, is used for routine cleaning and disinfection of clinical furniture and medical equipment. In a series of bleach and NaDCC exposure tests on grades 304L (Grade 304L denotes a lower carbon content, usually with no difference in corrosion resistance) and 316 stainless steel coupons18 demonstrated the corrosive effects of bleach solutions on medical device disinfection. The NaDCC solutions did not show corrosive effects on either grade of stainless steel. The substitution of NaDCC for bleach17 is considered a safe alternative19 and can reduce the frequency of equipment replacement and the costs and can maintain the required levels of disinfection18.
Steps to minimise the corrosion risk to stainless steel include selecting the appropriate grade of stainless steel for the specific need17. Recommendations for a cleaning solution on stainless steel include the avoidance of chlorides and bleach, pH levels less than 5 or greater than 12, and boiling water above 60˚C20. The use of chloride-based chemicals for disinfection in healthcare settings may contribute to the reduced resistance appearing as damage to the unknown grade of stainless steel WDTs and other equipment over time.
One Australian manufacturer of stainless steel clinical furniture advises against the use of a commonly used cleaning and disinfection cloth as its incorrect use can cause tea staining and corrosion20. The manufacturers of the product state it is alcohol and chlorine free and ideal for cleaning and disinfecting all non-porous surfaces21. There are, however, three additives that can be damaging to stainless steel if this wipe is used as recommended on grade 304 stainless steel20.
Regular audits of new and existing WDTs for signs of corrosion and those that do not meet specifications should be replaced and/or repurposed. Where minor corrosion has occurred on existing and prospective new suitable WDTs, a remedial restoration process using a proprietary solution will need to be finessed to manage any damage that may develop in the future largely due to disinfection agents.
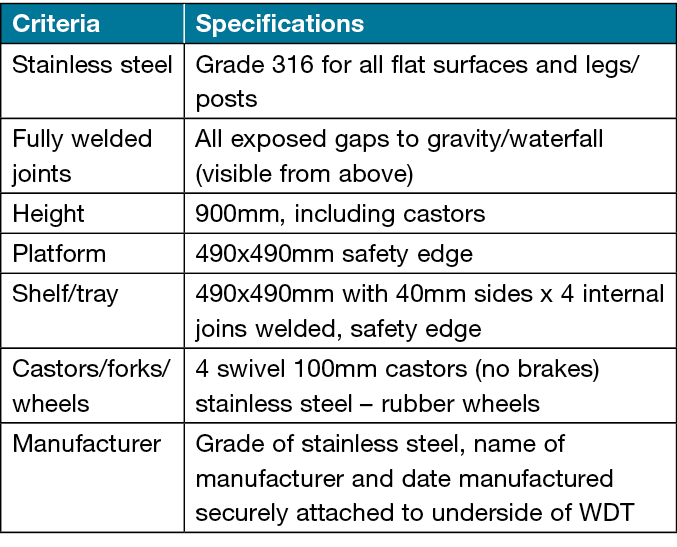
In May 2022, an audit by IPCU of procedure trolleys at an offsite outpatient clinical area reported some vertical parts had visible corrosion streaks similar to that observed in the original survey. In July 2022, as a result of IPCU audit and discussion at the interdisciplinary PEC, a second WDT using the modified specifications and upgraded to 16 stainless steel was commissioned and tested by the same team (Figure 3). The higher grade of stainless steel was considered as it may be able to resist and minimise the effects of chloride and other disinfecting agents. Grade 304 stainless steel is typically a more affordable option7 and the selection of grade 316 added approximately 10% to the cost of original grade 304 WDT. The regional PEC endorsed the final specifications (Table 3) in September 2022, and included upgrading the legs to grade 316 stainless steel as this area appears to be the main sites of corrosion. The specifications will be recommended for all WDT procurement in the Tasmanian Health Service (THS) North and shared with key wound clinicians in the THS South and North West.

Figure 3. The recommended grade 316 stainless steel with a 40mm high shelf
Table 3. Specification for WDTs
Conclusions
The introduction of WDT specifications will assist clinicians select the best equipment to meet most clinical needs. An awareness of the potential damage from cleaning and disinfection agents will also guide choice in selecting the grade of stainless steel of the WDT even if all the other specifications are not adopted. The advent of a permanent tag detailing the grade of stainless steel, manufacturer’s name, and date of manufacture as a foundation will be a standard and allow for performance auditing and value for money of WDTs over time. The larger sterile field in the commercial wound dressing packs will enhance wound dressing procedures and increase concordance with ANTT®15 and wound dressing procedures as described in the third iteration of the 2020 consensus document22.
Acknowledgements
Holly Dodd, Clinical Nurse Educator, IPCU Launceston General Hospital. National and local colleagues and key clinicians who provided their time and ideas
Conflict of interest
The author declares no conflicts of interest.
Ethics statement
An ethics statement is not applicable.
Funding
The author received no funding for this study.
Author contribution
The author is the sole contributor.
Author(s)
Clarissa A Young*
Launceston General Hospital, Launceston, TAS 7250, Australia
*Corresponding author email Clarissa.young@bigpond.com
References
- Aperam. What is stainless steel?; 2021 [cited 2021 Jun 9]. Available from: https://www.aperam.com/stainless/what-is-stainless-steel/
- Thomas PG. History of stainless steel – celebrating 100 years; 2013 [cited 2021 Jun 16]. Available from: http:www.azom.com/article.aspx?ArticleID-8307
- Metal Supermarkets. The difference between 304 and 316 stainless steel; 2018 [cited 2021 Jun 15]. Available from: https://www.metalsupermarkets.com
- Team Stainless. Benefits of stainless steel; 2020 [cited 2021 Jun 15]. Available from: https://wwww.teamstainless.org.
- ASSDA (Australian Stainless Steel Development Assoc). What is stainless steel? 2020 [cited 2022 Jul 19]. Available from: https://www.assda.asn.au/stainless-steel/what-is-stainless-steel
- Keclik B. Top 10 reasons stainless steel is right for healthcare design; 2020 [cited 2021 Aug 20]. Available from: https://www.medicaldesignbriefs.com/component/content/article/mdb/features/tecgnology-leaders/36925
- Atlas Steels. Grade data sheet; 2011 [cited 2021 Jun 9]. Available from: https://www.atlassteels.com.au/documents/Atlas_Grade_datasheet_316_rev_Jan_2011.pdf
- Werdohl UH / Euroinox (The European Stainless Steel Development Association). Stainless steel – where health comes first; 2009 [cited 2021 Jun 9]. Available from: https://www.worldstainless.org/Files/issf/non-image-files/PDF/Euro_Inox/WhenHealthComesFirst_EN.pdf
- ASSDA (Australian Stainless Steel Development Assoc). Preventing coastal corrosion (tea staining); 2010 [cited 2022 Jul 12]. Available from: https://www.assda.asn.au/images/2020/FAQs/ASSDA_Technical_FAQ_6_-_Preventing_Coastal_Corrosion.pdf
- Taylor M, McNicholas C, Nicoly C, Darzi A, Bell D, Reed J. Systematic review of the application of the plan–do–study–act method to improve quality in healthcare. BMJ Qual Saf 2014;23:290–298. Available from: https://qualitysafety.bmj.com/content/qhc/23/4/290.full.pdf
- HealthNavigator. PDSA cycle; 2022 [cited 2022 Oct 10]. Available from: https://www.healthnavigator.org.nz/clinicians/p/pdsa-cycle/
- Australasian Health Infrastructure Alliance. Part D – infection prevention and control; 2016 [cited 2021 Sep 5]. Available from: https://healthfacilityguidelines.com.au/part/part-d-infection-prevention-and-control-0
- Al Aboud K. Dressing trolleys in the healthcare centers: a perspective. Malaysian J Nurs 2014;3(2):3–4.
- Golvardi Yazdi MS, Naderizadeh M, Afzalipour R, Abedi HA, Radmehr M, Golvardi Yazdi R, Salari Pashaghi S. Designing and developing automatic trolley for washing and dressing the wounds. J Biomed Physics Engineer 2017;7(4):409–413.
- Rowley S, Clare S. Improving standards of aseptic practice through an ANTT trust-wide implementation process: a matter of prioritisation and care. J Infection Prevent 2009;10(10):S18–23.
- Emery Industries. Medical search; 2022 [cited 2022 Aug 11]. Available from: https://www.medicalsearch.com.au/what-is-tea-staining/f/23422
- Hollands W, Postlewaite J. Are your stainless steel surfaces being corroded by repeated bleach use? CEMAG; 2014 [cited 2022 Aug 23]. Available from: https://www.texwipe.com/Content/Images/uploaded/documents/Technical-Data/Stainless_Steel_Bleach.pdf
- TURI. NaDCC tablets for disinfection; 2020 [cited 2022 Jul 2]. Available from: https://www.turi.org/content/download/13101/202372/file/Fact+Sheet.+NaDCC.+2020.pdf
- Signature Clinical Furniture. Caring for your stainless steel; 2022. [cited 2022 Sep 21]. Available from: https://www.clinicalfurniture.com.au/caring-for-your-stainless-steel-products.html
- EBOS Healthcare. Tuffie 5 hospital grade disinfectant wipes; 2022 [cited 2022 Jun 12]. Available from: https://www.eboshealthcare.com.au/brands/vernacare/tuffie5-universal-sanitising-wipes/
- Jones V. The use of gauze: will it ever change? Int Wound J 2006 Jun;3(2):79–86. doi:10.1111/j.1742-4801.2006.00215.x. Available from: https://pubmed.ncbi.nlm.nih.gov/17007339/
- Wounds Australia. Application of aseptic technique in wound dressing procedure: a consensus document, 3rd ed. Canberra: Wounds Australia, 2020.



